Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacopa monnieri Extract
2.2. Animal Design
2.3. Chemicals
2.4. Prenatal Stress
2.5. Behavioral Test
2.5.1. Elevated Plus Maze (EPM)
2.5.2. Y-Maze Test
2.6. Hormone Assay
2.7. Total RNA Isolation
2.8. Protein Isolation
2.9. Quantitative Real-Time PCR (qRT-PCR)
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Behavioral Results
3.1.1. Elevated Plus Maze
3.1.2. Spatial Memory
3.2. Hormonal Results
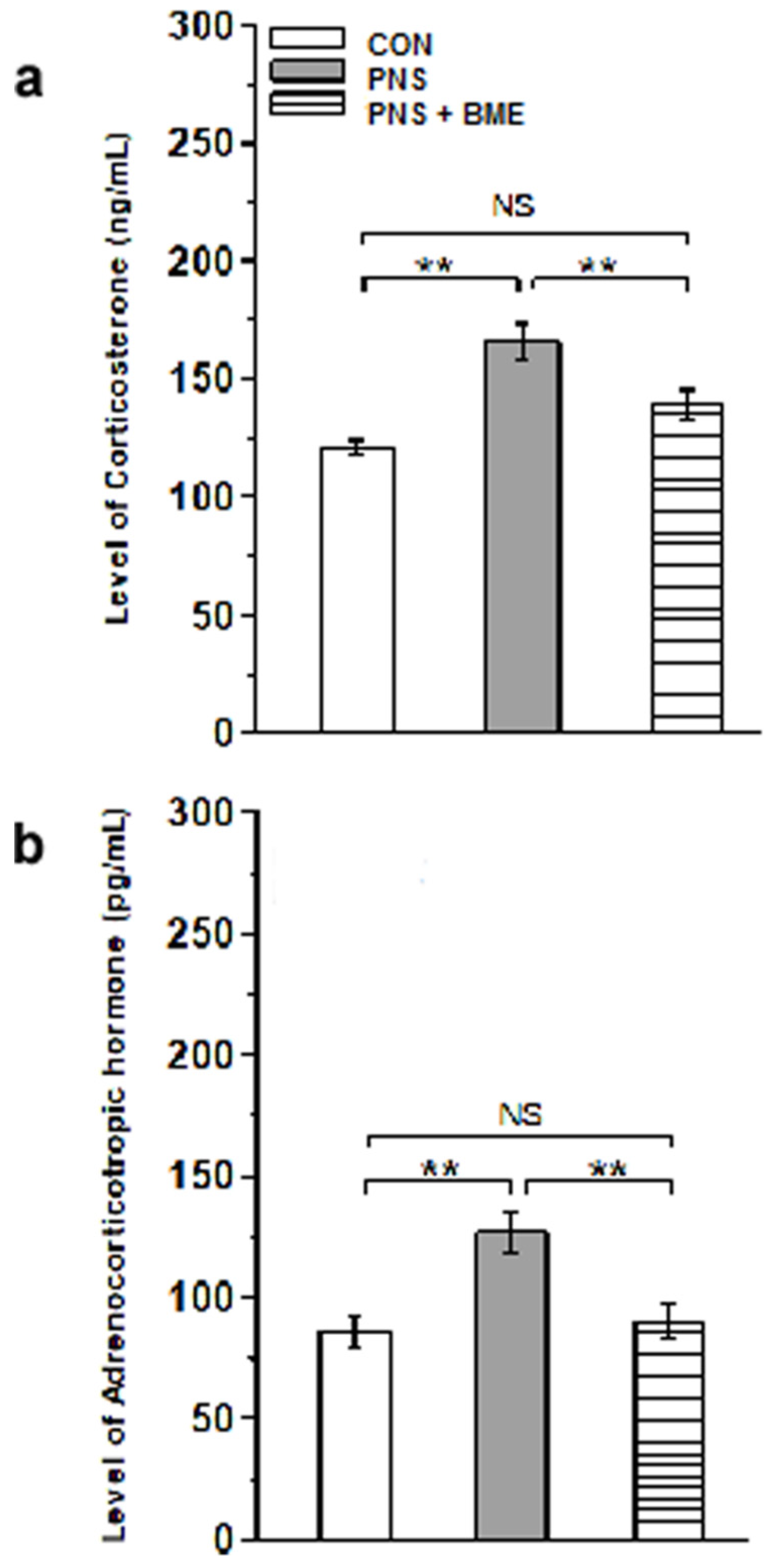
3.2.1. Effect of Exposure to BME Treatment on Prenatal Stress-Induced Changes in Corticosterone and Adrenocorticotropic Hormone
3.2.2. Effect of Exposure to BME Treatment on Prenatal Stress-Induced Changes on Glucocorticoid Receptor
3.3. Western Blot Results
3.3.1. Effect of Exposure to BME Treatment on Prenatal Stress-Induced Neuronal Apoptosis
3.3.2. Effect of Exposure to BME Treatment on Prenatal Stress-Induced Changes in Synaptophysin and Synaptotagmin-1
3.3.3. Effect of Exposure to BME Treatment on Prenatal Stress-Induced Changes on 5-HT1A and 5-HT2C Receptors
3.3.4. Effect of Exposure to BME Treatment on Gestational Stress-Induced Changes on Neurogranin (Ng) and CaMKII Signaling
3.3.5. Effect of Exposure to BME Treatment on Gestational Stress-Induced Changes on N-Methyl-D-Aspartate Receptors (2A,2B)
3.3.6. Effect of Exposure to BME Treatment on Postnatal Stress-Induced Changes in Postsynaptic Density Protein-95
3.3.7. Effect of Exposure to BME Treatment on Prenatal Stress-Induced Changes in Expression of Pro and Mature Brain-Derived Neurotrophic Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newport, D.J.; Stowe, Z.N.; Nemeroff, C.B. Parental depression, animal models of an adverse life event. Am. J. Psychiatry 2002, 159, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.R.; Bale, T.L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008, 28, 9055–9065. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Vera, T.M.; Battaglia, G. Prenatal exposure to fluoxetine (Prozac) produces site-specific and age-dependent alterations in brain serotonin transporters in rat progeny, evidence from autoradiographic studies. J. Pharmacol. Exp. Ther. 1998, 286, 1474–1481. [Google Scholar] [PubMed]
- Forcelli, P.A.; Heinrichs, S.C. Teratogenic effects of maternal antidepressant exposure on neural substrates of drug-seeking behavior in offspring. Addict. Biol. 2008, 13, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, Y.; Li, Y.; Zhu, H.; Wang, L.; Zhu, M.Y. Chronic social defeat up-regulates expression of the serotonin transporter in rat dorsal raphe nucleus and projection regions in a glucocorticoid-dependent manner. J. Neurochem. 2012, 123, 6. [Google Scholar] [CrossRef]
- Miyagawa, K.; Tsuji, M.; Ishii, D.; Takeda, K.; Takeda, H. Prenatal stress induces vulnerability to stress together with the disruption of central serotonin neurons in mice. Behav. Brain Res. 2015, 277, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, Z.; Ren, M.; Wang, J.; Gao, J.; Guo, Y.; Xu, K.; Li, F.; Zhu, D.; Zhang, H.; et al. Social defeat stress before pregnancy induces depressive-like behaviours and cognitive deficits in adult male offspring, correlation with neurobiological changes. BME Neurosci. 2018, 19, 1–22. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse, the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012, 13, 22–37. [Google Scholar] [CrossRef]
- Weinstock, M. Prenatal stressors in rodents, effects on behavior. Neurobiol. Stress 2017, 6, 3–13. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Li, L.; Wang, P.; Ji, X.; Ai, H.; Zhang, L.; Li, L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 2010, 83, 196–201. [Google Scholar] [CrossRef]
- Sasaki, T.; Kitagawa, K.; Yagita, Y.; Sugiura, S.; Omura-Matsuoka, E.; Tanaka, S.; Matsushita, K.; Okano, H.; Tsujimoto, Y.; Hori, M. Bcl2 enhances survival of newborn neurons in the normal and ischemic hippocampus. Neurosci. Res. 2006, 84, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Price, M.L.; Curtis, A.L.; Kirby, L.G.; Valentino, R.J.; Lucki, I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology 1998, 18, 492–502. [Google Scholar] [CrossRef]
- Higuchi, Y.; Soga, T.; Parhar, I.S. Regulatory Pathways of Monoamine Oxidase A during Social Stress. Front. Neurosci. 2017, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, S.; Vernon, M.M.; Pan, J.; Chen, L.; Barish, P.A.; Zhang, Y.; Acharya, A.P.; Yu, J.; Govindarajan, S.S.; et al. Curcumin prevents corticosterone-induced neurotoxicity and abnormalities of neuroplasticity via 5-HT receptor pathway. J. Neurochem. 2011, 118, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.X.; Huang, G.D.; Su, F.; Wang, H.; Zhang, C.; Yu, X. Vortioxetine administration attenuates cognitive and synaptic deficits in 5×FAD mice. Psychopharmacology 2020, 237, 1233–1243. [Google Scholar] [CrossRef]
- Gassowska, M.; Baranowska-Bosiacka, I.; Moczydlowska, J.; Frontczak-Baniewicz, M.; Gewartowska, M.; Struzynska, L.; Gutowska, I.; Chlubek, D.; Adamczyk, A. Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring. Toxicology 2016, 373, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model, Gender Difference and Metabolomics Study. Front. Pharmacol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Bader, L.R.; Carboni, J.D.; Burleson, C.A.; Cooper, M.A. 5-HT1A receptor activation reduces fear-related behaviour following social defeat in Syrian hamsters. Pharmacol. Biochem. Behav. 2014, 122, 182–190. [Google Scholar] [CrossRef][Green Version]
- Akatsu, S.; Ishikawa, C.; Takemura, K.; Ohtani, A.; Shiga, T. Effects of prenatal stress and neonatal handling on anxiety, spatial learning and serotonergic system of male offspring mice. Neurosci. Res. 2015, 101, 15–23. [Google Scholar] [CrossRef]
- Mikhalienko, V.A.; Butkevich, I.P. Prenatal stimulation of 5-HT 1A receptors improves adaptive behaviour in prenatally stressed rats. Bull. Exp. Biol. Med. 2019, 166, 306–330. [Google Scholar] [CrossRef]
- Heisler, L.K.; Zhou, L.; Bajwa, P.; Hsu, J.; Tecott, L.H. Serotonin 5-HT2C receptors regulate anxiety-like behaviour. Genes Brain Behav. 2007, 6, 491–496. [Google Scholar] [CrossRef]
- Purkayastha, S.; Ford, J.; Kanijilal, B.; Diallo, S.; Inigo, D.R.J.; Neuwirth, L.; El Idrissi, A.; Ahmed, Z.; Wierasko, A.; Azmitia, E.C.; et al. Clozapine functions through the prefrontal cortex serotonin 1A receptor to heighten neuronal activity via calmodulin kinase II–NMDA receptor interactions. J. Neurochem. 2012, 120, 396–407. [Google Scholar] [CrossRef]
- Sanhueza, M.; Fernndez-Vilalobos, G.; Stein, I.S.; Kasumova, G.; Zhang, P.; Bayer, K.U.; Otmakhov, N.; Hell, J.W.; Lisman, J. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci. 2011, 31, 9170–9178. [Google Scholar] [CrossRef] [PubMed]
- Incontro, S.; Diaz-Alonso, J.; Lafrati, J.; Vieria, M.; Asensio, C.S.; Sohal, V.S.; Roche, K.W.; Bender, K.J.; Nicoll, R.A. The CaMKII/NMDA receptor complex control hippocampal synaptic transmission by kinase-dependent and independent mechanism. Nat. Commun. 2018, 9, 2069. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; Ishikawa, Y.; Yagi, H.; Iguchi, T.; Oka, Y.; Kuroda, K.; Iwata, K.; Kiyonari, H.; Matsuda, S.; Matsuzaki, H.; et al. PIP3-PhIdb2 is crucial for LTP regulating synaptic NMDA and AMPA receptor density and PSD95 turnover. Sci. Rep. 2019, 9, 4305. [Google Scholar] [CrossRef]
- Yeh, C.M.; Huang, C.C.; Hsu, K.S. Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. J. Physiol. 2012, 590, 991–1010. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, W.; Zhang, W.; Dong, E. Gestational stress induces depressive-like and anxiety phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics 2016, 11, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, N.; Rastogi, R.P.; Dhar, M.L. Chemical examination of Bacopa monniera Wettst. The constitution of Bacoside A. Indian J. Chem. 1965, 3, 24–29. [Google Scholar]
- Basu, N.; Rastogi, R.P.; Dhar, M.L. Chemical examination of Bacopa monniera, Wettst: Part III Bacoside B. Indian J. Chem. 1967, 5, 84–86. [Google Scholar]
- Singh, H.K.; Rastogi, R.P.; Srimal, R.C.; Dhawan, B.N. Effect of bacoside A and B on avoidance responses in rats. Phytother. Res. 1988, 2, 70–75. [Google Scholar] [CrossRef]
- Banerjee, R.; Hazra, S.; Ghosh, A.K.; Mondal, A.C. Chronic administration of Bacopa monniera increases BDNF protein and mRNA expressions, a study in chronic unpredictable stress induced animal model of depression. Psychiatry Investig. 2014, 11, 3. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Mondal, A.C. Neuroprotective, neurotrophic and anti-oxidative role of Bacopa monnieri on CUS induced model of depression in rat. Neurochem. Res. 2016, 41, 11. [Google Scholar] [CrossRef]
- Calabrese, C.; Gregory, W.L.; Leo, M.; Kraemer, D.; Bone, K.; Oken, B. Effects of standardized Bacopa monnieri extract on congnitive performance, anxiety, and depression in the elderly, a randomized, double–blind, placebo-controlled trail. J. Altern. Complement. Med. 2008, 14, 707–713. [Google Scholar] [CrossRef]
- Benson, S.; Downey, L.A.; Stough, C.; Wetherell, M.; Zangara, A.; Scholey, A. An acyte, double-blind, placebo-controlled cross-over study of 320 mg and 640 mg doses of Bacopa monnieri (CDRI 08) on multitasking stress reactivity and mood. Phytother. Res. 2014, 28, 551–559. [Google Scholar] [CrossRef]
- Liu, J.; Mori, A. Stress, aging, and brain oxidative damage. Neurochem. Res. 1999, 24, 1479–1497. [Google Scholar] [CrossRef]
- Plaza-Briceño, W.; Estay, S.F.; de la Fuente-Ortega, E.; Gutierrez, C.; Sanchez, G.; Hidalgo, C.; Chavez, A.E.; Haeger, P.A. N-Methyl-d-Aspartate Receptor Modulation by Nicotinamide Adenine Dinucleotide Phosphate Oxidase Type 2 Drives Synaptic Plasticity and Spatial Memory Impairments in Rats Exposed Pre- and Postnatally to Ethanol. Antioxid. Redox Signal. 2020, 32, 602–617. [Google Scholar] [CrossRef]
- Patel, S.K.; Singh, S.; Singh, H.K.; Singh, S.K. Effect of standardized extract of Bacopa monnieri (CDRI-08) on testicular functions in adult male mice. J. Ethnopharmacol. 2017, 197, 101–109. [Google Scholar] [CrossRef]
- Sfikakis, A.; Galanopoulou, P.; Konstandi, M.; Tsakayannis, D. Stress through handling for vaginal screening, serotonin, and ACTH response to ether. Pharmacol. Biochem. Behav. 1996, 53, 965–970. [Google Scholar] [CrossRef]
- Arakawa, H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behav. Brain Res. 2018, 341, 98–108. [Google Scholar] [CrossRef]
- Lee, Y.A.; Kim, Y.J.; Goto, Y. Cognitive and affective alterations by prenatal and postnatal stress interaction. Physiol. Behav. 2016, 165, 146–153. [Google Scholar] [CrossRef]
- Walf, A.; Cheryl, A.F. The use of the elevated plus maze as as assay of anxiety-related behaviour in rodents. Nat. Protoc. 2007, 2, 322. [Google Scholar] [CrossRef]
- Conrad, C.D.; Galea, L.A.; Kuroda, Y.; McEwen, B.S. Chronic stress impairs rat spatial memory on the Y maze and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996, 110, 1321–1334. [Google Scholar] [CrossRef]
- Mohri, M.; Rezapoor, H. Effects of heprin citrate and EDTA plasma biochemistry of sheep comparison with serum. Res. Vet. Sci. 2009, 86, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Zapala, M.A.; Hovatta, I.; Ellison, J.A.; Wodicka, L.; Del Rio, J.A.; Tennant, R.; Tynan, W.; Broide, R.S.; Helton, R.; Stoveken, B.S.; et al. Adult mouse brain gene expression patterns bear an embryologic imprint. Proc. Natl. Acad. Sci. USA 2005, 102, 10357–10362. [Google Scholar] [CrossRef] [PubMed]
- Preethi, J.; Singh, H.K.; Charles, P.D.; Rajan, K.E. Participation of microRNA124-CREB pathway, a parallel memory enhancing mechanism of standardized extract of Bacopa moniera (BESEB CDRI-08). Neurochem. Res. 2012, 10, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Bogdanowicz, W.; Haupt, M.; Marimuthu, G.; Rajan, K.E. Role of olfactory bulb serotonin in olfactory learning in the greater short-nosed fruit bat, Cynopterus sphinx (Chiroptera, Pteropodidae). Brain Res. 2010, 1352, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, S.; Bogdanowicz, W.; Raghuram, H.; Marimuthu, G.; Rajan, K.E. Structure of distress call, implication for specificity and activation of dopaminergic system. J. Comp. Physiol. A 2016, 202, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Raguvarman, D.; Rajan, K.E. Environmental enrichment reduces anxiety by differently activating serotonergic and neuropeptide Y (NPY)-ergic system in Indian field mouse (Musbooduga), an animal model for post-traumatic stress disorder. PLoS ONE 2015, 10, e0127945. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bock, J.; Rether, K.; Groger, N.; Xie, L.; Braun, K. Perinatal programming of emotional brain circuits, An integrative from from system to moldecules. Front. Neurosci. 2014, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Davis, E.P.; Shahbaba, B.; Pruessner, J.C.; Head, K.; Sandman, C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problem. Proc. Natl. Acad. Sci. USA 2012, 109, E1312–E1319. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.M.; Buitelaar, J.K.; de Weerth, C. Mechanisms underlying the effects of prenatal psychological stress on child outcomes, Beyond the HPA axis. Eur. Child. Adolesc. Psychiatry 2014, 23, 943–956. [Google Scholar] [CrossRef]
- Radtke, K.M.; Ruf, M.; Gunter, H.M.; Dohrmann, K.; Schauer, M.; Meyer, A.L.; Elbert, T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticod receptor. Trans. Psychiatry 2011, 1, e21. [Google Scholar] [CrossRef]
- Wilson, C.A.; Vazdarjanova, A.; Terry, A.V., Jr. Exposure to variable prenatal stress in rats, effets on anxiety-related behaviours, innate and contextual fear and fear extinction. Behav. Brain Res. 2013, 238, 279–288. [Google Scholar] [CrossRef]
- Matrisciano, F.; Tueting, P.; Dalal, I.; Kadriu, B.; Grayson, D.R.; Davis, J.M.; Nicoletti, F.; Guidotti, A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology 2013, 68, 184–194. [Google Scholar] [CrossRef]
- Aziz, N.A.; Kendall, D.A.; Pardon, M.C. Prenatal exposure to chronic mild stress increases corticosterone levels in the amniotic fluid and induces cognitive deficits in female offspring, improved by treatment with the antidepressant drug amitriptyline. Behav. Brain Res. 2012, 231, 29–39. [Google Scholar] [CrossRef]
- Drake, A.J.; Tang, J.I.; Nyirenda, M.J. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin. Sci. 2007, 113, 219–232. [Google Scholar] [CrossRef]
- Sheikh, N.; Ahmad, A.; Siripurapu, K.B.; Kuchibhotla, V.K.; Singh, S.; Palit, G. Effect of Bacopa monniera on stress induced changes in plasma corticosterone and brain monoamines in rats. J. Ethnopharmacol. 2007, 111, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.L.; Conway-Campbell, B.L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 2010, 11, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Hapgood, J.P.; Avenant, C.; Moliki, J.M. Glucocorticoid-independent modulation of GR activity: Implications for immunotherapy. Pharmacol. Ther. 2016, 165. [Google Scholar] [CrossRef]
- Pérez-Nievas, B.G.; García-Bueno, B.; Caso, J.R.; Menchén, L.; Leza, J.C. Corticosterone as a marker of susceptibility to oxidative/nitrosative cerebral damage after stress exposure in rats. Psychoneuroendocrinology 2007, 32, 703–711. [Google Scholar] [CrossRef]
- Orlovsky, M.A.; Dosenko, V.E.; Spiga, F.; Skiibo, G.C.; Lightman, S.L. Hippocampus remodeling by chronic stress accompanied by GR, proteasome and caspase-3 overexpression. Brain Res. 2014, 1593, 83–94. [Google Scholar] [CrossRef]
- Filipovic, D.; Zlatkovic, J.; Inta, D.; Bjelobaba, I.; Stojiljkovic, M.; Gass, P. Chronic isolation stress predisposes the frontal cortex but not the hippocampus to the potentially detrimental release of cytochrome c from mitochondria and the activation of caspase-3. J. Neurosci. Res. 2011, 89, 9. [Google Scholar] [CrossRef]
- Höschl, C.; Hajek, T. Hippocampal damage mediated by corticosteroids—A neuropsychiatric research challenge. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 81–88. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Sheng, C.; Peng, W.; Hui, S.; Gong, W.; Chen, S. Icarin prevents amyloid beta-induced via the PI3K/Akt pathway in PC-12 cells. Evid. Based Complement. Altern. Med. 2015, 2015, 235265. [Google Scholar] [CrossRef]
- Means, J.C.; Venkatesan, A.; Gerdes, B.; Fan, J.Y.; Bjes, E.S.; Price, J.L. Drosophila spaghetti and doubletime link the circadian clock and light to caspases, apoptosis and tauopathy. PLoS Genet. 2015, 11, e1005171. [Google Scholar] [CrossRef]
- Krishna, G.; Hosamani, R. Muralidhara Bacopa monnieri supplements offset paraquat-induced behavioural phenotype and brain oxidative pathways in mice. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 57–66. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family, Bcl2 proteins and apoptosis, an update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 family, key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef]
- Mondal, P.; Trigun, S.K. Bacopa monnieri Extract (CDRI-08) modulates the NMDA receptor subunits and nNOS-apoptosis axis in cerebellum of hepatic encephalopathy rats. Evid. Based Complement. Altern. Med. 2015, 535013. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, S.; Yadav, S.K.; Verma, R.; Singh, S.P.; Mahdi, A.A. Role of ethanolic extract of Bacopa monnieri against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced mice model via inhibition of apoptotic pathways of dopaminergic neurons. Brain Res. Bull. 2017, 135, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.H.; Matthews, S.G. Programming of the hypothalamo-pituitary-adrenal axis, serotonergic involvement. Stress 2004, 7, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Gemmel, M.; Rayen, I.; Lotus, T.; van Donkelaar, E.; Steinbusch, H.W.; De Lacalle, S.; Kokras, N.; Dalla, C.; Pawluski, J.L. Developmental fluoxetine and prenatal stress effects on serotonin, dopamine, and synaptophysin density in the PFC and hippocampus of offspring at weaning. Dev. Psychobiol. 2016, 58, 315–327. [Google Scholar] [CrossRef]
- Gemmel, M.; Kokras, N.; Dalla, C.; Pawluski, J.L. Perinatal fluoxetine prevents the effect of pre-gestational maternal stress on 5-HT in the PFC, but maternal stress has enduring effects on mPFC synaptic structure in offspring. Neuropharmacology 2018, 128, 168–180. [Google Scholar] [CrossRef]
- Flak, J.N.; Ostrander, M.M.; Tasker, J.G.; Herman, J.P. Chronic stress-induced neurotransmitter plasticity in the PVN. J. Comp. Neurol. 2009, 517, 156–165. [Google Scholar] [CrossRef]
- Hescham, S.; Grace, L.; Kellaway, L.A.; Bugarith, K.; Russell, V.A. Effect of exercise on synaptophysin and calcium/calmodulin-dependent protein kinase levels in prefrontal cortex and hippocampus of a rat model of developmental stress. Metab. Brain Dis. 2009, 24, 701–709. [Google Scholar] [CrossRef]
- Ge, J.F.; Qi, C.C.; Zhou, J.N. Imbalance of leptin pathway and hypothalamus synaptic plasticity markers are associated with stress-induced depression in rats. Behav. Brain Res. 2013, 249, 38–43. [Google Scholar] [CrossRef]
- Ge, J.F.; Xu, Y.Y.; Qin, G.; Peng, Y.N.; Zhang, C.F.; Liu, X.R.; Liang, L.C.; Wang, Z.Z.; Chen, F.H. Depression-like Behavior Induced by Nesfatin-1 in Rats, Involvement of Increased Immune Activation and Imbalance of Synaptic Vesicle Proteins. Front. Neurosci. 2015, 9, 429. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Biou, V.; Xu, W.; Schluter, O.; Malenka, R.C. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat. Neurosci. 2009, 12, 172–181. [Google Scholar] [CrossRef]
- Chai, G.S.; Jiang, X.; Ni, Z.F.; Ma, Z.W.; Xie, A.J.; Cheng, X.S.; Wang, J.Z.; Liu, G.P. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J. Neurochem. 2013, 124, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, Z.; Rodriguez, S.; Hyde, K.; Alford, S.; Hamm, H.E. Gβγ Binds to the Extreme C Terminus of SNAP25 to Mediate the Action of Gi/o-Coupled G Protein-Coupled Receptors. Mol. Pharmacol. 2016, 89, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Alford, S.; Hamm, H.; Rodriguez, S.; Zurawski, Z. Gβγ SNARE Interactions and Their Behavioral Effects. Neurochem. Res. 2019, 44, 636–649. [Google Scholar] [CrossRef]
- Jørgensen, H.; Knigge, U.; Kjær, A.; Møller, M.; Warberg, J. Serotonergic stimulation of corticotropin-releasing hormone and pro-opiomelanocortin gene expression. J. Neuroendocrinol. 2002, 14, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.A. Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats, a possible mechanism by which stress influences brain development. Pharmacol. Biochem. Behav. 1990, 35, 943–947. [Google Scholar] [CrossRef]
- Van den Hove, D.L.A.; Lauder, J.M.; Scheepens, A.; Prickaerts, J.H.; Blanco, C.E.; Steinbusch, H.W.M. Prenatal stress in the rat alters 5-HT1A receptor binding in the ventral hippocampus. Brain Res. 2006, 1090, 29–34. [Google Scholar] [CrossRef]
- Morrison, K.E.; Cooper, M.A. A role for 5-HT1A receptors in the basolateral amygdala in the development of conditioned defeat in Syrian hamsters. Pharmacol. Biochem. Behav. 2012, 10, 3. [Google Scholar] [CrossRef]
- Akimova, E.; Lanzenberger, R.; Kasper, S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiatry 2009, 66, 7. [Google Scholar] [CrossRef]
- Fairchild, G.; Leitch, M.M.; Ingram, C.D. Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology 2003, 45, 925–934. [Google Scholar] [CrossRef]
- Lanfumey, L.; Mongeau, R.; Cohen-Salmon, C.; Hamon, M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci. Biobehav. Rev. 2008, 32, 1174–1184. [Google Scholar] [CrossRef]
- Krishnakumar, A.; Anju, T.R.; Abraham, P.M.; Paulose, C.S. Alteration in 5-HT 2C, NMDA receptor and IP3 in cerebral cortex of epileptic rats, restorative role of Bacopa monnieri. Neurochem. Res. 2015, 40, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, F.; Pasini, M.; Frasca, A.; Drago, F.; Racagni, G.; Riva, M.A. Prenatal stress alters glutamatergic system responsiveness in adult rat prefrontal cortex. J. Neurochem. 2009, 109, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Morinobu, S.; Yamamoto, S.; Matsumoto, T.; Takei, S.; Fujita, Y.; Yamawaki, S. Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder. Psychopharmacology 2013, 229, 1. [Google Scholar] [CrossRef] [PubMed]
- Gerendasy, D.G.; Sutcliffe, J.G. RC3/neurogranin, a postsynaptic calpacitin forsetting the response threshold to calcium influxes. Mol. Neurobiol. 1997, 15, 131–163. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.P.; Huang, F.L.; Jager, T.; Li, J.; Reymann, K.G.; Balschun, D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J. Neurosci. 2004, 24, 10660–10669. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, D.; Chen, R.; Cai, Q.; Jia, N.; Su, Q.; Zhang, H.; Zhu, Z.; Zeng, J.; Li, H. Expression of neurogranin in hippocampus of rat offspring exposed to restraint stress and pulsed magnetic fields. Brain Res. 2014, 1570, 26–34. [Google Scholar] [CrossRef]
- Jones, K.J.; Templet, S.; Zemoura, K.; Kuzniewska, B.; Pena, F.X.; Hwang, H.; Lei, D.J.; Haensgen, H.; Nguyen, S.; Saenz, C.; et al. Rapid, experience-dependent translation of neurogranin enables memory encoding. Proc. Natl. Acad. Sci. USA 2018, 115, E5805–E5814. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Xu, H.; Yang, H.; Chen, T.; Chen, S.; Chen, X. Chronic unpredictable stress before pregnancy reduce the expression of brain-derived neurotrophic factor and N-methyl-D-aspartate receptor in hippocampus of offspring rats associated with impairment of memory. Neurochem. Res. 2010, 35, 7. [Google Scholar] [CrossRef]
- Sun, H.; Guan, L.; Zhu, Z.; Li, H. Reduced levels of NR1 and NR2A with depression-like behaviour in different brain regions in prenatally stressed juvenile offspring. PLoS ONE 2013, 8, e81775. [Google Scholar] [CrossRef]
- Badihian, N.; Daniali, S.S.; Kelishadi, R. Transcriptional and epigenetic changes of brain derived neurotrophic factor following prenatal stress: A systematic review of animal studies. Neurosci. Biobehav. Rev. 2019. [Google Scholar] [CrossRef]
- Preethi, J.; Singh, H.K.; Rajan, K.E. Possible involvement of standardized Bacopa monniera extract (CDRI-08) in epigenetic regulation of reelin and brain-derived neurotropic factor to enhance memory. Front. Pharmacol. 2016, 7, 166. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivasangari, K.; Rajan, K.E. Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring. Antioxidants 2020, 9, 1229. https://doi.org/10.3390/antiox9121229
Sivasangari K, Rajan KE. Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring. Antioxidants. 2020; 9(12):1229. https://doi.org/10.3390/antiox9121229
Chicago/Turabian StyleSivasangari, Karunanithi, and Koilmani Emmanuvel Rajan. 2020. "Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring" Antioxidants 9, no. 12: 1229. https://doi.org/10.3390/antiox9121229
APA StyleSivasangari, K., & Rajan, K. E. (2020). Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring. Antioxidants, 9(12), 1229. https://doi.org/10.3390/antiox9121229




