Prevention of Fine Dust-Induced Vascular Senescence by Humulus lupulus Extract and Its Major Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. FD
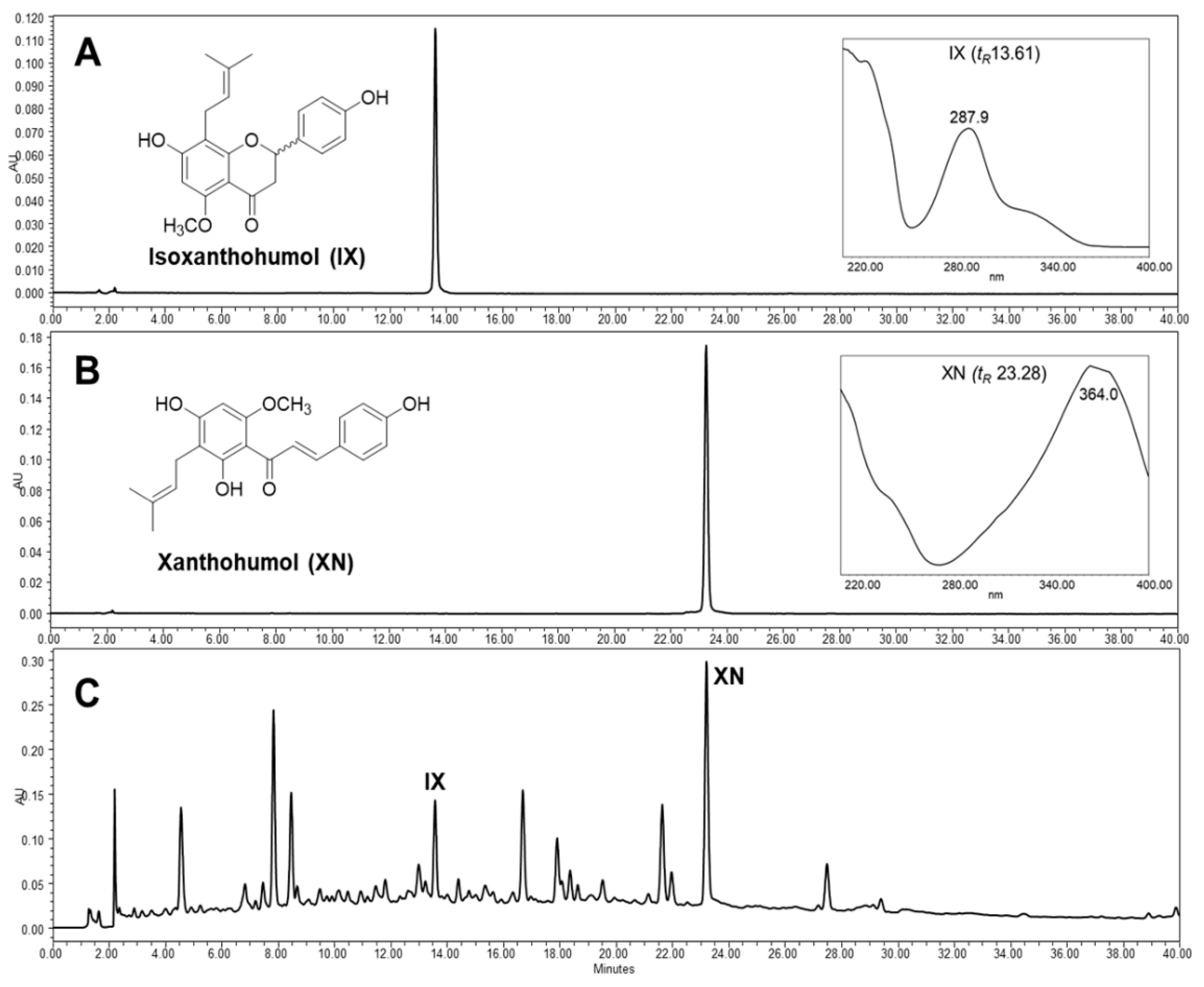
2.2. Preparation of Extract and Isolation of Hop Prenylflavonoids
2.3. Cell Culture
2.4. Detection of Senescence-Associated β-Galactosidase Activity (SA-β-gal)
2.5. Proliferation Assay
2.6. Western Blot Analysis
2.7. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels in Porcine Coronary ECs
2.8. Vascular Reactivity Study
2.9. Statistical Analysis
3. Result and Discussion
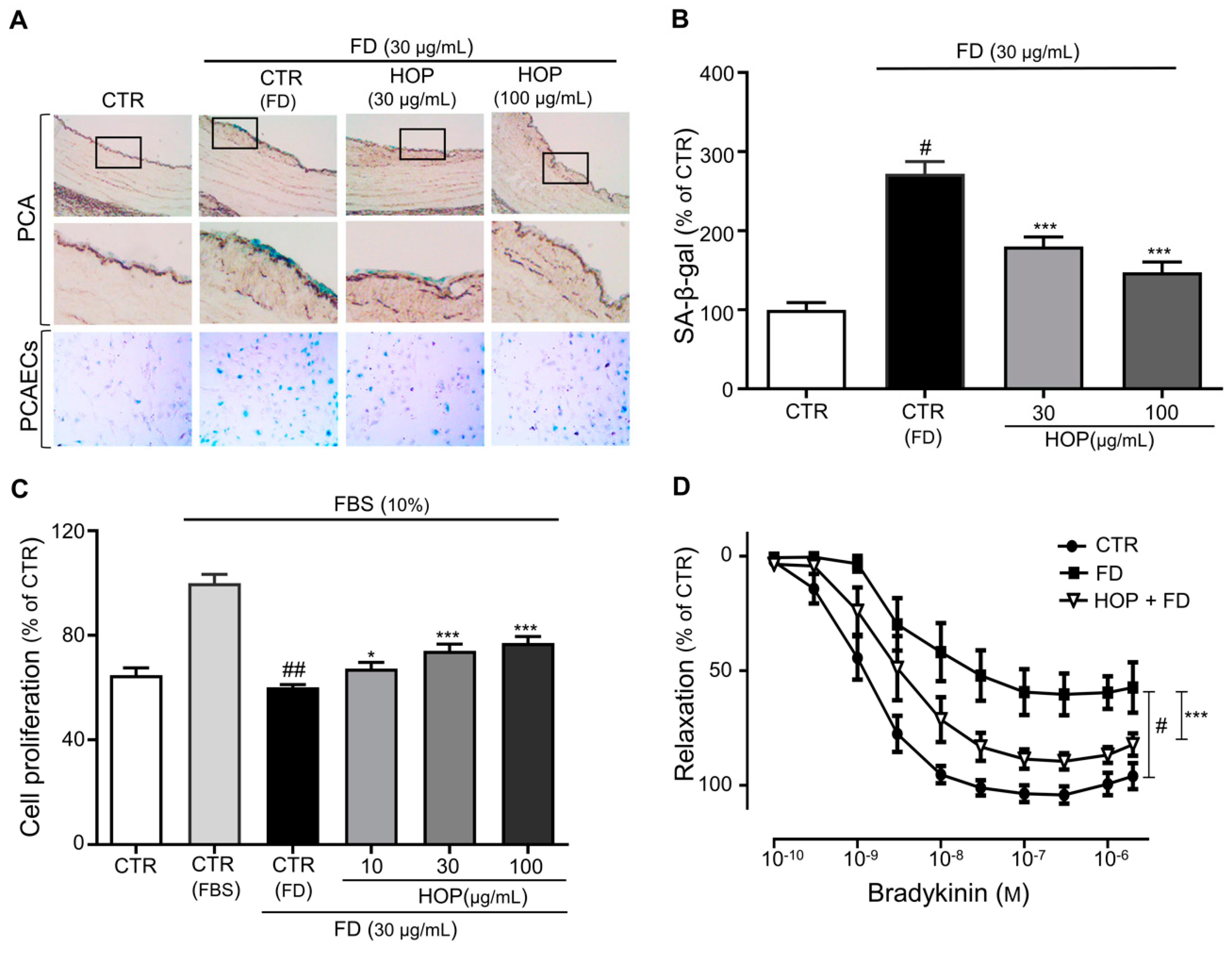
3.1. Hop Extract (HOP) Prevents FD-Induced Premature Endothelial Senescence and Dysfunction in Porcine Coronary Artery
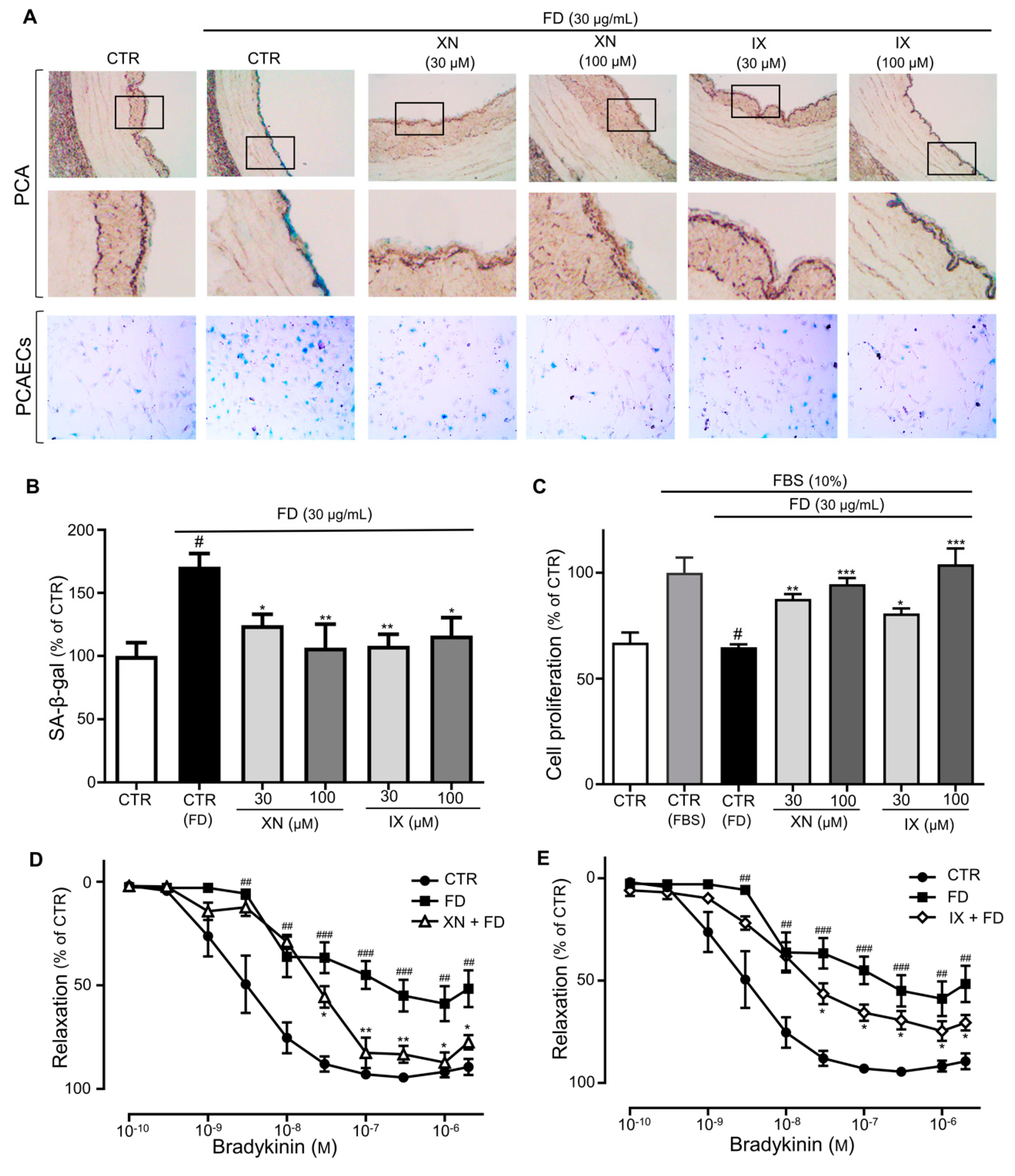
3.2. Xanthohumol and Isoxanthohumol in HOP Prevent FD-Induced Endothelial Premature Senescence
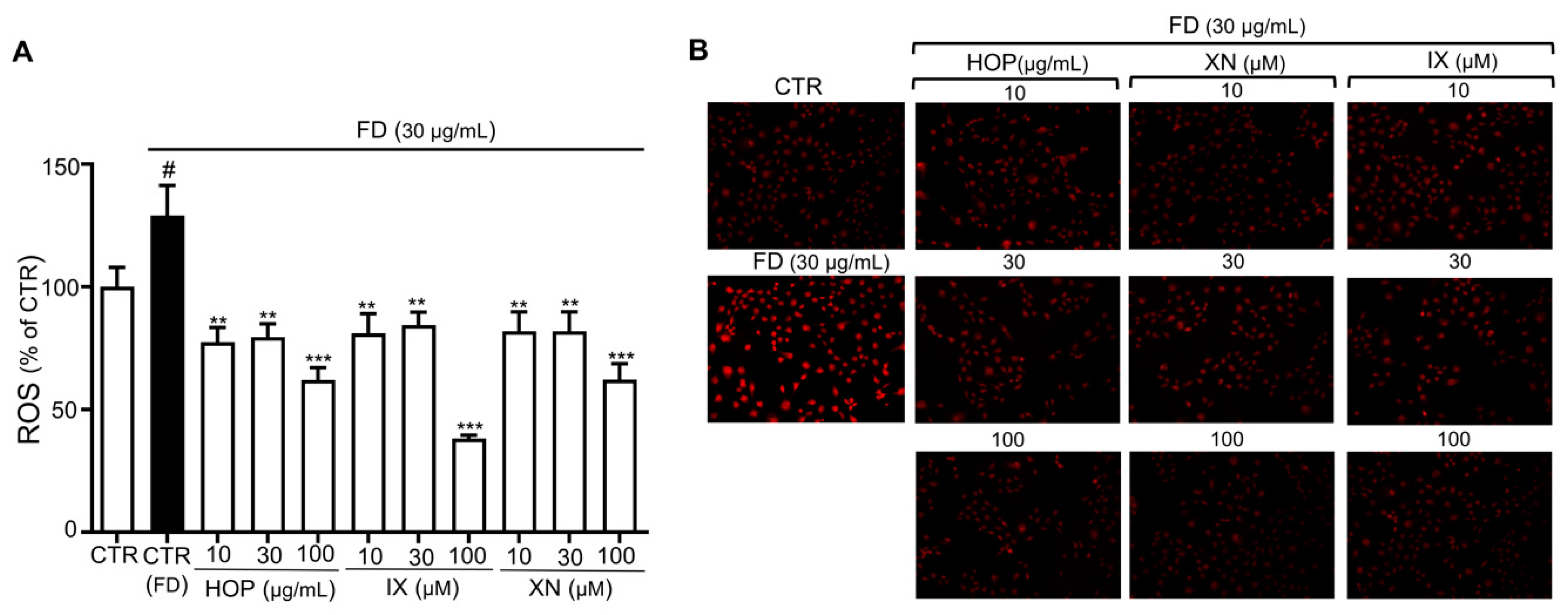
3.3. HOP and Its Major Components XN and IX Decrease FD-Induced Oxidative Stress Levels
3.4. HOP and Its Major Compounds XN and IX Downregulate Redox-Sensitive Endothelial Senescence Markers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 2015, 8, E8–E19. [Google Scholar]
- Brook, R.D.; Rajagopalan, S.; PopeIII, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.J.; Kim, B.; Lee, K. Air pollution exposure and cardiovascular disease. Toxicol. Res. 2014, 30, 71–75. [Google Scholar] [CrossRef]
- Cosselman, K.E.; Navas-Acien, A.; Kaufman, J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 627–642. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef] [Green Version]
- Finch, J.; Conklin, D.J. Air Pollution-induced vascular dysfunction: Potential role of endothelin-1 (ET-1) system. Cardiovasc. Toxicol. 2016, 16, 260–275. [Google Scholar] [CrossRef] [Green Version]
- Bhayadia, R. Senescence-induced oxidative stress causes endothelial dysfunction. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 71, 161–169. [Google Scholar] [CrossRef]
- Herrera, M.D.; Mingorance, C.; Rodríguez-Rodríguez, R.; Alvarez de Sotomayor, M. Endothelial dysfunction and aging: An update. Ageing Res. Rev. 2010, 9, 142–152. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health. Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Kim, K.S.; Kim, J.E.; Choi, K.J.; Bae, S.; Kim, D.H. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int. J. Radiat. Biol. 2014, 90, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, H.; Ha, Y.; Tilton, R.G.; Zhang, W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed. Res. Int. 2014, 2014, 902842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, M.; Hayashi, T.; Mizuno, N.; Hattori, Y.; Kuzuya, M. Intermittent high glucose implements stress-induced senescence in human vascular endothelial cells: Role of superoxide production by NADPH oxidase. PLoS ONE 2015, 10, e0123169. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Bai, X.; Chen, X. Angiotensin II induces endothelial cell senescence via the activation of mitogen-activated protein kinases. Cell Biochem. Funct. 2008, 26, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat. Res. 2016, 186, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, K.; Lee, H.H.; Gong, D.S.; Park, S.H.; Yi, E.; Schini-Kerth, V.; Oak, M.H. Fine air pollution particles induce endothelial senescence via redox-sensitive activation of local angiotensin system. Environ Pollut. 2019, 252, 317–329. [Google Scholar] [CrossRef]
- Monsalve, B.; Concha-Meyer, A.; Palomo, I.; Fuentes, E. Mechanisms of Endothelial Protection by Natural Bioactive Compounds from Fruit and Vegetables. An Acad. Bras. Ciênc, 2017; 89, 615–633. [Google Scholar]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Gormaz, J.G.; Valls, N.; Sotomayor, C.; Turner, T.; Rodrigo, R. Potential role of polyphenols in the prevention of cardiovascular diseases: Molecular bases. Curr. Med. Chem. 2016, 23, 115–128. [Google Scholar] [CrossRef]
- Schini-Kerth, V.B.; Etienne-Selloum, N.; Chataigneau, T.; Auger, C. Vascular protection by natural product-derived polyphenols: In vitro and in vivo evidence. Planta Med. 2011, 77, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti- inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [Green Version]
- Koetter, U.; Biendl, M. Hops (Humulus lupulus): A review of its historic and medicinal uses. HerbalGram 2010, 87, 44–57. [Google Scholar]
- Luzak, B.; Kassassir, H.; Roj, E.; Stanczyk, L.; Watala, C.; Golanski, J. Xanthohumol from hop cones (Humulus lupulus L.) prevents ADP-induced platelet reactivity. Arch. Physiol. Biochem. 2017, 123, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Komaitis, M. 45-Antioxidant Capacity of Hops. In Beer in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 467–474. [Google Scholar]
- Jiang, C.H.; Sun, T.L.; Xiang, D.X.; Wei, S.S.; Li, W.Q. Anticancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Front Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Strathmann, J.; Gerhauser, C. Anti-proliferative and Apoptosis-Inducing Properties of Xanthohumol, a Prenylated Chalcone from Hops (Humulus lupulus L.). Natural Compounds as Inducers of Cell Death: Volume 1, Diederich, M., Noworyta, K., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 69–93. [Google Scholar]
- Philips, N.; Samuel, P.; Lozano, T.; Gvaladze, A.; Guzman, B.; Siomyk, H.; Haas, G. Effects of Humulus lupulus extract or its components on viability, lipid peroxidation, and expression of vascular endothelial growth factor in melanoma cells and fibroblasts. Madridge J. Clin. Res. 2017, 1, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Biendl, M. Commercial hop extracts rich in xanthohumol. In Proceedings of the Scientific Commission of International Hop Grower’s Convention, Tettnang, Germany, 24–28 June 2007. [Google Scholar]
- Stevens, J.F.; Ivancic, M.; Hsu, V.L.; Deinzer, M.L. Prenylflavonoids from Humulus lupulus. Phytochemistry 1997, 44, 1575–1585. [Google Scholar] [CrossRef]
- Park, S.H.; Shim, B.S.; Yoon, J.S.; Lee, H.H.; Lee, H.W.; Yoo, S.B.; Wi, A.J.; Park, W.S.; Kim, H.J.; Kim, D.W.; et al. Vascular protective effect of an ethanol extract of Camellia japonica fruit: Endothelium-dependent relaxation of coronary artery and reduction of smooth muscle cell migration. Oxid. Med. Cell Longev. 2016, 2016, 6309565. [Google Scholar] [CrossRef] [Green Version]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.; Szczepanowska, J.; Koopman, W.J.H.; Wieckowski, M.R. Quantifying ROS levels using CM-H(2)DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef]
- Haberzettl, P.; Conklin, D.J.; Abplanalp, W.T.; Bhatnagar, A.; O’Toole, T.E. Inhalation of Fine Particulate Matter Impairs Endothelial Progenitor Cell Function Via Pulmonary Oxidative Stress. Arter. Thromb Vasc. Biol. 2018, 38, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Briet, M.; Collin, C.; Laurent, S.; Tan, A.; Azizi, M.; Agharazii, M.; Jeunemaitre, X.; Alhenc-Gelas, F.; Boutouyrie, P. Endothelial function and chronic exposure to air pollution in normal male subjects. Hypertension 2007, 50, 970–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Porath, I.; Weinberg, R. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Martens-Lobenhoffer, J.; Täger, M.; Schröder, H.; Scalera, F. Aspirin reduces endothelial cell senescence. Biochem. Biophys. Res. Commun. 2005, 334, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jia, F.; He, J.; Xie, X.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, D.Z.; Cowan, P.J.; et al. Ambient fine particulate matter suppresses in vivo proliferation of bone marrow stem cells through reactive oxygen species formation. PLoS One 2015, 10, e0127309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Bourdrel, T.; Bind, M.A.; Béjot, Y.; Morel, O.; Argacha, J.F. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017, 110, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Eccles, M.; Li, C.G. Senescence Associated β-galactosidase Staining. Bio-Protocol 2012, 2, e247. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Żołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol—Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef]
- Hohn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; Konig, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ma, Y.; Wu, X.; Garcia, J.; Wang, T. Particulate matter promotes epithelial-to-mesenchymal transition in human lung epithelial cells via ROS pathway. Eur. Respir. J. 2016, 48, PA4267. [Google Scholar]
- Wang, Q.; Zou, M.H. Measurement of reactive oxygen species (ROS) and mitochondrial ROS in AMPK knockout mice blood vessels. Methods Mol. Biol. 2018, 1732, 507–517. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, aging and Alzheimer’s disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Mamarbachi, A.M.; Villeneuve, L.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech. Ageing Dev. 2008, 129, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Mattera, R.; Benvenuto, M.; Giganti, M.G.; Tresoldi, I.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Masuelli, L.; Manzari, V.; Modesti, A.; et al. Effects of polyphenols on oxidative stress-mediated injury in cardiomyocytes. Nutrients 2017, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; McCall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Potaniec, B.; Grabarczyk, M.; Stompor, M.; Szumny, A.; Zieliński, P.; Żołnierczyk, A.K.; Anioł, M. Antioxidant activity and spectroscopic data of isoxanthohomol oxime and related compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Świtalska, M.; Podgórski, R.; Uram, Ł.; Aebisher, D.; Wietrzyk, J. Synthesis and biological evaluation of 4’-O-acetyl-isoxanthohumol and its analogues as antioxidant and antiproliferative agents. Acta Biochim. Pol. 2017, 64, 577–583. [Google Scholar] [CrossRef]
- Seliger, J.M.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzyme Inhib. Med. Chem. 2018, 33, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tronina, T.; Bartmańska, A.; Filip-Psurska, B.; Wietrzyk, J.; Popłoński, J.; Huszcza, E. Fungal metabolites of xanthohumol with potent antiproliferative activity on human cancer cell lines in vitro. Bioorg. Med. Chem. 2013, 21, 2001–2006. [Google Scholar] [CrossRef]
- Gerhauser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Neumann, I.; Scherf, H.R.; Frank, N.; Bartsch, H.; et al. Cancer chemopreventive activity of Xanthohumol, a natural product derived from hop. Mol. Cancer Ther 2002, 1, 959–969. [Google Scholar] [PubMed]
- Büchter, C.; Havermann, S.; Koch, K.; Wätjen, W. Isoxanthohumol, a constituent of hop (Humulus lupulus L.), increases stress resistance in Caenorhabditis elegans dependent on the transcription factor DAF-16. Eur. J. Nutr. 2016, 55, 257–265. [Google Scholar]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the regulation of cellular senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [Green Version]
- Schilder, Y.D.; Heiss, E.H.; Schachner, D.; Ziegler, J.; Reznicek, G.; Sorescu, D.; Dirsch, V.M. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic. Biol. Med. 2009, 46, 1598–1606. [Google Scholar] [CrossRef]
- Lassègue, B.; Griendling, K.K. NADPH oxidases: Functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Acosta, O.; de los Angeles Fortis-Barrera, M.; Barrios-Maya, M.A.; Ramírez, A.R.; Aguilar, F.J.A.; El-Hafidi, M. Reactive oxygen species from NADPH oxidase and mitochondria participate in the proliferation of aortic smooth muscle cells from a model of metabolic syndrome. Oxid. Med. Cell Longev. 2018, 2018, 5835072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, G.C.; Abbas, M.; Khemais-Benkhiat, S.; Burban, M.; Ribeiro, T.P.; Toti, F.; Idris-Khodja, N.; Côrtes, S.F.; Schini-Kerth, V.B. Replicative senescence promotes prothrombotic responses in endothelial cells: Role of NADPH oxidase- and cyclooxygenase-derived oxidative stress. Exp. Gerontol. 2017, 93, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Harrison, D.G. Angiotensin II and Superoxide Generation. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1255–1271. [Google Scholar]
- Hasan, H.; Abbas, M.; Auger, C.; Belcastro, E.; Farooq, M.A.; Park, S.H.; Ohlmann, P.; Toti, F.; Schini-Kerth, V.; Morel, O.; et al. Atrial endothelial cells senescence promotes thrombogenicity, inflammation and extracellular matrix remodeling: Role of the local Ang II/AT1 receptor pathway. Arch. Cardiovasc. Dis. Suppl. 2018, 10, 223. [Google Scholar] [CrossRef]
- Meili Wang, X.Y.; Suli, Z.; Chenfeng, M.; Ning, C.; Xiaochun, Y.; Jingwei, B.; Weiwei, H.; Qian, F.; Huirong, L. Autoantibodies against AT1 receptor contribute to vascular aging and endothelial cell senescence. Aging Dis. 2019, 10, 1012–1025. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiwakoti, S.; Adhikari, D.; Lee, J.P.; Kang, K.-W.; Lee, I.-S.; Kim, H.J.; Oak, M.-H. Prevention of Fine Dust-Induced Vascular Senescence by Humulus lupulus Extract and Its Major Bioactive Compounds. Antioxidants 2020, 9, 1243. https://doi.org/10.3390/antiox9121243
Shiwakoti S, Adhikari D, Lee JP, Kang K-W, Lee I-S, Kim HJ, Oak M-H. Prevention of Fine Dust-Induced Vascular Senescence by Humulus lupulus Extract and Its Major Bioactive Compounds. Antioxidants. 2020; 9(12):1243. https://doi.org/10.3390/antiox9121243
Chicago/Turabian StyleShiwakoti, Saugat, Deepak Adhikari, Jeong Pyo Lee, Ki-Woon Kang, Ik-Soo Lee, Hyun Jung Kim, and Min-Ho Oak. 2020. "Prevention of Fine Dust-Induced Vascular Senescence by Humulus lupulus Extract and Its Major Bioactive Compounds" Antioxidants 9, no. 12: 1243. https://doi.org/10.3390/antiox9121243
APA StyleShiwakoti, S., Adhikari, D., Lee, J. P., Kang, K.-W., Lee, I.-S., Kim, H. J., & Oak, M.-H. (2020). Prevention of Fine Dust-Induced Vascular Senescence by Humulus lupulus Extract and Its Major Bioactive Compounds. Antioxidants, 9(12), 1243. https://doi.org/10.3390/antiox9121243






