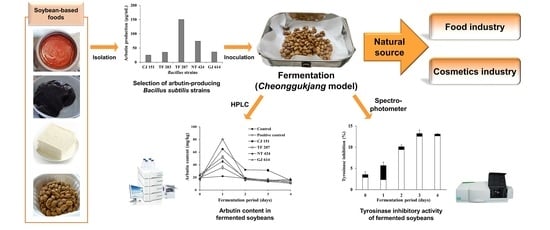
Tyrosinase Inhibitory Activity of Soybeans Fermented with Bacillus subtilis Capable of Producing a Phenolic Glycoside, Arbutin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacillus Strains from Soybean-Based Products
2.2. Preparation of Assay Medium for Arbutin Production and Arbutin Culture for HPLC Analysis
2.3. Preparation of Bacterial Suspension for Soybean Fermentation
2.4. Soybean Fermentation with Arbutin-Producing B. subtilis Using a Cheonggukjang Model
2.5. Treatment of Arbutin Cultures and Soybean Samples for Arbutin Analysis and Tyrosinase Inhibitory Activity Assay
2.6. Chromatographic Separation
2.7. Tyrosinase Inhibitory Activity Assay
2.8. Physicochemical and Microbial Analyses
2.9. Statistical Analyses
3. Results and Discussion
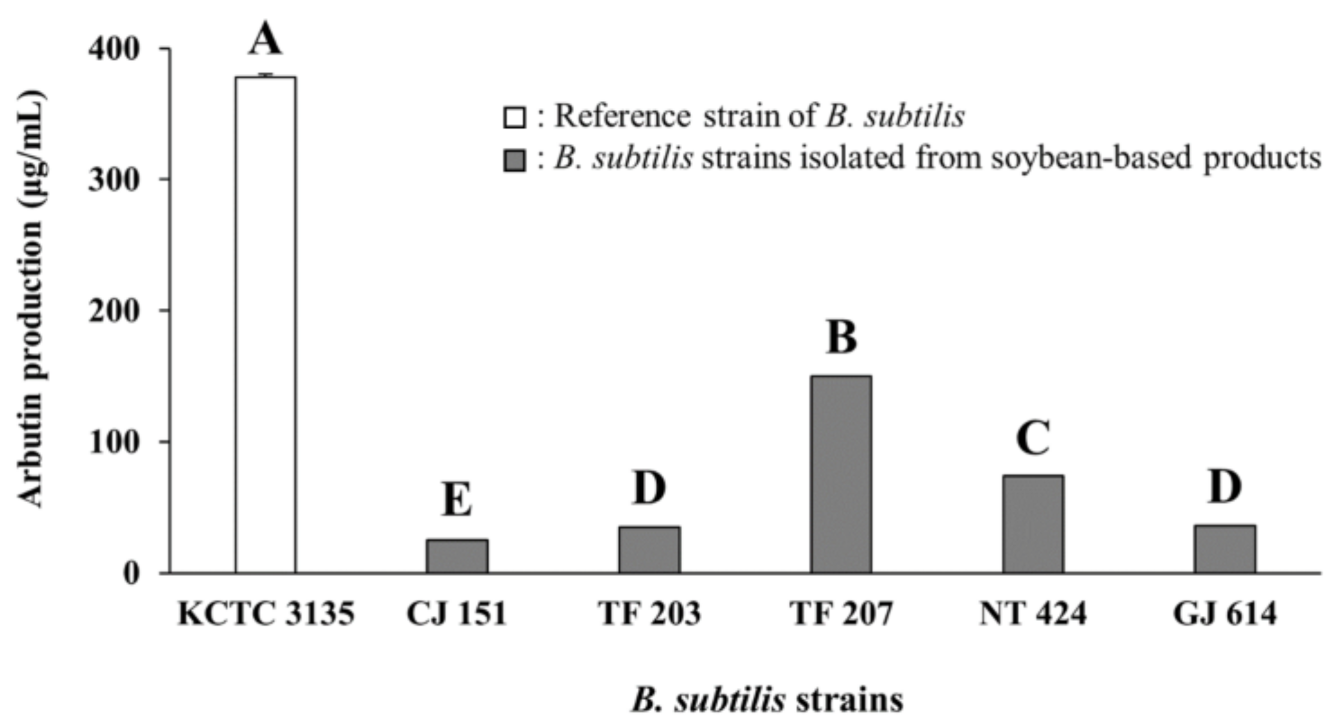
3.1. Arbutin Production by Bacillus Strains Isolated from Soybean-Based Products
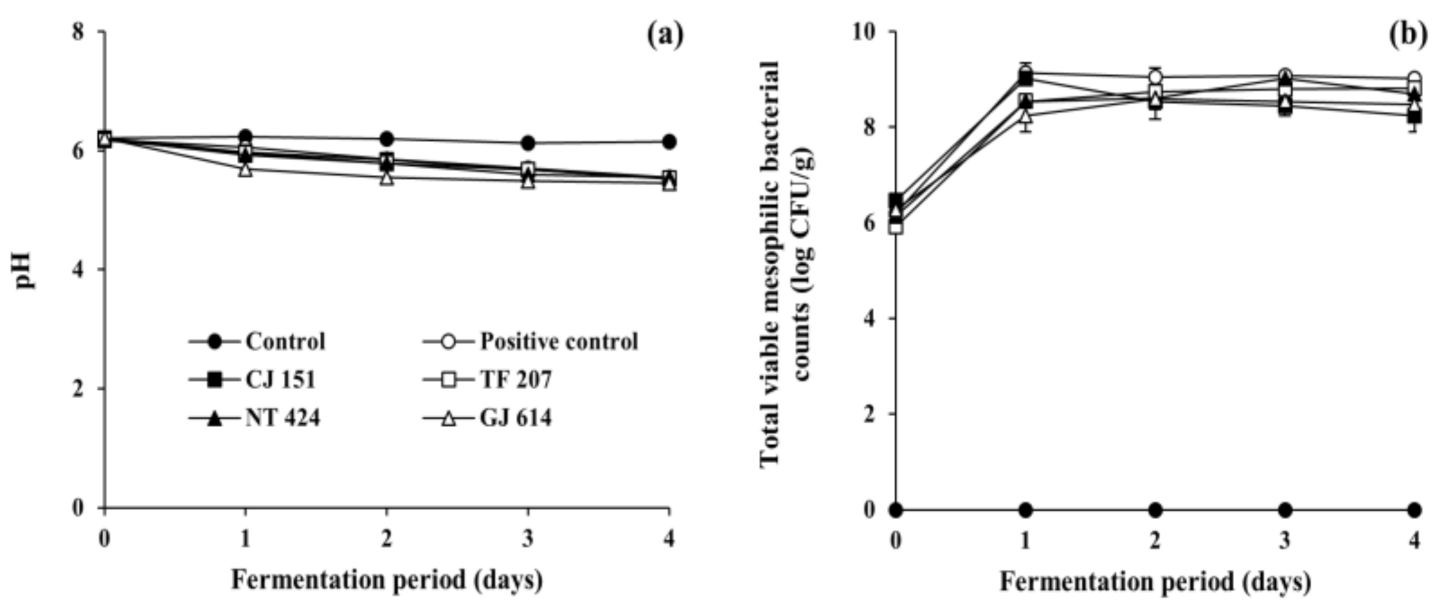
3.2. Changes in Physicochemical and Microbial Properties during Soybean Fermentation with Arbutin-Producing B. subtilis Strains
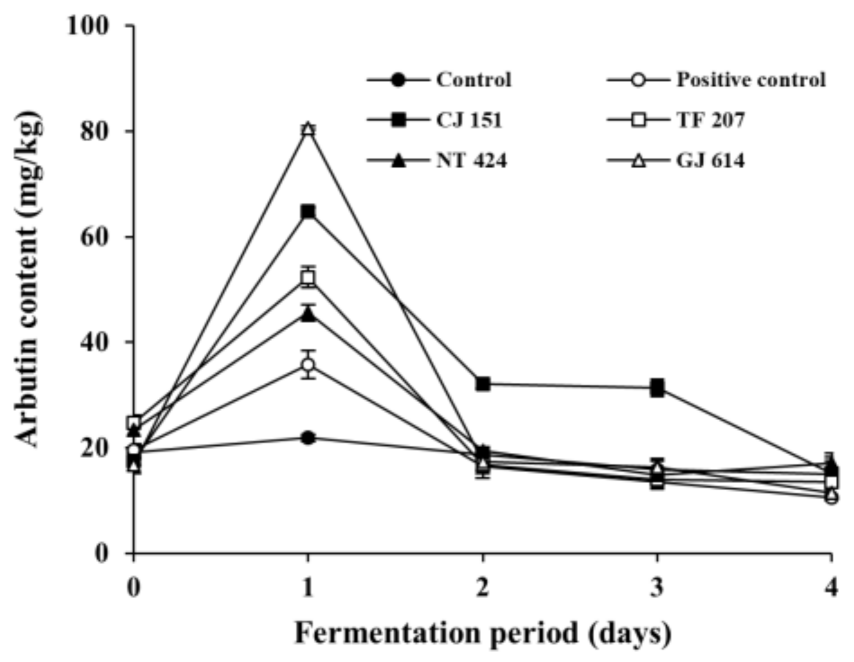
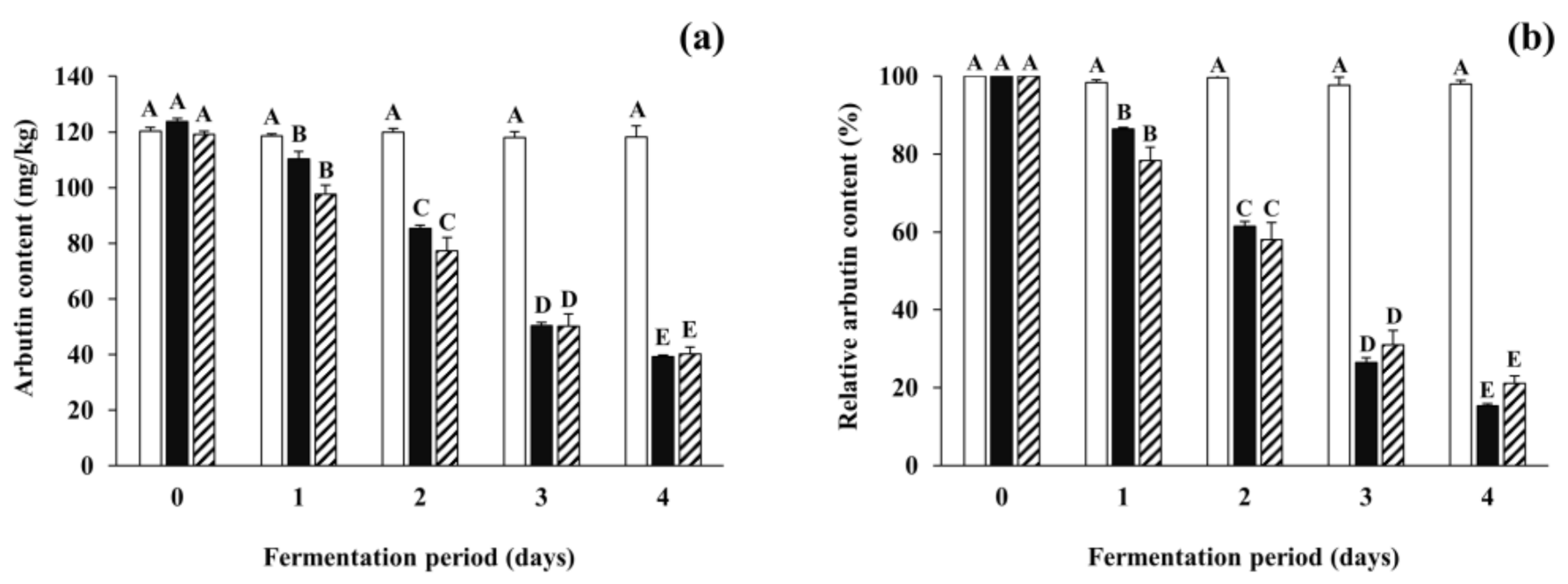
3.3. Changes in Arbutin Content during Soybean Fermentation with Arbutin-Producing B. subtilis Strains
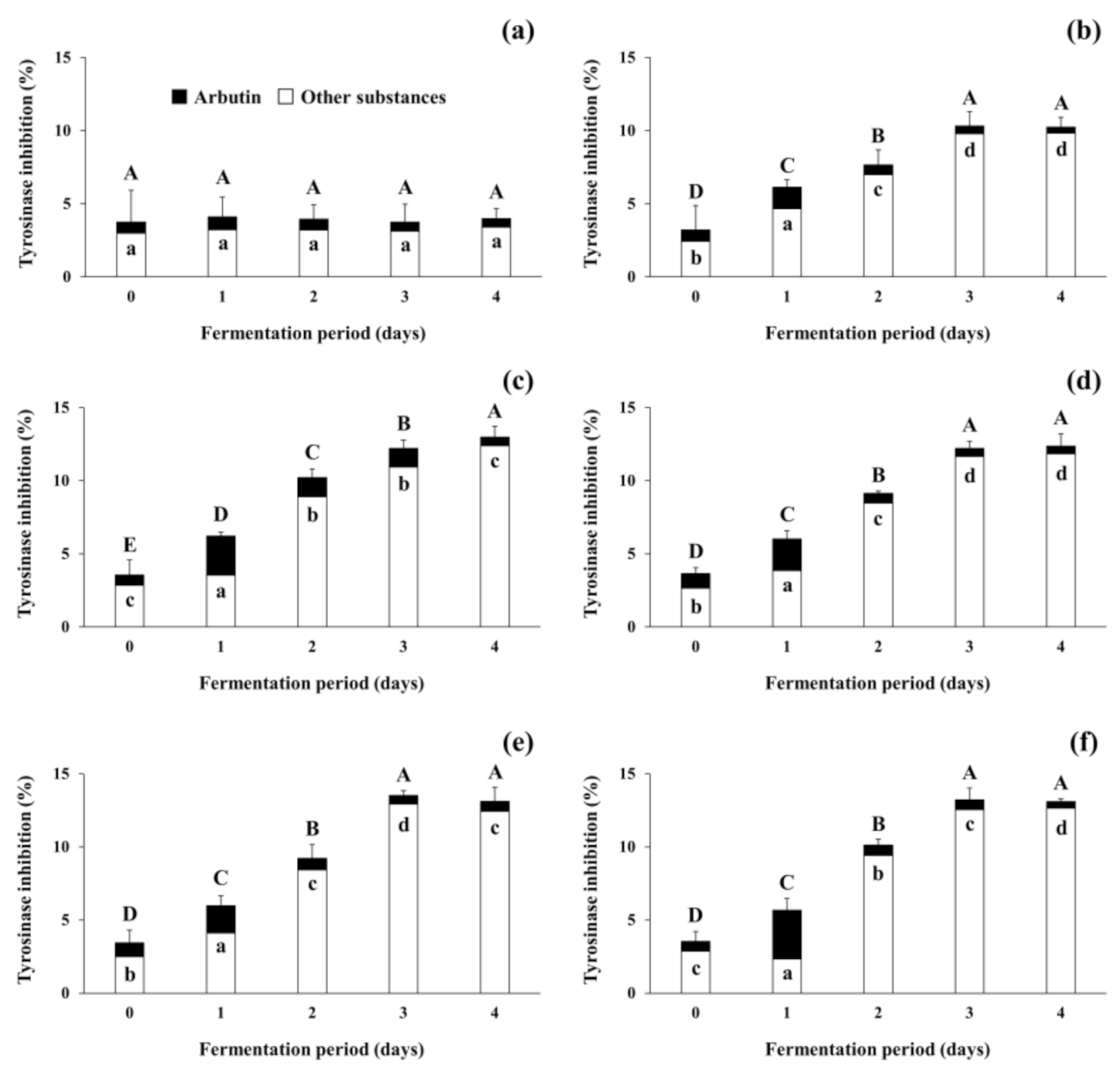
3.4. Changes in Tyrosinase Inhibitory Activity during Soybean Fermentation with Arbutin-Producing B. subtilis Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bertazzo, A.; Favretto, D.; Costa, C.V.L.; Allegri, G.; Traldi, P. Effects of ultraviolet irradiation on melanogenesis from tyrosine, Dopa and dopamine: A matrix-assisted laser desorption/ionization mass spectrometric study. Rapid Commun. Mass Spectrom. 2000, 14, 1862–1868. [Google Scholar] [CrossRef]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Invest. Dermatol. 2011, 131, E8–E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson’s disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexoudi, A.; Alexoudi, I.; Gatzonis, S. Parkinson’s disease pathogenesis, evolution and alternative pathways: A review. Rev. Neurol. 2018, 174, 699–704. [Google Scholar] [CrossRef]
- Zecca, L.; Zucca, F.A.; Wilms, H.; Sulzer, D. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003, 26, 578–580. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. S. Afr. J. Bot. 2016, 103, 89–94. [Google Scholar] [CrossRef]
- Koirala, P.; Seong, S.H.; Zhou, Y.; Shrestha, S.; Jung, H.A.; Choi, J.S. Structure–activity relationship of the tyrosinase inhibitors kuwanon G, mulberrofuran G, and albanol B from Morus species: A kinetics and molecular docking study. Molecules 2018, 23, 1413. [Google Scholar] [CrossRef] [Green Version]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Giménez, J.; Cuadros, T.; Bové, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sequlveda, M.; Peñuelas, N.; Torra, A.; et al. Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat. Commun. 2019, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Puizina-Ivić, N.; Mirić, L.; Čarija, A.; Karlica, D.; Marasović, D. Modern approach to topical treatment of aging skin. Coll. Antropol. 2010, 34, 1145–1153. [Google Scholar]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Ding, Y.; Kong, D.; Zhou, T.; Yang, N.; Xin, C.; Xu, J.; Wang, Q.; Zhang, H.; Wu, Q.; Lu, X.; et al. α-Arbutin protects against Parkinson’s disease-associated mitochondrial dysfunction in vitro and in vivo. Neuromol. Med. 2020, 22, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bellad, K.A.; Nanjwade, B.K.; Kamble, M.S.; Srichana, T.; Idris, N.F. Development of cosmeceuticals. World J. Pharm. Pharm. Sci. 2017, 6, 643–691. [Google Scholar]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert Opin. Ther. Patents 2016, 26, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hwang, S.J.; Kang, Y.S.; Jung, J.; Park, S.; Hong, J.E.; Park, Y.; Lee, H.-J. Synthesis of arbutin–gold nanoparticle complexes and their enhanced performance for whitening. Arch. Pharm. Res. 2019, 42, 977–989. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Shi, X.-X.; Chen, G.-R.; Ren, Z.-H.; Luo, L.; Yan, J. A new synthesis of α-arbutin via Lewis acid catalyzed selective glycosylation of tetra-O-benzyl-α-D-glucopyranosyl trichloroacetimidate with hydroquinone. Carbohydr. Res. 2006, 341, 1945–1947. [Google Scholar] [CrossRef]
- Nishimura, T.; Kometani, T.; Takii, H.; Terada, Y.; Okada, S. Purification and some properties of α-amylase from Bacillus subtilis X-23 that glucosylates phenolic compounds such as hydroquinone. J. Ferment. Bioeng. 1994, 78, 31–36. [Google Scholar] [CrossRef]
- Seo, D.-H.; Jung, J.-H.; Lee, J.-E.; Jeon, E.-J.; Kim, W.; Park, C.-S. Biotechnological production of arbutins (α-and β-arbutins), skin-lightening agents, and their derivatives. Appl. Microbiol. Biotechnol. 2012, 95, 1417–1425. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, Y.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Recent progress on biological production of α-arbutin. Appl. Microbiol. Biotechnol. 2018, 102, 8145–8152. [Google Scholar] [CrossRef]
- Wang, S.; Fu, C.; Bilal, M.; Hu, H.; Wang, W.; Zhang, X. Enhanced biosynthesis of arbutin by engineering shikimate pathway in Pseudomonas chlororaphis P3. Microb. Cell Fact. 2018, 17, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, M.; Rogero, M.M.; Fisberg, M.; Waitzberg, D. Health impact of childhood and adolescent soy consumption. Nutr. Rev. 2017, 75, 500–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration (FDA). Food labeling: Health claims; Soy protein and coronary heart disease. Fed. Reg. 1999, 64, 57700–57733. [Google Scholar]
- Chae, G.Y.; Ha, B.J. The comparative evaluation of fermented and non-fermented soybean extract on antioxidation and whitening. Toxicol. Res. 2011, 27, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Han, S.S.; Hur, S.J.; Lee, S.K. A comparison of antioxidative and anti-inflammatory activities of sword beans and soybeans fermented with Bacillus subtilis. Food Funct. 2015, 6, 2736–2748. [Google Scholar] [CrossRef]
- De La Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-E.; Kang, Y.-G.; Park, J.S.; Lim, T.-G.; Lee, K.W. Review of soybean phytochemicals and their bioactive properties relevant for skin health. J. Food Nutr. Res. 2017, 5, 852–858. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.M.; Hong, S.Y.; Math, R.K.; Lee, J.H.; Kambiranda, D.M.; Kim, J.M.; Islam, S.M.A.; Yun, M.G.; Cho, J.J.; Lim, W.J.; et al. Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. Food Chem. 2009, 114, 413–419. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, J.H.; Yun, H.D.; Ahn, B.Y.; Kim, H.; Seo, W.T. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Phongphisutthinant, R.; Wiriyacharee, P.; Preunglampoo, S.; Leelapat, P.; Kanjanakeereetumrong, P.; Lamyong, S. Selection of Bacillus spp. for isoflavone aglycones enriched Thua-nao, a traditional Thai fermented soybean. J. Pure Appl. Microbiol. 2015, 9, 59–68. [Google Scholar]
- Zhu, Y.P.; Fan, J.F.; Cheng, Y.Q.; Li, L.T. Improvement of the antioxidant activity of Chinese traditional fermented okara (Meitauza) using Bacillus subtilis B2. Food Control 2008, 19, 654–661. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Dahal, N.R.; Ndungutse, V. Bacillus fermentation of soybean: A review. J. Food Sci. Technol. Nepal 2010, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-K.; Lim, Y.-S.; Kim, Y.-S.; Park, S.-Y.; Lee, C.-H.; Hwang, K.W.; Kwon, D.Y. Free-radical-scavenging and tyrosinase-inhibition activities of Cheonggukjang samples fermented for various times. Food Chem. 2008, 106, 564–568. [Google Scholar] [CrossRef]
- Pyo, Y.H.; Jin, Y.J. Monascus-mediated fermentation improves the nutricosmetic potentials of soybeans. Food Sci. Biotechnol. 2016, 25, 883–891. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.; Kim, D.H.; Hong, S.Y.; Lee, J.S.; Kim, M. Total phenolic content, antioxidant, tyrosinase and α-glucosidase inhibitory activities of water soluble extracts of noble starter culture Doenjang, a Korean fermented soybean sauce variety. Food Control 2016, 59, 854–861. [Google Scholar] [CrossRef]
- Ponnusha, B.S.; Subramaniyam, S.; Pasupathi, P. Antioxidant and antimicrobial properties of Glycine Max-A review. Int. J. Curr. Biol. Med. Sci. 2011, 1, 49–62. [Google Scholar]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar]
- International Organization for Standardization. ISO 7218:2007. Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations; ISO: Geneva, Switzerland, 2007; Available online: https://www.iso.org/standard/36534.html (accessed on 15 August 2007).
- Claus, D.; Berkeley, R.C.W. Genus Bacillus Cohn. In Bergey’s Manual of Systematic Bacteriology, 1st ed.; Sneath, P.H.A., Mair, N.S., Sharpe, M.E., Holt, J.G., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1986; Volume 2, pp. 1105–1139. [Google Scholar]
- Liu, C.-Q.; Deng, L.; Zhang, P.; Zhang, S.-R.; Liu, L.; Xu, T.; Wang, F.; Tan, T.-W. Screening of high α-arbutin producing strains and production of α-arbutin by fermentation. World J. Microbiol. Biotechnol. 2013, 29, 1391–1398. [Google Scholar] [CrossRef]
- Sinnelä, M.T.; Park, Y.K.; Lee, J.H.; Jeong, K.C.; Kim, Y.W.; Hwang, H.-J.; Mah, J.-H. Effects of calcium and manganese on sporulation of Bacillus species involved in food poisoning and spoilage. Foods 2019, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Jeon, A.R.; Lee, J.H.; Mah, J.-H. Biogenic amine formation and bacterial contribution in Cheonggukjang, a Korean traditional fermented soybean food. LWT Food Sci. Technol. 2018, 92, 282–289. [Google Scholar] [CrossRef]
- Park, Y.K.; Jin, Y.H.; Lee, J.-H.; Byun, B.Y.; Lee, J.; Jeong, K.C.; Mah, J.-H. The role of Enterococcus faecium as a key producer and fermentation condition as an influencing factor in tyramine accumulation in Cheonggukjang. Foods 2020, 9, 915. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, E.J.; Park, S.H.; Son, K.H.; Yang, S.J.; Kim, S.M.; Park, S.R.; Kim, Y.H.; Gong, G.H.; Woo, M.H. Guideline for analytical method of preservatives and sunscreen ingredients in cosmetics-Analysis of arbutin and adenosine in cosmetics. Regul. Res. Food Drug Cosmet. 2010, 5, 23–28. [Google Scholar]
- Piao, X.L.; Baek, S.H.; Park, M.K.; Park, J.H. Tyrosinase-inhibitory furanocoumarin from Angelica dahurica. Biol. Pharm. Bull. 2004, 27, 1144–1146. [Google Scholar] [CrossRef] [Green Version]
- Chettri, R.; Tamang, J.P. Functional properties of Tungrymbai and Bekang, naturally fermented soybean foods of North East India. Int. J. Fermented Foods 2014, 3, 87–103. [Google Scholar] [CrossRef]
- Korean Collection for Type Cultures (KCTC). Available online: https://kctc.kribb.re.kr/search/resourceDetail?sn=3135&kind=Bacteria (accessed on 2 November 2020).
- Chiorcea-Paquim, A.-M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Setlow, B.; Cabrera-Hernandez, A.; Cabrera-Martinez, R.M.; Setlow, P. Identification of aryl-phospho-β-D-glucosidases in Bacillus subtilis. Arch. Microbiol. 2004, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011–2015). Expert Opin. Ther. Patents 2016, 26, 347–362. [Google Scholar] [CrossRef]
- Cui, J.; Xia, P.; Zhang, L.; Hu, Y.; Xie, Q.; Xiang, H. A novel fermented soybean, inoculated with selected Bacillus, Lactobacillus and Hansenula strains, showed strong antioxidant and anti-fatigue potential activity. Food Chem. 2020, 333, 127527. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.H.; Jeon, A.R.; Mah, J.-H. Tyrosinase Inhibitory Activity of Soybeans Fermented with Bacillus subtilis Capable of Producing a Phenolic Glycoside, Arbutin. Antioxidants 2020, 9, 1301. https://doi.org/10.3390/antiox9121301
Jin YH, Jeon AR, Mah J-H. Tyrosinase Inhibitory Activity of Soybeans Fermented with Bacillus subtilis Capable of Producing a Phenolic Glycoside, Arbutin. Antioxidants. 2020; 9(12):1301. https://doi.org/10.3390/antiox9121301
Chicago/Turabian StyleJin, Young Hun, Ah Ran Jeon, and Jae-Hyung Mah. 2020. "Tyrosinase Inhibitory Activity of Soybeans Fermented with Bacillus subtilis Capable of Producing a Phenolic Glycoside, Arbutin" Antioxidants 9, no. 12: 1301. https://doi.org/10.3390/antiox9121301
APA StyleJin, Y. H., Jeon, A. R., & Mah, J.-H. (2020). Tyrosinase Inhibitory Activity of Soybeans Fermented with Bacillus subtilis Capable of Producing a Phenolic Glycoside, Arbutin. Antioxidants, 9(12), 1301. https://doi.org/10.3390/antiox9121301