Medicinal Profile, Phytochemistry, and Pharmacological Activities of Murraya koenigii and its Primary Bioactive Compounds
Abstract
:1. Introduction
2. Murraya koenigii (M. koenigii)
2.1. Traditional Uses of M. koenigii
2.2. Medicinal Uses of M. koenigii
2.3. Phytochemistry of M. koenigii
2.4. Bioavailability Study of M. koenigii-Derived Active Constituents
3. Molecular Mechanism and Activities of M. koenigii
3.1. Antioxidants
3.2. Oxidative Stress
3.3. Mitochondrial Dysfunction
3.4. Inflammation
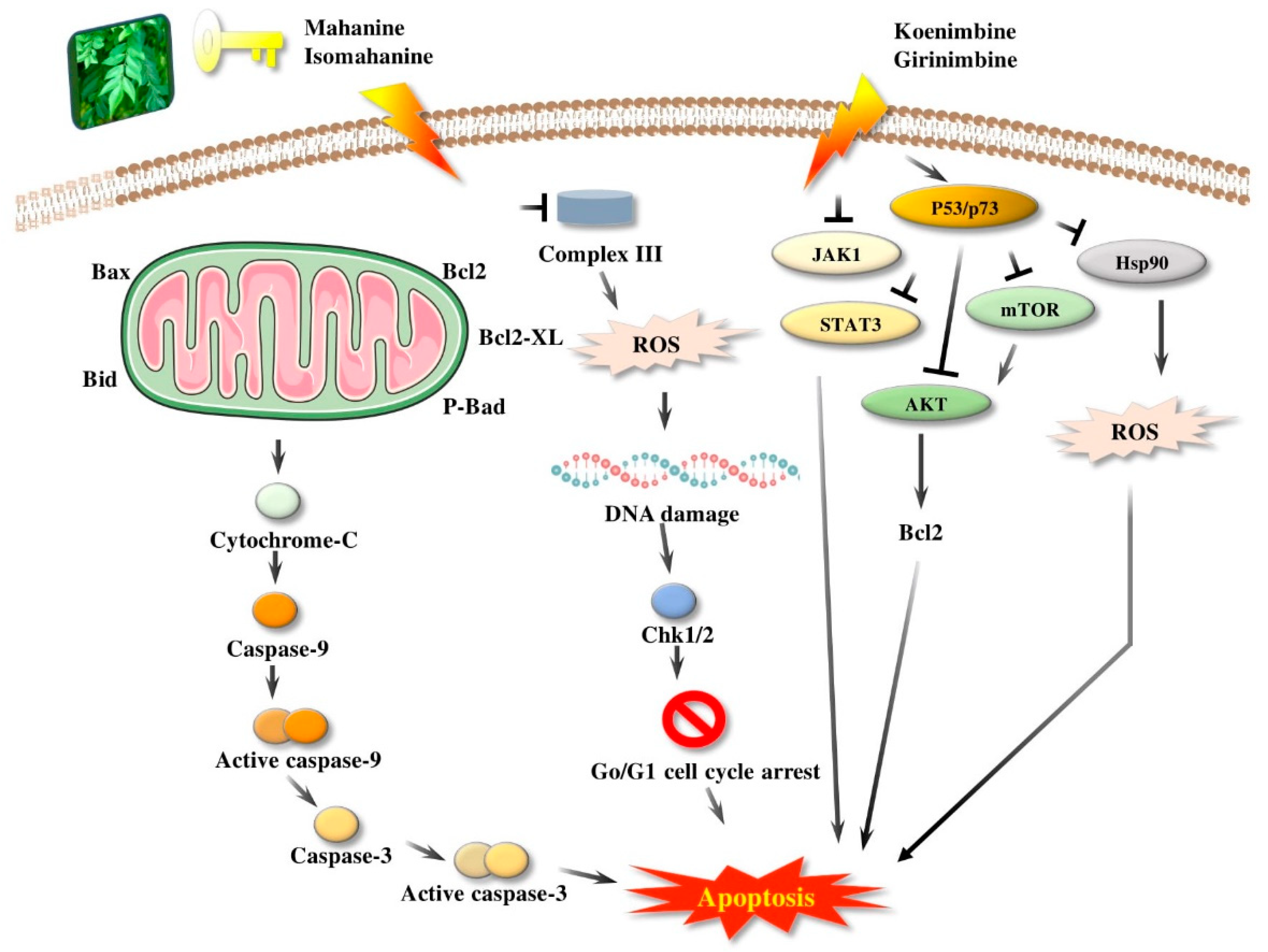
3.5. Apoptosis
4. Beneficial Pharmacological Activities of M. koenigii and Its Primary Active Derivatives
4.1. Antifungal Activity
4.2. Antibacterial Activity
4.3. Hepatoprotective Effect
4.4. Immunomodulatory Activity
4.5. Nephroprotective Activity
4.6. Antidiabetic Activity
4.7. Anticancer Activity (In Vivo and In Vitro)
4.8. Neuroprotective Activity
4.9. Radioprotective and Chemoprotective Activity
4.10. Wound Healing Effect
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Handral, H.K.; Pandith, A.; Shruthi, S.D. A review on Murraya koenigii: Multipotential medicinal plant. Asian J. Pharm. Clin. Res. 2012, 5, 5–14. [Google Scholar]
- Ujowundu, C.O.; Okafor, O.E.; Agha, N.C.; Nwaogu, L.A.; Igwe, K.O.; Igwe, C.U. Phytochemical and chemical composition of Combretum zenkeri leaves. J. Med. Plants Res. 2010, 4, 965–968. [Google Scholar]
- Kang, W.; Wang, Y. China Digital Governance Development Review Over the Past Two Decades. Int. J. Public Adm. Digit. Age 2018, 5, 92–106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Du, S.; Duan, X.; Zhang, S. Effects of ultrahigh pressure processing on the physicochemical characteristics of Taibai Kudzu starch. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2007, 2017. [Google Scholar] [CrossRef]
- Phumthum, M.; Balslev, H. Thai Ethnomedicinal Plants Used for Diabetes Treatment. OBM Integr. Complement. Med. 2018, 3, 1–25. [Google Scholar] [CrossRef]
- Gunjan, M.; Naing, T.W.; Saini, R.S.; Ahmad, A.; Naidu, J.R.; Kumar, I. Marketing trends & future prospects of herbal medicine in the treatment of various disease. World J. Pharm. Res. 2015, 4, 132–155. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 140–149. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Sisodia, R.; Walia, S.; Sati, O.P.; Kumar, J.; Kundu, A. Chemical analysis of essential oils of Eupatorium adenophorum and their antimicrobial, antioxidant and phytotoxic properties. J. Pest Sci. (2004) 2014, 87, 341–349. [Google Scholar] [CrossRef]
- Yankuzo, H.; Ahmed, Q.U.; Santosa, R.I.; Akter, S.F.U.; Talib, N.A. Beneficial effect of the leaves of Murraya koenigii (Linn.) Spreng (Rutaceae) on diabetes-induced renal damage in vivo. J. Ethnopharmacol. 2011, 135, 88–94. [Google Scholar] [CrossRef]
- Husna, F.; Suyatna, F.D.; Arozal, W.; Poerwaningsih, E.H. Anti-Diabetic Potential of Murraya koenigii (L) and its Antioxidant Capacity in Nicotinamide-Streptozotocin Induced Diabetic Rats. Drug Res. (Stuttg) 2018, 68, 631–636. [Google Scholar] [CrossRef]
- Amna, U.; Halimatussakdiah, P.W.; Saidi, N.; Nasution, R. Evaluation of cytotoxic activity from Temurui (Murraya koenigii [Linn.] Spreng) leaf extracts against HeLa cell line using MTT assay. J. Adv. Pharm. Technol. Res. 2019, 10, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.K.; Abu, N.; Mohamad, N.E.; Beh, B.K.; Ho, W.Y.; Ebrahimi, S.; Yusof, H.M.; Ky, H.; Tan, S.W.; Alitheen, N.B. Chemopreventive and immunomodulatory effects of Murraya koenigii aqueous extract on 4T1 breast cancer cell-challenged mice. BMC Complement. Altern. Med. 2015, 4, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nooron, N.; Ohba, K.; Takeda, K.; Shibahara, S.; Chiabchalard, A. Dysregulated expression of MITF in subsets of hepatocellular carcinoma and cholangiocarcinoma. Tohoku J. Exp. Med. 2017, 242, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, R.; Bhattacharya, K.; Samanta, S.K.; Pal, B.C.; Mandal, C. Improved chemosensitivity in cervical cancer to cisplatin: Synergistic activity of mahanine through STAT3 inhibition. Cancer Lett. 2014, 351, 81–90. [Google Scholar] [CrossRef]
- Bhandari, P. Curry leaf (Murraya koenigii) or Cure leaf: Review of its curative properties. J. Med. Nutr. Nutraceuticals 2012, 2, 92–97. [Google Scholar] [CrossRef]
- Desai, S.N.; Patel, D.K.; Devkar, R.V.; Patel, P.V.; Ramachandran, A.V. Hepatoprotective potential of polyphenol rich extract of Murraya koenigii L.: An in vivo study. Food Chem. Toxicol. 2012, 50, 310–314. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Patel, D.K.; Devkar, R.V.; Ramachandran, A.V. Flavonoid rich extract of Murraya koenigii alleviates in-vitro LDL oxidation and oxidized LDL induced apoptosis in raw 264.7 Murine macrophage cells. J. Food Sci. Technol. 2015, 52, 3367–3375. [Google Scholar] [CrossRef] [Green Version]
- Goutam, M.P.; Purohit, R.M. Antimicrobial activity of the essential oil of the leaves of Murraya koenigii (Linn) Spreng (Indian curry leaf). Indian J. Pharm. 1974, 2, 48–51. [Google Scholar]
- Maswada, H.F.; Abdallah, S.A. In vitro antifungal activity of three geophytic plant extracts against three post-harvest pathogenic fungi. Pakistan J. Biol. Sci. 2013, 16, 1698–1705. [Google Scholar] [CrossRef]
- Bonde, S.D.; Nemade, L.S.; Patel, M.R.; Patel, A.A. Murraya koenigii (Curry leaf): Ethnobotany, Phytochemistry and Pharmacology—A Review. Int. J. Pharm. Phytopharm. Res. 2011, 1, 23. [Google Scholar]
- Ma, Q.G.; Xu, K.; Sang, Z.P.; Wei, R.R.; Liu, W.M.; Su, Y.L.; Yang, J.B.; Wang, A.G.; Ji, T.F.; Li, L.J. Alkenes with antioxidative activities from Murraya koenigii (L.) Spreng. Bioorg. Med. Chem. Lett. 2016, 26, 799–803. [Google Scholar] [CrossRef]
- Chatterjee, P.; Seal, S.; Mukherjee, S.; Kundu, R.; Bhuyan, M.; Barua, N.C.; Baruah, P.K.; Babu, S.P.S.; Bhattacharya, S. A carbazole alkaloid deactivates mTOR through the suppression of rictor and that induces apoptosis in lung cancer cells. Mol. Cell. Biochem. 2015, 405, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Bhattacharya, K.; Sarkar, S.; Samanta, S.K.; Pal, B.C.; Mandal, C. Mahanine synergistically enhances cytotoxicity of 5-fluorouracil through ROS-mediated activation of PTEN and p53/p73 in colon carcinoma. Apoptosis 2014, 19, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Amin, K.S.; Jagadeesh, S.; Baishay, G.; Rao, P.G.; Barua, N.C.; Bhattacharya, S.; Banerjee, P.P. Mahanine restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in prostate cancer cells. Mol. Cancer 2013, 12, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.; Nayak, A.; Kar, M.; Banerjee, S.K.; Das, A.; Upadhyay, S.N.; Singh, R.K.; Banerji, A.; Banerji, J. Antidiarrhoeal activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia 2010, 81, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Ningappa, M.B.; Dhananjaya, B.L.; Dinesha, R.; Harsha, R.; Srinivas, L. Potent antibacterial property of APC protein from curry leaves (Murraya koenigii L.). Food Chem. 2010, 118, 747–750. [Google Scholar] [CrossRef]
- Tripathi, Y.; Anjum, N.; Rana, A. Chemical Composition and In vitro Antifungal and Antioxidant Activities of Essential Oil from Murraya koenigii (L.) Spreng. Leaves. Asian J. Biomed. Pharm. Sci. 2018, 8, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Mahipal, P.; Pawar, R.S. Nephroprotective effect of Murraya koenigii on cyclophosphamide induced nephrotoxicity in rats. Asian Pac. J. Trop. Med. 2017, 10, 808–812. [Google Scholar] [CrossRef]
- Rautela, R.; Das, G.K.; Khan, F.A.; Prasad, S.; Kumar, A.; Prasad, J.K.; Ghosh, S.K.; Dhanze, H.; Katiyar, R.; Srivastava, S.K. Antibacterial, anti-inflammatory and antioxidant effects of Aegle marmelos and Murraya koenigii in dairy cows with endometritis. Livest. Sci. 2018, 214, 142–148. [Google Scholar] [CrossRef]
- Mani, V.; Ramasamy, K.; Ahmad, A.; Wahab, S.N.; Jaafar, S.M.; Kek, T.L.; Salleh, M.Z.; Majeed, A.B.A. Effects of the total alkaloidal extract of Murraya koenigii leaf on oxidative stress and cholinergic transmission in aged mice. Phyther. Res. 2013, 27, 46–53. [Google Scholar] [CrossRef]
- Sathaye, S.; Bagul, Y.; Gupta, S.; Kaur, H.; Redkar, R. Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp. Toxicol. Pathol. 2011, 63, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.A.; Shahnawaz, M.; Qazi, P.H.; Qazi, H. General overview of medicinal plants: A review. J. Phytopharm. 2017, 6, 349–351. [Google Scholar]
- Mani, V.; Ramasamy, K.; Ahmad, A.; Parle, M.; Shah, S.A.A.; Majeed, A.B.A. Protective effects of total alkaloidal extract from Murraya koenigii leaves on experimentally induced dementia. Food Chem. Toxicol. 2012, 50, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Tamilselvam, K.; Sulthana, A.; Mohankumar, T.; Manimaran, D.; Elangovan, N. Isolongifolene Attenuates Oxidative Stress and Behavioral Impairment in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Nutr. Pharmacol. Neurol. Dis. 2018, 8, 53–58. [Google Scholar]
- Zang, Y.D.; Li, C.J.; Song, X.Y.; Ma, J.; Yang, J.Z.; Chen, N.H.; Zhang, D.M. Total synthesis and neuroprotective effect of O-methylmurrayamine A and 7-methoxymurrayacine. J. Asian Nat. Prod. Res. 2017, 19, 623–629. [Google Scholar] [CrossRef]
- Patil, R.; Dhawale, K.; Gound, H.; Gadakh, R. Protective effect of leaves of Murraya koenigii on reserpine-induced orofacial dyskinesia. Iran. J. Pharm. Res. 2012, 11, 635–641. [Google Scholar]
- Erkan, N.; Tao, Z.; Vasantha Rupasinghe, H.P.; Uysal, B.; Oksal, B.S. Antibacterial activities of essential oils extracted from leaves of Murraya koenigii by solvent-free microwave extraction and hydro-distillation. Nat. Prod. Commun. 2012, 7, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Natural medicines from plant source used for therapy of diabetes mellitus: An overview of its pharmacological aspects. Asian Pac. J. Trop. Dis. 2012, 2, 139–150. [Google Scholar] [CrossRef]
- Nalli, Y.; Khajuria, V.; Gupta, S.; Arora, P.; Riyaz-Ul-Hassan, S.; Ahmed, Z.; Ali, A. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org. Biomol. Chem. 2016, 14, 3322–3332. [Google Scholar] [CrossRef]
- Joshi, T.; Jain, T.; Mahar, R.; Singh, S.K.; Srivastava, P.; Shukla, S.K.; Mishra, D.K.; Bhatta, R.S.; Banerjee, D.; Kanojiya, S. Pyranocarbazoles from Murraya koenigii (L.) Spreng. as antimicrobial agents. Nat. Prod. Res. 2018, 32, 430–434. [Google Scholar] [CrossRef]
- Sharma, S.; Handu, S.; Dubey, A.; Sharma, P.; Mediratta, P.; Ahmed, Q. Anti-anxiety and anti-depressant like effects of Murraya koenigii in experimental models of anxiety and depression. Anc. Sci. Life 2017, 36, 215–219. [Google Scholar] [PubMed]
- Adebajo, A.C.; Ayoola, O.F.; Iwalewa, E.O.; Akindahunsi, A.A.; Omisore, N.O.A.; Adewunmi, C.O.; Adenowo, T.K. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine 2006, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Tembhurne, S.V.; Sakarkar, D.M. Hypoglycemic effects of fruit juice of Murraya koenigii (L) in alloxan induced diabetic mice. Int. J. PharmTech Res. 2009, 1, 1589–1593. [Google Scholar]
- Sim, K.M.; Teh, H.M. A new carbazole alkaloid from the leaves of Malayan Murraya koenigii. J. Asian Nat. Prod. Res. 2011, 13, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Igara, C.; Omoboyowa, D.; Ahuchaogu, A.; Orji, N.; Ndukwe, M. Phytochemical and nutritional profile of Murraya koenigii (Linn) Spreng leaf. J. Pharmacogn. Phytochem. 2016, 5, 7–9. [Google Scholar]
- Samanta, S.K.; Kandimalla, R.; Gogoi, B.; Dutta, K.N.; Choudhury, P.; Deb, P.K.; Devi, R.; Pal, B.C.; Talukdar, N.C. Phytochemical portfolio and anticancer activity of Murraya koenigii and its primary active component, mahanine. Pharmacol. Res. 2018, 129, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Ramsewak, R.S.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L.; Nitiss, J.L. Biologically active carbazole alkaloids from Murraya koenigii. J. Agric. Food Chem. 1999, 47, 444–447. [Google Scholar] [CrossRef]
- Joshi, B.S.; Kamat, V.N.; Gawad, D.H. On the structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron 1970, 26, 1475–1482. [Google Scholar] [CrossRef]
- Gahlawat, D.K.; Jakhar, S.; Dahiya, P. Murraya koenigii (L.) Spreng: An ethnobotanical, phytochemical and pharmacological review. J. Pharmacogn. Phytochem. 2014, 3, 109–119. [Google Scholar]
- Tachibana, Y.; Kikuzaki, H.; Lajis, N.H.; Nakatani, N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001, 49, 5589–5594. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Srivastava, S.D. New constituents and biological activity of the roots of Murraya koenigii. J. Indian Chem. Soc. 1993, 2, 607–627. [Google Scholar]
- Noolu, B.; Gogulothu, R.; Bhat, M.; Qadri, S.S.Y.H.; Sudhakar Reddy, V.; Bhanuprakash Reddy, G.; Ismail, A. In Vivo Inhibition of Proteasome Activity and Tumour Growth by Murraya koenigii Leaf Extract in Breast Cancer Xenografts and by Its Active Flavonoids in Breast Cancer Cells. Anticancer Agents Med. Chem. 2016, 16, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Tian, J.; Yang, J.; Wang, A.; Ji, T.; Wang, Y.; Su, Y. Bioactive carbazole alkaloids from Murraya koenigii (L.) Spreng. Fitoterapia 2013, 87, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Hattori, I.; Miyase, T.; Ueno, A.; Hara, S.; Kageyama, C. Studies on the Constituents of Leaves of Citrus unshiu Marcov. Chem. Pharm. Bull. 1988, 36, 5004–5008. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Khlebnikov, V. Carotenoids and Degraded Carotenoids, VIII-Synthesis of (+)-Dihydroactinidiolide, (+)- and (−)-Actinidiolide, (+)- and (−)-Loliolide as well as (+)- and (−)-Epiloliolide. Liebigs Ann. Chem. 1993, 1993, 77–82. [Google Scholar] [CrossRef]
- Tan, S.P.; Ali, A.M.; Nafiah, M.A.; Amna, U.; Ramli, S.A.; Ahmad, K. Terpenes and Phenolic Compounds of Murraya koenigii. Chem. Nat. Compd. 2017, 53, 980–981. [Google Scholar] [CrossRef]
- ChV, S. Antioxidant and Biological Activities of Three Morphotypes of Murraya koenigii L. from Uttarakhand. J. Food Process. Technol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Patel, O.P.S.; Mishra, A.; Maurya, R.; Saini, D.; Pandey, J.; Taneja, I.; Raju, K.S.R.; Kanojiya, S.; Shukla, S.K.; Srivastava, M.N.; et al. Naturally Occurring Carbazole Alkaloids from Murraya koenigii as Potential Antidiabetic Agents. J. Nat. Prod. 2016, 79, 1276–1284. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Samanta, S.K.; Tripathi, R.; Mallick, A.; Chandra, S.; Pal, B.C.; Shaha, C.; Mandal, C. Apoptotic effects of mahanine on human leukemic cells are mediated through crosstalk between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways. Biochem. Pharmacol. 2010, 79, 361–372. [Google Scholar] [CrossRef]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoidal, J.R. Reactive oxygen species and cell signaling. Am. J. Respir. Cell Mol. Biol. 2001, 25, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Gill, N.S.; Sharma, B. Study on antioxidant potential of Murraya koenigii leaves in wistar rats. Pakistan J. Biol. Sci. 2013, 17, 126–129. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, I.W.; Kuspradini, H.; Arung, E.T.; Aryani, F.; Min, Y.H.; Kim, J.S.; Kim, Y.U. Biological Activity and Phytochemical Analysis of Three Indonesian Medicinal Plants, Murraya koenigii, Syzygium polyanthum and Zingiber purpurea. J. Acupunct. Meridian Stud. 2011, 4, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.J.M.; Ramalakshmi, K.; Borse, B.B.; Raghavan, B. Antioxidant and radical-scavenging carbazole alkaloids from the oleoresin of curry leaf (Murraya koenigii Spreng.). Food Chem. 2007, 100, 742–747. [Google Scholar] [CrossRef]
- Gupta, S.; Paarakh, P.M.; Gavani, U. Antioxidant activity of Murraya koenigii linn leaves. Pharmacologyonline 2009, 1, 474–478. [Google Scholar]
- Zahin, M.; Aqil, F.; Husain, F.M.; Ahmad, I. Antioxidant capacity and antimutagenic potential of Murraya koenigii. BioMed Res. Int. 2013, 2013, 263509. [Google Scholar] [CrossRef] [Green Version]
- Yogesh, K.; Jha, S.N.; Yadav, D.N. Antioxidant Activities of Murraya koenigii (L.) Spreng Berry Extract: Application in Refrigerated (4 ± 1 °C) Stored Meat Homogenates. Agric. Res. 2012, 1, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, S.T.; Sharmistha, B.; Shuchi, K. Assessment of antioxidant activity of leaves of Murraya koenigii extracts and it’s comparative efficacy analysis in different solvents. J. Pharm. Sci. Res. 2017, 9, 288–291. [Google Scholar]
- Waghmare, A.N.; Tembhurne, S.V.; Sakarar, D.M. Phytochemical Analysis and In vitro Antioxidant Properties of Murraya koenigii (L.) Fruits. Am. J. Phytomedicine Clin. Ther. 2015, 3, 403–416. [Google Scholar]
- Dexter, D.T.; Wells, F.R.; Lee, A.J.; Agid, F.; Agid, Y.; Jenner, P.; Marsden, C.D. Increased Nigral Iron Content and Alterations in Other Metal Ions Occurring in Brain in Parkinson’s Disease. J. Neurochem. 1989, 52, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Gomberg-Maitland, M.; Maitland, M.L.; Rich, S.; Garcia, J.G.N.; Weir, E.K. Mitochondrial metabolism, redox signaling, and fusion: A mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H570–H578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 53, 885–892. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Ichijo, H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal. 2005, 7, 472–481. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Del Valle, N.R.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [Green Version]
- Arulselvan, P.; Subramanian, S.P. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chem. Biol. Interact. 2007, 165, 155–164. [Google Scholar] [CrossRef]
- Khan, B.A.; Abraham, A.; Leelamma, S. Anti-oxidant effects of Curry leaf, Murraya koenigii and mustard seeds, Brassica juncea in rats fed with high fat diet. Indian J. Exp. Biol. 1997, 35, 148–150. [Google Scholar]
- Mitra, E.; Ghosh, A.K.; Ghosh, D.; Mukherjee, D.; Chattopadhyay, A.; Dutta, S.; Pattari, S.K.; Bandyopadhyay, D. Protective effect of aqueous Curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 2012, 50, 1340–1353. [Google Scholar] [CrossRef]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Beal, M.F. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Park. Relat. Disord. 2009, 3, S189–S194. [Google Scholar] [CrossRef]
- Kushnareva, Y.; Murphy, A.N.; Andreyev, A. Complex I-mediated reactive oxygen species generation: Modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 2002, 368, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ischiropoulos, H.; Beckman, J.S. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Investig. 2003, 111, 163–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; De Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Iman, V.; Mohan, S.; Abdelwahab, S.I.; Karimian, H.; Nordin, N.; Fadaeinasab, M.; Noordin, M.I.; Noor, S.M. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des. Dev. Ther. 2017, 2017, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Arun, A.; Patel, O.P.S.; Saini, D.; Yadav, P.P.; Konwar, R. Anti-colon cancer activity of Murraya koenigii leaves is due to constituent murrayazoline and O-methylmurrayamine A induced mTOR/AKT downregulation and mitochondrial apoptosis. Biomed. Pharmacother. 2017, 93, 510–521. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Elangovan, N.; Mohankumar, T.; Nataraj, J.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Akbar, M.; Sattar Khan, M.A. Isolongifolene attenuates rotenone-induced mitochondrial dysfunction, oxidative stress and apoptosis. Front. Biosci. (Sch. Ed.) 2018, 10, 248–261. [Google Scholar]
- Bashkatova, V.; Alam, M.; Vanin, A.; Schmidt, W.J. Chronic administration of rotenone increases levels of nitric oxide and lipid peroxidation products in rat brain. Exp. Neurol. 2004, 186, 235–241. [Google Scholar] [CrossRef]
- Gupta, S.; George, M.; Singhal, M.; Sharma, G.; Garg, V. Leaves extract of Murraya koenigii linn for anti-inflammatory and analgesic activity in animal models. J. Adv. Pharm. Technol. Res. 2010, 1, 68–77. [Google Scholar]
- Darvekar, V.M.; Patil, V.R.; Choudhari, A.B.; Road, D. Anti-inflammatory Activity of Murraya koenigii Spreng on Experimental Animals. J. Nat. Prod. Plant Resour. 2011, 1, 65–69. [Google Scholar]
- Mani, V.; Ramasamy, K.; Abdul Majeed, A.B. Anti-inflammatory, analgesic and anti-ulcerogenic effect of total alkaloidal extract from Murraya koenigii leaves in animal models. Food Funct. 2013, 4, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Sikha, M.S.; Ramesh, K.; Venkatesh, P.; Godugu, C. Modulation of cerulein-induced pancreatic inflammation by hydroalcoholic extract of curry leaf (Murraya koenigii). Phyther. Res. 2019, 33, 1510–1525. [Google Scholar] [CrossRef] [PubMed]
- Oben, K.Z.; Alhakeem, S.S.; McKenna, M.K.; Brandon, J.A.; Mani, R.; Noothi, S.K.; Jinpeng, L.; Akunuru, S.; Dhar, S.K.; Singh, I.P.; et al. Oxidative stress-induced JNK/AP-1 signaling is a major pathway involved in selective apoptosis of myelodysplastic syndrome cells by Withaferin-A. Oncotarget 2017, 8, 77436–77452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yin, X.; Lu, C.; Chen, X.; Ba, H.; Cai, J.; Sun, J. Mahanine induces apoptosis, cell cycle arrest, inhibition of cell migration, invasion and PI3K/AKT/mTOR signalling pathway in glioma cells and inhibits tumor growth in vivo. Chem. Biol. Interact. 2019, 299, 1–7. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, X.; Ran, Q.; Yang, K.; Wen, Y.; Li, H.; Wang, F. Globularifolin exerts anticancer effects on glioma U87 cells through inhibition of Akt/mTOR and MEK/ERK signaling pathways in vitro and inhibits tumor growth in vivo. Biochimie 2017, 141, 144–151. [Google Scholar] [CrossRef]
- Utaipan, T.; Athipornchai, A.; Suksamrarn, A.; Jirachotikoon, C.; Yuan, X.; Lertcanawanichakul, M.; Chunglok, W. Carbazole alkaloids from Murraya koenigii trigger apoptosis and autophagic flux inhibition in human oral squamous cell carcinoma cells. J. Nat. Med. 2017, 71, 158–169. [Google Scholar] [CrossRef]
- Syam, S.; Abdul, A.B.; Sukari, M.A.; Mohan, S.; Abdelwahab, S.I.; Wah, T.S. The growth suppressing effects of girinimbine on hepg2 involve induction of apoptosis and cell cycle arrest. Molecules 2011, 16, 7155–7170. [Google Scholar] [CrossRef] [Green Version]
- Xin, Q.; Muer, A. Girinimbine inhibits the proliferation of human ovarian cancer cells in vitro via the phosphatidylinositol-3-kinase (PI3K)/akt and the mammalian target of rapamycin (mTOR) and Wnt/β-catenin signaling pathways. Med. Sci. Monit. 2018, 24, 5480–5487. [Google Scholar] [CrossRef]
- Ahmadipour, F.; Noordin, M.I.; Mohan, S.; Arya, A.; Paydar, M.; Looi, C.Y.; Keong, Y.S.; Siyamak, E.N.; Fani, S.; Firoozi, M.; et al. Koenimbin, a natural dietary compound of Murraya koenigii (L) spreng: Inhibition of MCF7 breast cancer cells and targeting of derived MCF7 breast cancer stem cells (CD44+/CD24-/low): An in vitro study. Drug Des. Dev. Ther. 2015, 9, 1193–1208. [Google Scholar]
- Ito, C.; Itoigawa, M.; Nakao, K.; Murata, T.; Tsuboi, M.; Kaneda, N.; Furukawa, H. Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine 2006, 13, 359–365. [Google Scholar] [CrossRef]
- Kumar, N.S.; Mukherjee, P.K.; Bhadra, S.; Saha, B.P.; Pal, B.C. Acetylcholinesterase inhibitory potential of a carbazole alkaloid, mahanimbine, from Murraya koenigii. Phyther. Res. 2010, 24, 629–631. [Google Scholar]
- Deb, S. Synthesis of silver nano particles using Murraya koenigii (Green Curry leaves), Zea mays (Baby corn) and its antimicrobial activity against pathogens. Int. J. PharmTech Res. 2014, 6, 91–96. [Google Scholar]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial Effect of Silver Nanoparticles Synthesized Using Murraya koenigii (L.) against Multidrug-Resistant Pathogens. Bioinorg. Chem. Appl. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankar Ganesh, P.; Rai Vittal, R. In vitro antibiofilm activity of Murraya koenigii essential oil extracted using supercritical fluid CO2 method against Pseudomonas aeruginosa PAO1. Nat. Prod. Res. 2015, 29, 2295–2298. [Google Scholar] [CrossRef]
- Rath, S.; Padhy, R.N. Monitoring in vitro antibacterial efficacy of 26 Indian spices against multidrug resistant urinary tract infecting bacteria. Integr. Med. Res. 2014, 13, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.K.; Mohanty, S.; Padhi, A.; Pati, R.; Sonawane, A. Evaluation of antibacterial and cytotoxic activity of Artemisia nilagirica and Murraya koenigii leaf extracts against mycobacteria and macrophages. BMC Complement. Altern. Med. 2014. [Google Scholar] [CrossRef]
- Manfo, F.P.T.; Nantia, E.A.; Kuete, V. Hepatotoxicity and Hepatoprotective Effects of African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Yaoundé, Cameroon, 2014; pp. 223–256. [Google Scholar]
- Shah, P.; Singh, S.P.; Kumar, A. Combined effect of hydroethanolic extracts of Murraya koenigii and Phyllanthus niruri leaves on paracetamol and ethanol-induced toxicity in HepG2 cell line. Curr. Sci. 2015, 109, 1320. [Google Scholar] [CrossRef]
- Kaufmann, T.; Simon, H.U. Targeting disease by immunomodulation. Cell Death Differ. 2015, 22, 185–186. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.S.; Wakade, A.S.; Juvekar, A.R. Immunomodulatory activity of methanolic extract of Murraya koenigii (L) Spreng. leaves. Indian J. Exp. Biol. 2008, 46, 505–509. [Google Scholar]
- Paul, S.; Bandyopadhyay, T.K.; Bhattacharyya, A. Immunomodulatory effect of leaf extract of Murraya koenigii in diabetic mice. Immunopharmacol. Immunotoxicol. 2011, 33, 691–699. [Google Scholar] [CrossRef]
- Punuru, P.; Sujatha, D.; Kumari, B.P.; Charisma, V.V.L. Evaluation of aqueous extract of Murraya koenigii in unilateral renal ischemia reperfusion injury in rats. Indian J. Pharmacol. 2014, 46, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, M.Z.; Attuluri, V.; Qureshi, I.A.; Ghazi, I.A. Antioxidant and α-glucosidase inhibitory activities of Murraya koenigii leaf extracts. Pharmacogn. J. 2012, 4, 65–72. [Google Scholar] [CrossRef]
- Sarkar, S.; Dutta, D.; Samanta, S.K.; Bhattacharya, K.; Pal, B.C.; Li, J.; Datta, K.; Mandal, C.; Mandal, C. Oxidative inhibition of Hsp90 disrupts the super-chaperone complex and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int. J. Cancer 2013, 132, 695–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, C.; He, Q.; Liang, S.; Gong, X. Mahanimbine Exerts Anticancer Effects on Human Pancreatic Cancer Cells by Triggering Cell Cycle Arrest, Apoptosis, and Modulation of AKT/Mammalian Target of Rapamycin (mTOR) and Signal Transducer and Activator of Transcription 3 (STAT3) Signalling Pathway. Med. Sci. Monit. 2018, 1, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Noolu, B.; Ajumeera, R.; Chauhan, A.; Nagalla, B.; Manchala, R.; Ismail, A. Murraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cells. BMC Complement. Altern. Med. 2013, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.; Noolu, B.; Gogulothu, R.; Perugu, S.; Rajanna, A.; Babu, S.K. Cytotoxicity and Proteasome Inhibition by Alkaloid Extract from Murraya koenigii Leaves in Breast Cancer Cells-Molecular Docking Studies. J. Med. Food 2016, 19, 1155–1165. [Google Scholar] [CrossRef]
- Mohan, S.; Abdelwahab, S.I.; Cheah, S.C.; Sukari, M.A.; Syam, S.; Shamsuddin, N.; Rais Mustafa, M. Apoptosis effect of girinimbine isolated from Murraya koenigii on lung cancer cells in vitro. Evid.-Based Complement. Altern. Med. 2013, 2013, 689865. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, M.; Parle, M. Antiamnesic potential of Murraya koenigii leaves. Phyther. Res. 2009, 23, 308–316. [Google Scholar] [CrossRef]
- Mittal, J. Curry Leaf (Murraya koenigii): A Spice with Medicinal Property. MOJ Biol. Med. 2017, 2, 236–256. [Google Scholar] [CrossRef] [Green Version]
- Iyer, D.; Uma, D.P. Effect of Murraya koenigii (L.) on radiation induced rate of lipid peroxidation in Swiss albino mice. Indian Drugs 2009, 46, 160–162. [Google Scholar]
- Nagappan, T.; Segaran, T.C.; Wahid, M.E.A.; Ramasamy, P.; Vairappan, C.S. Efficacy of carbazole alkaloids, essential oil and extract of Murraya koenigii in enhancing subcutaneous wound healing in rats. Molecules 2012, 17, 14449–14463. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Compound | Molecular Formula | Plant Part | References |
|---|---|---|---|
| Alkaloids | |||
| Mahanine | C23H25NO2 | Leaves, stem bark, and seeds | [45,46,47,48,49] |
| Mahanimbine | C23H25NO | Leaves, roots, seeds, and fruits | [47,48,49] |
| Murrayanol | C24H29NO2 | Leaves, roots, and fruits | [47,48,49] |
| Koenimbine | C19H19NO2 | Leaves, seeds, and fruits | [46,47,48,49] |
| O-Methylmurrayamine A | C19H20NO2 | Leaves | [45,46,47,48] |
| Koenigicine | C20H21NO3 | Leaves | [45,46,47,48] |
| Koenigine | C19H19NO3 | Leaves and stem bark | [47,48,49] |
| Murrayone (Coumarine) | C15H14O4 | Leaves | [45,46,47,48] |
| Mahanimbicine | C23H25NO | Leaves | [45,46,47,48] |
| Bicyclomahanimbicine | C23H25NO | Leaves | [45,46,47,48] |
| Phebalosin | C15H14O4 | Leaves | [45,46,47,48] |
| Isomahanimbine | C23H25NO | Leaves and roots | [45,46,48] |
| Koenimbidine | C20H21NO3 | Leaves and roots | [45,46,48] |
| Euchrestine B | C24H29NO2 | Leaves | [45,46] |
| Bismurrayafoline E | C48H56N2O4 | Leaves | [45,46] |
| Isomahanine | C23H25NO2 | Leaves, seeds, and fruits | [45,46,49] |
| Mahanimbinine | C23H27NO2 | Leaves and seeds | [45,46,49] |
| Girinimbilol | C18H19NO | Leaves | [45,46] |
| Pyrayafoline-d | C23H25NO2 | Leaves and stem bark | [45,46,49] |
| Glycozoline | C14H13NO | Leaves | [45,46] |
| Cyclomahanimbine | C23H25NO | Leaves | [45,46] |
| Isomurrayazoline | C23H25NO | Leaves | [45,46] |
| Mahanimboline | C23H25NO2 | Leaves | [49] |
| Mukonicine | C20H21NO3 | Leaves | [49] |
| Isolongifolene | C15H24 | Leaves | [49] |
| Mukonal | C13H9NO2 | Stems | [49] |
| Mukeic acid | C14H11NO3 | Stems | [49] |
| 9-Carbethoxy-3-methyl carbazole | C16H15NO2 | Roots and stems | [49] |
| 9-Formyl-3-methyl carbazole | C14H11NO | Roots and stems | [49] |
| Murrayazolinol | C23H25NO2 | Stems bark | [49,50] |
| Mahanimbinol | C23H27NO | Stems bark | [49,50] |
| Mukoeic acid | C14H11NO3 | Stem bark | [49,50] |
| Osthol | C15H16O3 | Stem bark | [49,50] |
| Umbelliferone | C9H6O3 | Stem bark | [49,50] |
| Murrayanine | C14H11NO2 | Stem bark | [49,50] |
| Mukoenine-A | C18H19NO | Roots and stem bark | [49,50] |
| Mukoenine-B | C23H25NO2 | Roots and stem bark | [49,50] |
| Mukoline | C14H13NO2 | Roots | [49,50] |
| Mukolidine | C14H11NO2 | Roots and stem bark | [49,50] |
| (M)-murrastifoline-F | C28H24N2O2 | Roots and stem bark | [49,50] |
| 3-Methyl-9H-carbazole-9-carbaldehyde | C14H11NO | Roots | [49,50] |
| Bismahanine | C46H48N2O4 | Roots and stem bark | [49,50] |
| Bikoeniquinone A | C27H20N2O3 | Roots and stem bark | [49,50] |
| Bismurrayaquinone | C26H16N2O4 | Roots and stem bark | [49,50] |
| 3-Methylcarbazole | C13H11N | Roots | [49] |
| Murrayafoline A | C14H13NO | Roots | [49] |
| Murrayakonine A | C37H36N2O2 | Leaves and stems | [39] |
| Murrayakonine B | C23H23NO2 | Leaves and stems | [39] |
| Murrayakonine C | C24H25NO3 | Leaves and stems | [39] |
| Murrayakonine D | C23H25NO2 | Leaves and stems | [39] |
| Girinimbine | C18H17NO | Roots, stem bark, and seeds | [49,51] |
| Murrayacine | C18H15NO2 | Stem and bark | [49,51] |
| Murrayazoline | C23H25NO | Stem and bark | [49,51] |
| Flavonoids | |||
| Quercetin | C15H10O7 | Leaves | [52] |
| Apigenin | C15H10O5 | Leaves | [52] |
| Kaempferol | C15H10O6 | Leaves | [52] |
| Rutin | C27H30O16 | Leaves | [52] |
| Catechin | C15H14O6 | Leaves | [52] |
| Myricetin | C15H10O8 | Leaves | [52] |
| 4-O-β-d-Rutinosyl-3-methoxyphenyl-1-propanone | C22H32O12 | Leaves | [53] |
| 1-O-β-d-Rutinosyl-2(R)-ethyl-1-pentanol | C19H36O10 | Leaves | [53] |
| 8-Phenylethyl-O-β-d-rutinoside | C20H30O10 | Leaves | [54] |
| Terpenoids | |||
| Blumenol A | C13H20O3 | Leaves | [53] |
| Icariside B1 | C19H30O8 | Leaves | [53] |
| Loliolide | C11H16O3 | Leaves | [53] |
| Blumenol A | C13H20O3 | Leaves | [53] |
| Icariside B1 | C19H30O8 | Leaves | [53] |
| (−)-Epiloliolide | C11H16O3 | Leaves | [55] |
| (−)-α-pinene | C10H16 | Leaves | [55] |
| (−)-β-pinene | C10H16 | Leaves | [55] |
| (+)-β-pinene | C10H16 | Leaves | [55] |
| (+)-sabinene | C10H16 | Leaves | [55] |
| Squalene | C30H50 | Leaves and bark | [56] |
| β-sitosterol | C29H50O | Leaves and bark | [56,57] |
| Polyphenols | |||
| Selin-11-en-4α-ol | C15H26O | Leaves and bark | [56] |
| 2-hydroxy-4-methoxy-3,6-dimethylbenzoic acid | C10H12O4 | Bark | [56] |
| Serial No. | Constituent | Constituent Structure | Activity |
|---|---|---|---|
| 1 | Mahanine | 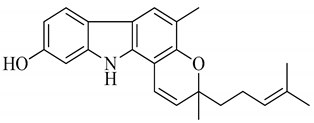 | Cytotoxicity, anti-microbial, and anti-cancer |
| 2 | Mahanimbine |  | Cytotoxicity, anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 3 | Isomahanine | 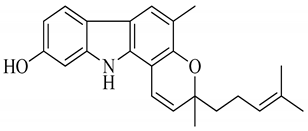 | Cytotoxicity, anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 4 | koenimbine | 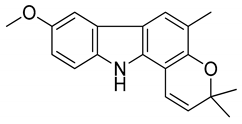 | Cytotoxicity and anti-diarrhea |
| 5 | Girinimbine | 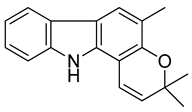 | Anti-tumor |
| 6 | Isolongifolene | 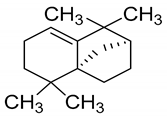 | Anti-oxidant and neuroprotective |
| 7 | Pyrayafoline D | 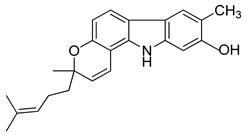 | Anti-cancer and anti-bacterial |
| 8 | Murrayafoline | 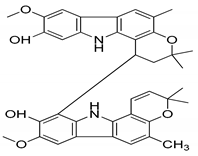 | Cytotoxicity and anti-inflammatory |
| 9 | Murrayazoline |  | Cytotoxicity and anti-tumor |
| 10 | Koenoline | 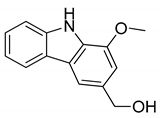 | Cytotoxicity |
| 11 | 9-formyl-3-methyl carbazole | 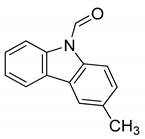 | Anti-oxidant |
| 12 | O-Methylmurrayamine |  | Anti-oxidant and neuroprotective |
| 13 | Koenine |  | Anti-oxidant |
| 14 | Koenigine | 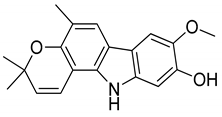 | Anti-oxidant |
| 15 | Mukonicine | 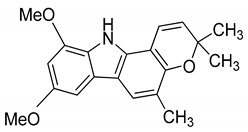 | Anti-oxidant |
| 16 | Mahanimbinine | 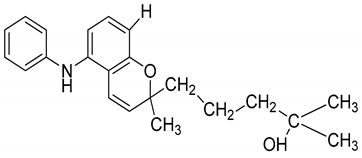 | Anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 17 | Murrayacinine | 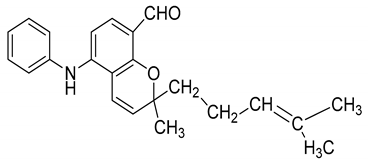 | Anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 18 | Mahanimboline | 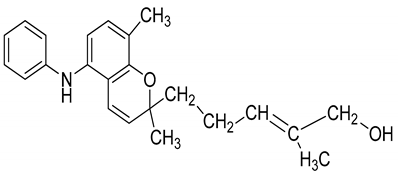 | Cytotoxicity, anti-oxidant, anti-microbial, anti-diabetic, and hyperlipidemic |
| 19 | Mukoeic acid | 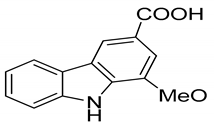 | Anti-oxidant |
| 20 | Murrayanine |  | Anti-oxidant |
| Pharmacological Activities | Plant Parts | Extract | Bioactive Compounds | Model | Main Finding | Reference |
|---|---|---|---|---|---|---|
| In vitro studies | ||||||
| Antifungal | Leaves | Essential oil | − | Disc diffusion method | Essential oil extracted from M. koenigii exhibited activities with MIC in the range of 25.5 to 75 μg/mL against pathogenic fungi A. niger, F. moniliforme, P. notatum, M. mucedo, and P. funiculosum | [27] |
| Antibacterial | Leaves | Solvent-free microwave extraction | − | Soy agar | Minimum inhibitory concentrations (MIC) of solvent-free microwave extraction (SFME) and hydro-distilled oil from M. koenigii with values of 400 and 600 μg/mL against L. innocua SFME-essential oil at 300 μg/mL provided 92% inhibition, indicating its antibacterial potential | [37] |
| Antibacterial | Leaves | Methanol | Koenine, koenigine, and mahanine | Broth micro-dilution assay | Koenine, koenigine, and mahanine extracted from M. koenigii exhibited activities with MIC values of 3.12–12.5 µg/mL against bacterial strains S. aureus and K. pneumonia | [40] |
| Antibacterial | Leaves | Aqueous | − | Agar diffusion assay | M. koenigii-AGNPs exhibited inhibitory activity against E. coli and S. aureus, with a value of 16 mm for M. koenigii-AgNPs and 15 mm for AgNO3 solution at 100 µg/well | [18] |
| Antibacterial | Leaves | Essential oil | − | Microtiter assay | Essential oil extracts of M. koenigii treatment resulted in a reduction of biofilm formation in P. aeruginosa PAO1. M. koenigii essential oil may effectively control Pseudomonas biofilms in indwelling medical device | [19] |
| Antibacterial | Leaves | Petroleum ether, ethanol, and water | − | Colony-forming unit (CFU) assay | Ethanol extracts of M. koenigii exhibited activity half maximal inhibitory concentration ((IC50) of 400 μg/mL) against the mycobacterium smegmatis compared to petroleum ether and water extracts | [20] |
| Hepatoprotective | Leaves | Aqueous | − | Hep G2 cell line | M. koenigii leaves preventing alcohol-induced cellular damage | [31] |
| Antioxidant | Leaves | Ethanol | − | DPPH free radical scavenging assay | exhibited activities with IC50 values of 21.4–49.5 µg/mL | [21] |
| Antioxidant | Leaves | Aqueous | − | TBARS, CAT, SOD, and glutathione (GSH) assay | Carbazole alkaloids from M. koenigii extract exhibited activity with IC50 values of 120 μg/mL in an ethanol-induced hepatotoxicity in vitro model | [31] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | DPPH free radical scavenging assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 36.46 μg/mL | [64] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | ABTS radical scavenging assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 11.55 μg/mL | [64] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | Superoxide assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 11.47 μg/mL | [64] |
| Antioxidant | Leaves | Aqueous/zinc oxide nanoparticles | − | H2O2 Assay | Zinc oxide nanoparticle-synthesized M. koenigii extract exhibited activity with an IC50 value of 54.06 μg/mL | [64] |
| Antioxidant | Leaves | Ethanoic | − | DPPH free radical scavenging assay | The ethanoic extract of M. koenigii showed an 80% scavenging activity, which was similar to the activities exhibited by the control antioxidant compound quercetin | [66] |
| Antioxidant | Leaves | Aqueous, alcohol, and acetone | − | DPPH free radical scavenging assay | The extracts of M. koenigii exhibited activities with an EC50 value of acetone of 4.7 µg/mL, alcohol of 4.1 µg/mL, and aqueous of 4.4 µg/mL, which were comparable to the EC50 value of 2.6 µg/mL exhibited by ascorbic acid, which was the positive control | [67] |
| Antioxidant | Leaves | Petroleum ether and ethyl acetate | − | Cupric-reducing antioxidant capacity | CUPRAC assays indicated the highest reducing potential in the benzene fraction, followed by petroleum ether and ethyl acetate | [68] |
| Antioxidant | Leaves | Benzene, ethyl acetate, acetone, methanol, and ethanol | − | DPPH free radical scavenging assay | Results showed that for 100 µg/mL, the benzene fraction extracted from M. koenigii showed 88.3% free radical scavenging activity, followed by ethyl acetate (79.5%), petrol ether (78.7%), acetone (66.1%), methanol (50.7%), and ethanol (53.0%) fractions, respectively, with the positive control being ascorbic acid (93.1%) | [69] |
| Antioxidant | Fruits | Aqueous | − | DPPH free radical scavenging assay | Fruit extracted from M. koenigii exhibited activities with an EC50 value of 2.6 mg/mL | [71] |
| Cytotoxicity | Stem bark and roots | Hexane, chloroform, and methanol | Girinimbine | MTT assay | Girinimbine was shown to significantly inhibit the proliferation of HT-29 cells with an IC50 value of 4.79 ± 0.74 μg/mL. | [85] |
| Cytotoxicity | Leaves | Ethanol | Murrayazoline and O-methylmurrayamine A | MTT assay | Murrayazoline and O-methylmurrayamine A exhibited activities with IC50 values of 5.7 and 17.9 mM in both HEK-293 and HaCaT cell lines, respectively | [86] |
| Cytotoxicity | − | − | Isolongifolene | MTT assay | Isolongifolene exhibited activities at 10 µM, showing a 90% viability in SH-SY5Y cells | [87] |
| Cytotoxicity | Leaves | Methanol | − | MTT assay | M. koenigii methanolic extract exhibited activities with IC50 values >400 µg/mL in the CLS-354 cell line | [96] |
| Cytotoxicity | Leaves | Ethanol | − | MTT assay | M. koenigii ethanolic extract exhibited activities with an IC50 value of 20 µg/mL in the mouse macrophage RAW 264.7 cell line | [20] |
| Cytotoxicity | Leaves | Hexane, ethyl acetate, and methanol | − | MTT assay | Three extracts of M. koenigii exhibited were very active, with values of <1 μg/mL to 2.25 μg/mL, and were thus proved to be potent cytotoxic activity agents against HeLa cancer cells | [11] |
| Anti-inflammatory | Stems | Methanol | Murrayakonine A, murrayanine, and O-methylmurrayamine-A | Human peripheral blood mononuclear cells | In vitro experiments showed murrayakonine A (IC50 10 µM), murrayanine (IC50 9.4 µM), and O-methylmurrayamine-A (IC50 7 µM) against TNF-α, and murrayanine (IC50 8.4 µM) and methylmurrayamine-A (IC50 8.4 µM) against IL-6, respectively | [39] |
| Anticancer (Colon) | Leaves | Ethanol | O-methylmurrayamine 5.7–17.9 µM | MCF-7 cells | O-methylmurrayamine A exhibited anti-colon cancer activity through downregulation of the Akt/mTOR survival pathway and activation of the intrinsic pathway of apoptosis | [86] |
| Anticancer (Oral) | Leaves | Methanol | Mahanine 15 μM | CLS-354 cells | Mahanine increased the expression of LC3B-II, cleaved caspase-3 proteins, and the inhibition of autophagic flux | [96] |
| Anticancer (Ovarian) | Stem bark | Methanol | Girinimbine 10 µM | Ovarian cancer cell line SKOV3/ SV40 | Girinimbine was found to be mainly due to the induction of apoptosis and cell cycle arrest due to the inhibition of the PI3K/AKT/mTOR and Wnt/b-catenin signaling pathways | [98] |
| Anticancer (Breast) | Leaves | Aqueous acetone | Koenimbin 4.89 μg/mL | MCF7 breast cancer stem cells | Koenimbin induced apoptosis in MCF7 cells that was mediated by cell death and regulated the mitochondrial membrane potential by downregulating Bcl2 and upregulating Bax, due to cytochrome c release from the mitochondria to the cytosol, and significantly downregulated the Wnt/β-catenin self-renewal pathway | [98] |
| Anticancer (Prostate) | Leaves | Aqueous acetone | Koenimbin 3.73 μg/mL | Prostate cancer stem cells | Koenimbin induced apoptosis through the intrinsic signaling pathway and suppression of the translocation of cytoplasmic NF-κB into the nucleus, in addition to displaying potential for targeting PCSCs, as affirmed by the prostasphere formation and Aldefluor assay | [99] |
| Anticancer | Leaves | Methanol | Mahanine 7.5 μM | Glioma HS 683 cells | Mahanine inhibited the cell migration and invasion and inhibited cell growth was simultaneous with the suppression of p-PI3K, p-AKT, and p-mTOR | [96] |
| Anticancer (Liver) | Leaves | Methanol | Mahanine 25 μM | HepG2, HuCCT1, and KKU-100 cells | Mahanine showed potent cytotoxicity, with increased expression levels of MITF balance between the cellular stresses | [13] |
| Anticancer (Cervical) | Leaves | Methanol | Mahanine 8.6 μM | HeLa (HPV-18) and SiHa (HPV-16) cell line | Mahanine and cisplatin synergistically displayed growth inhibitory activity in cervical cancer, the inhibition of STAT3 activation, cell migration, and induced apoptosis | [14] |
| Anticancer (Lung) | Leaves | Methanol | Mahanine 15 μM | NSCLC cancer cell line A549 | Mahanine induced the impairment of mTORC2 through rictor inhibition and the destruction of NSCLC cancer cells | [22] |
| Anticancer (Colon) | Leaves | Methanol | Mahanine 0–30 μM | HCT116, HCT116, SW480, and Vero | Mahanine synergistically activated the two tumor suppressors PTEN and p53/p73 and can potentially be used in combination therapy with 5-FU for the treatment of colon carcinoma | [23] |
| Anticancer (prostate) | Leaves | Methanol | Mahanine 10 μM | PC3 and LNCaP cell line | Mahanine selectively degraded DNMT1 and DNM T3B via the ubiquitin-proteasomal pathway in a dose-dependent manner upon the inactivation of Akt signaling | [24] |
| Neuroprotective | Leaves | Methanol | Isolongifolene 10 µM | SH-SY5Y cells | Isolongifolene was effectively attenuated in oxidative stress, mitochondrial dysfunction, and apoptosis | [87] |
| Neuroprotective | Leaves | Methanol | O-methylmurrayamine A | PC12 cells | O-methylmurrayamine A possibly protects against DNA damage, apoptosis, and high levels of cell viability | [35] |
| In vivo studies | ||||||
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract resulted in a significant reduction in the level of TBARS in both the plasma (3.64 ± 0.13) and pancreas (53.40 ± 2.13) of diabetic rats | [61] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract resulted in a significant increase in the level of GSH in both the plasma (24.16 ± 1.30) and pancreas (19.52 ± 1.09) of diabetic rats | [77] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract significantly restored the activity of SOD in the pancreas (3.69 ± 0.15) of diabetic rats | [77] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract significantly restored the activity of CAT in the pancreas (12.94 ± 0.54) of diabetic rats | [77] |
| Antioxidant | Leaves | Aqueous | − | Male albino Wistar rat | The oral administration of an M. koenigii leaf extract significantly restored the activity of GPx in the pancreas (5.86 ± 0.22) of diabetic rats | [67] |
| Antioxidant | Leaves | Ethanol | − | Sprague Dawley rats | For 200 and 400 µg/mL b.w, the M. koenigii extract showed 80% inhibited free radical generation and 75% restored GSH levels | [10] |
| Antioxidant | Leaves | Water | − | Male albino Wistar rat | Extract exhibited the potential to reduce lipid peroxidation activity in the liver (2.44 ± 0.029) and kidney (2.34 ± 0.09) in potassium dichromate-induced Wistar rats | [38] |
| Anti-inflammatory | Stem bark and roots | Hexane, chloroform, and methanol | Girinimbine | Adult zebrafish | Girinimbine treatment significantly suppressed the IL-1β and TNF-α levels induced by peritoneal fluid mice | [85] |
| Anti-inflammatory | Leaves | Ethanol | − | Sprague Dawley rats | Oral administration of an M. koenigii extract showed the reduced formation of oedema, with values of 43.28%, 59.67%, and 62.29% induced by carrageenan, histamine, and serotonin in rats | [30] |
| Hepatoprotective | Leaves | Hydro-ethanolic | − | Male Wistar rats | M. koenigii leaves significantly decreased CCl4 -induced hepatotoxic in a time- and dose-dependent manner | [16] |
| Nephroprotective | Leaves | Aqueous | − | Male Wistar rats | M. koenigii extract treatment significantly decreased the renal functional markers, like the blood urea nitrogen and creatinine level | [28] |
| Anti-Diabetic | Leaves | Ethanol | − | Swiss albino mice | M. koenigii possesses antidiabetic activity and has antioxidant effects on STZ-NA-induced diabetes mellitus and particularly significantly decreased the HOMA-IR index | [10] |
| Anticancer (Colon) | Stem bark and roots | Hexane, chloroform, and methanol | Girinimbine 1.5–100 µg/mL | Zebrafish and Male ICR mice | Girinimbine, supplementation specifically, resulted in the induction of apoptosis, the inhibition of inflammation, and a significant increase in cell numbers in the G0/G1 phase | [85] |
| Anticancer (Breast) | Leaves | Aqueous | − | Female BALB/c mice | M. koenigii aqueous extract has potential for cytotoxicity, anti-inflammatory, and immunomodulatory effects and delays rather than inhibits tumor formation | [12] |
| Neuroprotective | Leaves | Methanol | − | Male albino mice | M. koenigii is effective in attenuating memory impairment and oxidative stress and prevents abnormal oral movements | [36] |
| Neuroprotective | Leaves | Ethanol | − | Swiss albino mice | M. koenigii supplementation resulted in an improvement of acetylcholine (ACh) and reduction in acetylcholinesterase (AChE). In addition, a significant elevation of serum biomarkers, and decline in creatinine, total cholesterol, urea nitrogen, and glucose levels, ameliorated the hepatic and renal functions in the normal ageing process | [30] |
| Neuroprotective | Leaves | Ethanol | − | Male swiss albino mice | M. koenigii leaves elevated the acetylcholine level in the brain and ultimately improved memory impairment. In vitro, it showed BACE1 inhibition and was found to be a non-competitive inhibitor | [33] |
| Neuroprotective | Leaves | Methanol | Isolongifolene 10 mg/kg b.w. | Male albino Wistar rat | Isolongifolene effectively attenuated behavioral impairment and oxidative stress, acting as an antiaging agent | [34] |
| Anti-anxiety and anti-depressant | Leaves | Aqueous | − | Swiss albino mice | M. koenigii aqueous leaf extract reduced the despair behavior in experimental animal models, suggesting an anti-depressant-like activity and also reduced spontaneous locomotor activity | [41] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakrishnan, R.; Vijayraja, D.; Jo, S.-H.; Ganesan, P.; Su-Kim, I.; Choi, D.-K. Medicinal Profile, Phytochemistry, and Pharmacological Activities of Murraya koenigii and its Primary Bioactive Compounds. Antioxidants 2020, 9, 101. https://doi.org/10.3390/antiox9020101
Balakrishnan R, Vijayraja D, Jo S-H, Ganesan P, Su-Kim I, Choi D-K. Medicinal Profile, Phytochemistry, and Pharmacological Activities of Murraya koenigii and its Primary Bioactive Compounds. Antioxidants. 2020; 9(2):101. https://doi.org/10.3390/antiox9020101
Chicago/Turabian StyleBalakrishnan, Rengasamy, Dhanraj Vijayraja, Song-Hee Jo, Palanivel Ganesan, In Su-Kim, and Dong-Kug Choi. 2020. "Medicinal Profile, Phytochemistry, and Pharmacological Activities of Murraya koenigii and its Primary Bioactive Compounds" Antioxidants 9, no. 2: 101. https://doi.org/10.3390/antiox9020101
APA StyleBalakrishnan, R., Vijayraja, D., Jo, S.-H., Ganesan, P., Su-Kim, I., & Choi, D.-K. (2020). Medicinal Profile, Phytochemistry, and Pharmacological Activities of Murraya koenigii and its Primary Bioactive Compounds. Antioxidants, 9(2), 101. https://doi.org/10.3390/antiox9020101







