An Update on Thiol Signaling: S-Nitrosothiols, Hydrogen Sulfide and a Putative Role for Thionitrous Acid
Abstract
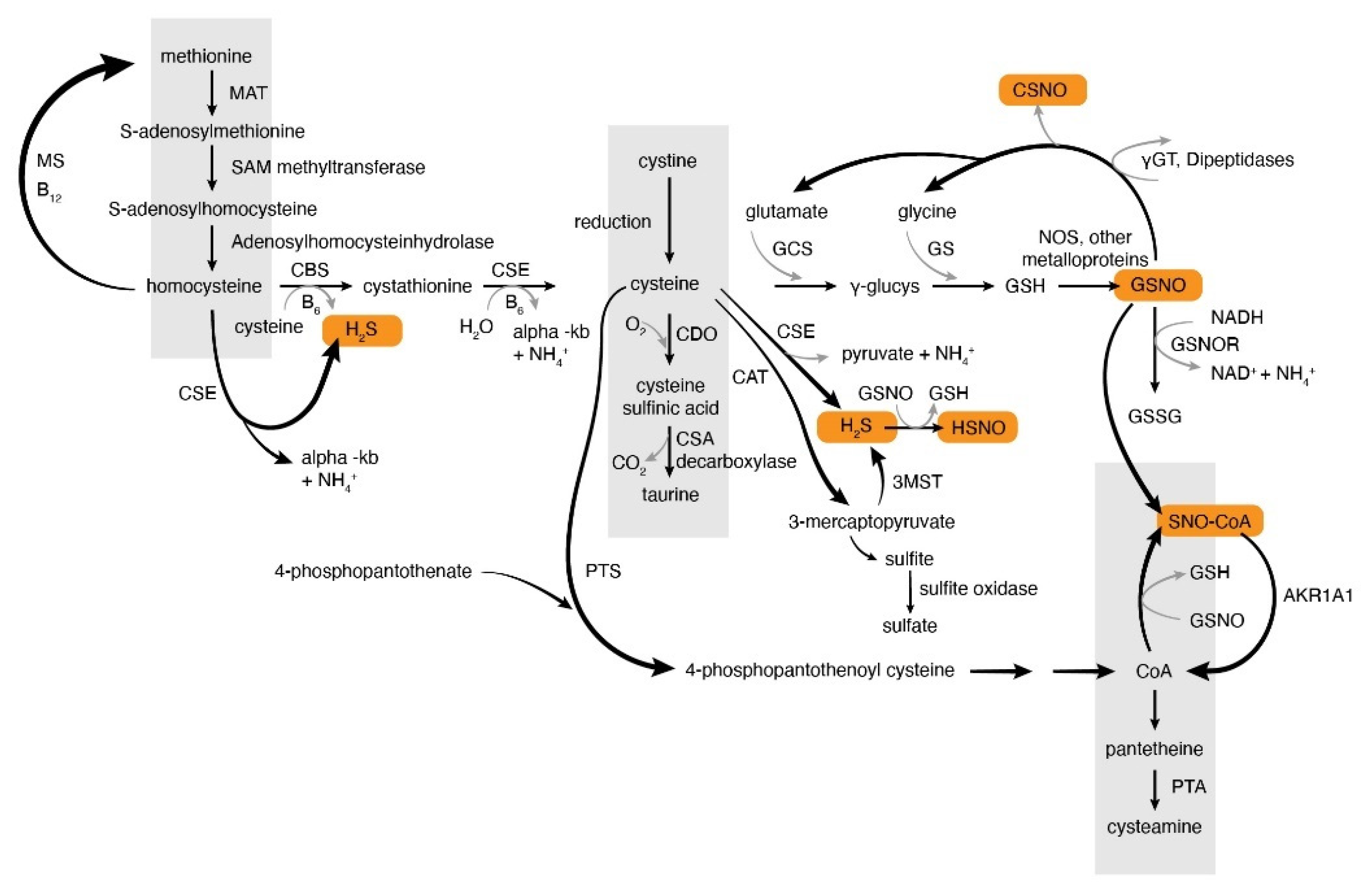
:1. Introduction
2. Overview of S-Nitrosothiol Metabolism
2.1. Production of S-Nitrosothiols and Other Nitrogen Oxides In Vivo
2.2. S-Nitrosylation Signaling and S-Nitrosothiols
2.3. Denitrosylation
3. Hydrogen Sulfide Production and Metabolism In Vivo. Similarities and Differences of NO and H2S Metabolism
Thionitrous Acid and Related Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hibbs, J.B., Jr.; Vavrin, Z.; Taintor, R.R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J. Immunol. 1987, 138, 550–565. [Google Scholar] [PubMed]
- Babu, B.R.; Frey, C.; Griffith, O.W. L-arginine binding to nitric-oxide synthase. The role of H-bonds to the nonreactive guanidinium nitrogens. J. Biol. Chem. 1999, 274, 25218–25226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southan, G.J.; Srinivasan, A. Nitrogen oxides and hydroxyguanidines: Formation of donors of nitric and nitrous oxides and possible relevance to nitrous oxide formation by nitric oxide synthase. Nitric Oxide 1998, 2, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Vicente, F.B.; Vespa, G.; Miller, A.; Haymond, S. Quantification of Arginine and Its Methylated Derivatives in Plasma by High-Performance Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS). Methods Mol. Biol. 2016, 1378, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Marozkina, N.V.; Gaston, B. S-Nitrosylation signaling regulates cellular protein interactions. Biochim. Biophys. Acta 2012, 1820, 722–729. [Google Scholar] [PubMed] [Green Version]
- Marozkina, N.V.; Gaston, B. Nitrogen chemistry and lung physiology. Annu. Rev. Physiol. 2015, 77, 431–452. [Google Scholar] [CrossRef]
- Smith, B.C.; Marletta, M.A. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 2012, 16, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Blonder, J.P.; Mutka, S.C.; Sun, X.; Qiu, J.; Green, L.H.; Mehra, N.K.; Boyanapalli, R.; Suniga, M.; Look, K.; Delany, C.; et al. Pharmacologic inhibition of S-nitrosoglutathione reductase protects against experimental asthma in BALB/c mice through attenuation of both bronchoconstriction and inflammation. BMC Pulm. Med. 2014, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Kobzik, L.; Bredt, D.S.; Lowenstein, C.J.; Drazen, J.; Gaston, B.; Sugarbaker, D.; Stamler, J.S. Nitric oxide synthase in human and rat lung: Immunocytochemical and histochemical localization. Am. J. Respir. Cell Mol. Biol. 1993, 9, 371–377. [Google Scholar] [CrossRef]
- Asano, K.; Chee, C.B.; Gaston, B.; Lilly, C.M.; Gerard, C.; Drazen, J.M.; Stamler, J.S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc. Natl. Acad. Sci. USA 1994, 91, 10089–10093. [Google Scholar]
- Guo, F.H.; De Raeve, H.R.; Rice, T.W.; Stuehr, D.J.; Thunnissen, F.B.; Erzurum, S.C. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 7809–7813. [Google Scholar] [PubMed] [Green Version]
- Gow, A.J.; Chen, Q.; Hess, D.T.; Day, B.J.; Ischiropoulos, H.; Stamler, J.S. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J. Biol. Chem. 2002, 277, 9637–9640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukahara, H.; Ishida, T.; Mayumi, M. Gas-phase oxidation of nitric oxide: Chemical kinetics and rate constant. Nitric Oxide 1999, 3, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller, M.J.; Joshi, M.S.; Thomas, D.D.; Lancaster, J.R., Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA 1998, 95, 2175–2179. [Google Scholar]
- Rosenfeld, R.J.; Bonaventura, J.; Szymczyna, B.R.; MacCoss, M.J.; Arvai, A.S.; Yates, J.R., III; Tainer, J.A.; Getzoff, E.D. Nitric-oxide synthase forms N-NO-pterin and S-NO-cys: Implications for activity, allostery, and regulation. J. Biol. Chem. 2010, 285, 31581–31589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, Y.; Joseph, J.; Kalyanaraman, B. Intramolecular electron transfer between tyrosyl radical and cysteine residue inhibits tyrosine nitration and induces thiyl radical formation in model peptides treated with myeloperoxidase, H2O2, and NO2-: EPR SPIN trapping studies. J. Biol. Chem. 2005, 280, 40684–40698. [Google Scholar] [CrossRef] [Green Version]
- Stamler, J.S.; Jia, L.; Eu, J.P.; McMahon, T.J.; Demchenko, I.T.; Bonaventura, J.; Gernert, K.; Piantadosi, C.A. Blood Flow Regulation by S-Nitrosohemoglobin in the Physiological Oxygen Gradient. Science 1997, 276, 2034–2037. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Akaike, T.; Miyamoto, Y.; Okamoto, T.; Sawa, T.; Otagiri, M.; Suzuki, S.; Yoshimura, T.; Maeda, H. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J. Biol. Chem. 1999, 274, 27069–27075. [Google Scholar]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Wilson, M.T.; Cooper, C.E. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2006, 103, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Whalen, E.J.; Foster, M.W.; Matsumoto, A.; Ozawa, K.; Violin, J.D.; Que, L.G.; Nelson, C.D.; Benhar, M.; Keys, J.R.; Rockman, H.A.; et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 2007, 129, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, B.; Drazen, J.M.; Jansen, A.; Sugarbaker, D.A.; Loscalzo, J.; Richards, W.; Stamler, J.S. Relaxation of human bronchial smooth muscle by S-nitrosothiols in vitro. J. Pharmacol. Exp. Ther. 1994, 268, 978–984. [Google Scholar] [PubMed]
- Carver, D.J.; Gaston, B.; Deronde, K.; Palmer, L.A. Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione. Am. J. Respir. Cell Mol. Biol. 2007, 37, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Straub, A.C.; Lohman, A.W.; Billaud, M.; Johnstone, S.R.; Dwyer, S.T.; Lee, M.Y.; Bortz, P.S.; Best, A.K.; Columbus, L.; Gaston, B.; et al. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature 2012, 491, 473–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, L.A.; Doctor, A.; Chhabra, P.; Sheram, M.L.; Laubach, V.E.; Karlinsey, M.Z.; Forbes, M.S.; Macdonald, T.; Gaston, B. S-nitrosothiols signal hypoxia-mimetic vascular pathology. J. Clin. Investig. 2007, 117, 2592–2601. [Google Scholar] [CrossRef]
- Antoniades, C.; Shirodaria, C.; Crabtree, M.; Rinze, R.; Alp, N.; Cunnington, C.; Diesch, J.; Tousoulis, D.; Stefanadis, C.; Leeson, P.; et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation 2007, 116, 2851–2859. [Google Scholar] [CrossRef] [Green Version]
- Antoniades, C.; Tousoulis, D.; Stefanadis, C. Effects of endothelial nitric oxide synthase gene polymorphisms on oxidative stress, inflammatory status, and coronary atherosclerosis: An example of a transient phenotype. J. Am. Coll. Cardiol. 2007, 49, 1226–1227. [Google Scholar] [CrossRef] [Green Version]
- Dikalova, A.; Aschner, J.L.; Kaplowitz, M.R.; Summar, M.; Fike, C.D. Tetrahydrobiopterin oral therapy recouples eNOS and ameliorates chronic hypoxia-induced pulmonary hypertension in newborn pigs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L743–L753. [Google Scholar] [CrossRef] [Green Version]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar]
- Jia, J.; Arif, A.; Terenzi, F.; Willard, B.; Plow, E.F.; Hazen, S.L.; Fox, P.L. Target-selective protein S-nitrosylation by sequence motif recognition. Cell 2014, 159, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Kornberg, M.D.; Sen, N.; Hara, M.R.; Juluri, K.R.; Nguyen, J.V.; Snowman, A.M.; Law, L.; Hester, L.D.; Snyder, S.H. GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 2010, 12, 1094–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.A.; Marletta, M.A. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat. Chem. Biol. 2005, 1, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paige, J.S.; Xu, G.; Stancevic, B.; Jaffrey, S.R. Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem. Biol. 2008, 15, 1307–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, B.; Reilly, J.; Drazen, J.M.; Fackler, J.; Ramdev, P.; Arnelle, D.; Mullins, M.E.; Sugarbaker, D.J.; Chee, C.; Singel, D.J.; et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc. Natl. Acad. Sci. USA 1993, 90, 10957–10961. [Google Scholar]
- Que, L.G.; Liu, L.; Yan, Y.; Whitehead, G.S.; Gavett, S.H.; Schwartz, D.A.; Stamler, J.S. Protection from experimental asthma by an endogenous bronchodilator. Science 2005, 308, 1618–1621. [Google Scholar] [CrossRef] [Green Version]
- Moore, P.E.; Ryckman, K.K.; Williams, S.M.; Patel, N.; Summar, M.L.; Sheller, J.R. Genetic variants of GSNOR and ADRB2 influence response to albuterol in African-American children with severe asthma. Pediatr. Pulmonol. 2009, 44, 649–654. [Google Scholar] [CrossRef]
- Seth, D.; Hess, D.T.; Hausladen, A.; Wang, L.; Wang, Y.J.; Stamler, J.S. A Multiplex Enzymatic Machinery for Cellular Protein S-nitrosylation. Mol. Cell 2018, 69, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Stamler, J.S.; Toone, E.J.; Lipton, S.A.; Sucher, N.J. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron 1997, 18, 691–696. [Google Scholar]
- Choi, Y.B.; Tenneti, L.; Le, D.A.; Ortiz, J.; Bai, G.; Chen, H.S.; Lipton, S.A. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 2000, 3, 15–21. [Google Scholar] [CrossRef]
- Lillo, M.A.; Himelman, E.; Shirokova, N.; Xie, L.H.; Fraidenraich, D.; Contreras, J.E. S-nitrosylation of Connexin43 hemichannels elicits cardiac stress induced arrhythmias in Duchenne Muscular Dystrophy mice. JCI Insight 2019, 4, 130091. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Schonhoff, C.; Papeta, N.; Ghafourifar, P.; Szibor, M.; Fang, K.; Gaston, B. S-Nitrosylation of mitochondrial caspases. J. Cell Biol. 2001, 154, 1111–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, N.S.; Calvert, J.W.; Elrod, J.W.; Gundewar, S.; Ji, S.Y.; Lefer, D.J. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2007, 104, 19144–19149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stsiapura, V.I.; Bederman, I.; Stepuro, I.I.; Morozkina, T.S.; Lewis, S.J.; Smith, L.; Gaston, B.; Marozkina, N. S-Nitrosoglutathione formation at gastric pH is augmented by ascorbic acid and by the antioxidant vitamin complex, Resiston. Pharm. Biol. 2018, 56, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, P.R.; Minor, R.L., Jr.; Guerra, R., Jr.; Bates, J.N.; Harrison, D.G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature 1990, 345, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, W.I. Endothelium-derived relaxing factor in brain blood vessels is not nitric oxide. Stroke 1992, 23, 1527–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batenburg, W.W.; de Vries, R.; Saxena, P.R.; Danser, A.H. L-S-nitrosothiols: Endothelium-derived hyperpolarizing factors in porcine coronary arteries? J. Hypertens. 2004, 22, 1927–1936. [Google Scholar] [CrossRef]
- Batenburg, W.W.; Popp, R.; Fleming, I.; de Vries, R.; Garrelds, I.M.; Saxena, P.R.; Danser, A.H. Bradykinin-induced relaxation of coronary microarteries: S-nitrosothiols as EDHF? Br. J. Pharmacol. 2004, 142, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Davisson, R.L.; Travis, M.D.; Bates, J.N.; Lewis, S.J. Hemodynamic effects of L- and D-S-nitrosocysteine in the rat. Stereoselective S-nitrosothiol recognition sites. Circ. Res. 1996, 79, 256–262. [Google Scholar]
- Lewis, S.J.; Travis, M.D.; Bates, J.N. Stereoselective S-nitrosocysteine recognition sites in rat brain. Eur. J. Pharmacol. 1996, 312, R3–R5. [Google Scholar] [CrossRef]
- Davisson, R.L.; Travis, M.D.; Bates, J.N.; Johnson, A.K.; Lewis, S.J. Stereoselective actions of S-nitrosocysteine in central nervous system of conscious rats. Am. J. Physiol. 1997, 272, H2361–H2368. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Hoque, A.; Bates, J.N. Differentiation of L- and D-S-nitrosothiol recognition sites in vivo. J. Cardiovasc. Pharmacol. 2005, 46, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.J.; Harvey, J.R.; Mulholland, E.L. Sodium (2-sulfonatoethyl) methanethiosulfonate prevents S-nitroso-l-cysteine activation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeca. Br. J. Pharmacol. 2003, 139, 1153–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, M.D.; Hoque, A.; Bates, J.N.; Lewis, S.J. Blockade of voltage-sensitive Ca(2+)-channels markedly diminishes nitric oxide- but not L-S-nitrosocysteine- or endothelium-dependent vasodilation in vivo. Eur. J. Pharmacol. 2000, 408, 289–298. [Google Scholar] [CrossRef]
- Seth, P.; Hsieh, P.N.; Jamal, S.; Wang, L.; Gygi, S.P.; Jain, M.K.; Coller, J.; Stamler, J.S. Regulation of MicroRNA Machinery and Development by Interspecies S-Nitrosylation. Cell 2019, 176, 1014–1025. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.; Johns, R.; Macdonald, T.; Kinter, M.; Gaston, B. S-nitrosoglutathione breakdown prevents airway smooth muscle relaxation in the guinea pig. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L716–L721. [Google Scholar]
- Benhar, M.; Forrester, M.T.; Hess, D.T.; Stamler, J.S. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 2008, 320, 1050–1054. [Google Scholar] [CrossRef] [Green Version]
- Haendeler, J.; Hoffmann, J.; Tischler, V.; Berk, B.C.; Zeiher, A.M.; Dimmeler, S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat. Cell Biol. 2002, 4, 743–749. [Google Scholar] [CrossRef]
- Bateman, R.L.; Rauh, D.; Tavshanjian, B.; Shokat, K.M. Human carbonyl reductase 1 is an S-nitrosoglutathione reductase. J. Biol. Chem. 2008, 283, 35756–35762. [Google Scholar]
- Trujillo, M.; Alvarez, M.N.; Peluffo, G.; Freeman, B.A.; Radi, R. Xanthine oxidase-mediated decomposition of S-nitrosothiols. J. Biol. Chem. 1998, 273, 7828–7834. [Google Scholar]
- Johnson, M.A.; Macdonald, T.L.; Mannick, J.B.; Conaway, M.R.; Gaston, B. Accelerated s-nitrosothiol breakdown by amyotrophic lateral sclerosis mutant copper, zinc-superoxide dismutase. J. Biol. Chem. 2001, 276, 39872–39878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, N.; Root, P.; Jiang, X.M.; Hogg, P.J.; Mutus, B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase. Proc. Natl. Acad. Sci. USA 2001, 98, 9539–9544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stomberski, C.T.; Anand, P.; Venetos, N.M.; Hausladen, A.; Zhou, H.L.; Premont, R.T.; Stamler, J.S. AKR1A1 is a novel mammalian S-nitroso-glutathione reductase. J. Biol. Chem. 2019, 294, 18285–18293. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Romieu, I.; Sienra-Monge, J.J.; Estela Del Rio-Navarro, B.; Anderson, D.M.; Jenchura, C.A.; Li, H.; Ramirez-Aguilar, M.; Del Carmen Lara-Sanchez, I.; London, S.J. Genetic variation in S-nitrosoglutathione reductase (GSNOR) and childhood asthma. J. Allergy Clin. Immunol. 2007, 120, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhry, S.; Que, L.G.; Yang, Z.; Liu, L.; Eng, C.; Kim, S.O.; Kumar, G.; Thyne, S.; Chapela, R.; Rodriguez-Santana, J.R.; et al. GSNO reductase and beta2-adrenergic receptor gene-gene interaction: Bronchodilator responsiveness to albuterol. Pharmacogenet. Genomics 2010, 20, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Marozkina, N.V.; Wang, X.Q.; Stsiapura, V.; Fitzpatrick, A.; Carraro, S.; Hawkins, G.A.; Bleecker, E.; Meyers, D.; Jarjour, N.; Fain, S.B.; et al. Phenotype of asthmatics with increased airway S-nitrosoglutathione reductase activity. Eur. Respir. J. 2015, 45, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Kolluru, G.K.; Yuan, S.; Kevil, C.G. Measurement of H2S in vivo and in vitro by the monobromobimane method. Methods Enzymol. 2015, 554, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Wintner, E.A.; Deckwerth, T.L.; Langston, W.; Bengtsson, A.; Leviten, D.; Hill, P.; Insko, M.A.; Dumpit, R.; VandenEkart, E.; Toombs, C.F.; et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 2010, 160, 941–957. [Google Scholar] [CrossRef] [Green Version]
- Sonobe, T.; Haouzi, P. H2S concentrations in the heart after acute H2S administration: Methodological and physiological considerations. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1445–H1458. [Google Scholar] [CrossRef]
- Prabhakar, N.R. Carbon monoxide (CO) and hydrogen sulfide (H(2)S) in hypoxic sensing by the carotid body. Respir. Physiol. Neurobiol. 2012, 184, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Vellecco, V.; Mancini, A.; Ianaro, A.; Calderone, V.; Attanasio, C.; Cantalupo, A.; Andria, B.; Savoia, G.; Panza, E.; Di Martino, A.; et al. Cystathionine beta-synthase-derived hydrogen sulfide is involved in human malignant hyperthermia. Clin. Sci. 2016, 130, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Calo, L.; Romero, L.C.; Garcia, I.; Gotor, C. An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cye homeostasis in Arabidopsis. Plant Physiol. 2010, 152, 656–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.; Stern, A.; Monteiro, H.P. S-nitrosothiols and H2S donors: Potential chemo-therapeutic agents in cancer. Redox Biol. 2019, 27, 101190. [Google Scholar] [CrossRef]
- Wilson, K.; Mudra, M.; Furne, J.; Levitt, M. Differentiation of the roles of sulfide oxidase and rhodanese in the detoxification of sulfide by the colonic mucosa. Dig. Dis. Sci. 2008, 53, 277–283. [Google Scholar] [CrossRef]
- Jackson, M.R.; Melideo, S.L.; Jorns, M.S. Human sulfide:quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 2012, 51, 6804–6815. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Kavalier, A.; Lukyanov, E.; Gross, S.S. S-sulfhydration/desulfhydration and S-nitrosylation/denitrosylation: A common paradigm for gasotransmitter signaling by HS and NO. Methods 2013, 62, 177–181. [Google Scholar] [CrossRef]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005, 7, 665–674. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suarez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Modis, K.; Panopoulos, P.; Asimakopoulou, A.; Gero, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szijarto, I.A.; Marko, L.; Filipovic, M.R.; Miljkovic, J.L.; Tabeling, C.; Tsvetkov, D.; Wang, N.; Rabelo, L.A.; Witzenrath, M.; Diedrich, A.; et al. Cystathionine gamma-Lyase-Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension 2018, 71, 1210–1217. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Cirino, G.; Vellecco, V.; Bucci, M. Nitric oxide and hydrogen sulfide: The gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017, 174, 4021–4031. [Google Scholar] [CrossRef] [Green Version]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef] [Green Version]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Zaid, A.; Giannogonas, P.; Cantalupo, A.; Dhayade, S.; Karalis, K.P.; Wang, R.; et al. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS ONE 2012, 7, e53319. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Pietri, R.; Roman-Morales, E.; Lopez-Garriga, J. Hydrogen sulfide and hemeproteins: Knowledge and mysteries. Antioxid. Redox Signal. 2011, 15, 393–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Yadav, P.K.; Kurthen, A.; Banerjee, R. Sulfide Oxidation by a Noncanonical Pathway in Red Blood Cells Generates Thiosulfate and Polysulfides. J. Biol. Chem. 2015, 290, 8310–8320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostelaar, T.; Vitvitsky, V.; Kumutima, J.; Lewis, B.E.; Yadav, P.K.; Brunold, T.C.; Filipovic, M.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. Hydrogen Sulfide Oxidation by Myoglobin. J. Am. Chem. Soc. 2016, 138, 8476–8488. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.S.; Traylor, T.G.; Gardiner, R.; Mizukami, H. Reaction of nitric oxide with heme proteins and model compounds of hemoglobin. Biochemistry 1987, 26, 3837–3843. [Google Scholar] [CrossRef]
- Jensen, B.; Fago, A. Reactions of ferric hemoglobin and myoglobin with hydrogen sulfide under physiological conditions. J. Inorg. Biochem. 2018, 182, 133–140. [Google Scholar] [CrossRef]
- Choi, Y.B.; Lipton, S.A. Redox modulation of the NMDA receptor. Cell. Mol. Life Sci. 2000, 57, 1535–1541. [Google Scholar] [CrossRef]
- Li, Q.; Lancaster, J.R., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, M.R.; Miljkovic, J.L.; Nauser, T.; Royzen, M.; Klos, K.; Shubina, T.; Koppenol, W.H.; Lippard, S.J.; Ivanović-Burmazović, I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012, 134, 12016–12027. [Google Scholar] [CrossRef]
- Bruce King, S. Potential biological chemistry of hydrogen sulfide (H2S) with the nitrogen oxides. Free Radic. Biol. Med. 2013, 55, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M.; et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 2015, 46, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, A.S.M.; Bhowmick, R.; Pal, K.; Katarkar, A.; Chaudhuri, K.; Ali, M. A Smart Molecule for Selective Sensing of Nitric Oxide: Conversion of NO to HSNO; Relevance of Biological HSNO Formation. Inorg. Chem. 2017, 56, 4324–4331. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Xu, S.; Radford, M.N.; Zhang, W.; Kelly, S.S.; Day, J.J.; Xian, M. O-->S Relay Deprotection: A General Approach to Controllable Donors of Reactive Sulfur Species. Angew. Chem. Int. Ed. Engl. 2018, 57, 5893–5897. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.; Martin-Drumel, M.A.; Lopez, C.A.; Crabtree, K.N.; Womack, C.C.; Nguyen, T.L.; Thorwirth, S.; Cummins, C.C.; Stanton, J.F.; McCarthy, M.C. Spontaneous and Selective Formation of HSNO, a Crucial Intermediate Linking H2S and Nitroso Chemistries. J. Am. Chem. Soc. 2016, 138, 11441–11444. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.V.; Anton, B.J.; Timerghazin, Q.K. On the possible biological relevance of HSNO isomers: A computational investigation. Phys. Chem. Chem. Phys. 2014, 16, 8476–8486. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marozkina, N.; Gaston, B. An Update on Thiol Signaling: S-Nitrosothiols, Hydrogen Sulfide and a Putative Role for Thionitrous Acid. Antioxidants 2020, 9, 225. https://doi.org/10.3390/antiox9030225
Marozkina N, Gaston B. An Update on Thiol Signaling: S-Nitrosothiols, Hydrogen Sulfide and a Putative Role for Thionitrous Acid. Antioxidants. 2020; 9(3):225. https://doi.org/10.3390/antiox9030225
Chicago/Turabian StyleMarozkina, Nadzeya, and Benjamin Gaston. 2020. "An Update on Thiol Signaling: S-Nitrosothiols, Hydrogen Sulfide and a Putative Role for Thionitrous Acid" Antioxidants 9, no. 3: 225. https://doi.org/10.3390/antiox9030225
APA StyleMarozkina, N., & Gaston, B. (2020). An Update on Thiol Signaling: S-Nitrosothiols, Hydrogen Sulfide and a Putative Role for Thionitrous Acid. Antioxidants, 9(3), 225. https://doi.org/10.3390/antiox9030225



