Plasma Non-Enzymatic Antioxidant Capacity (NEAC) in Relation to Dietary NEAC, Nutrient Antioxidants and Inflammation-Related Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Dietary Assessment and D-NEAC Estimation
2.4. Blood Samples and P-NEAC Measurements
2.5. Ascorbic Acid, Dehydroascorbic Acid and Total Vitamin C Determination
2.6. Fat-Soluble Antioxidant Compounds Determination
2.7. Inflammation Biomarker Measurements
2.8. Uric Acid Measurements
2.9. Statistical Analysis
3. Results
4. Discussion
4.1. D-NEAC and P-NEAC Relations
4.2. Dietary/Plasma NEAC Associations with Nutrient/Inflammation Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolarzyk, E.; Pietrzycka, A.; Zając, J.; Morawiecka-Baranek, J. Relationship between dietary antioxidant index (DAI) and antioxidants level in plasma of Kraków inhabitants. Adv. Clin. Exp. Med. 2017, 26, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Lee, S.-G.; Wang, Y.; Yang, M.; Davis, C.G.; Koo, S.I. Dietary Total Antioxidant Capacity Is Associated with Diet and Plasma Antioxidant Status in Healthy Young Adults. J. Acad. Nutr. Diet. 2012, 112, 1626–1635. [Google Scholar]
- Carrión-García, C.J.; Guerra-Hernández, E.J.; García-Villanova, B.; Molina-Montes, E. Non-enzymatic antioxidant capacity (NEAC) estimated by two different dietary assessment methods and its relationship with NEAC plasma levels. Eur. J. Nutr. 2017, 56, 1561–1576. [Google Scholar] [CrossRef]
- Serafini, M.; Del Rio, D. Understanding the association between dietary antioxidants, redox status and disease: Is the Total Antioxidant Capacity the right tool? Redox Rep. 2004, 9, 145–152. [Google Scholar] [CrossRef]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef]
- Serafini, M.; Jakszyn, P.; Luján-Barroso, L.; Agudo, A.; Bas Bueno-De-Mesquita, H.; Van Duijnhoven, F.J.B.; Jenab, M.; Navarro, C.; Palli, D.; Boeing, H.; et al. Dietary total antioxidant capacity and gastric cancer risk in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2012, 131, 544–554. [Google Scholar] [CrossRef]
- Shanahan, F.; van Sinderen, D.; O’Toole, P.W.; Stanton, C. Feeding the microbiota: Transducer of nutrient signals for the host. Gut 2017, 66, 1709–1717. [Google Scholar] [CrossRef] [Green Version]
- Babaei, M.; Dashti, N.; Lamei, N.; Abdi, K.; Nazari, F.; Abbasian, S.; Gerayeshnejad, S. Evaluation of plasma concentrations of homocysteine, IL-6, TNF-alpha, hs-CRP, and total antioxidant capacity in patients with end-stage renal failure. Acta Med. Iran. 2014, 52, 893–898. [Google Scholar]
- Romeu, M.; Aranda, N.; Giralt, M.; Ribot, B.; Nogues, M.R.; Arija, V. Diet, iron biomarkers and oxidative stress in a representative sample of Mediterranean population. Nutr. J. 2013, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Gawron-Skarbek, A.; Guligowska, A.; Prymont-Przymińska, A.; Nowak, D.; Kostka, T. Plasma and Salivary Non-Urate Total Antioxidant Capacity Does Not Depend on Dietary Vitamin C, E, or β-Carotene Intake in Older Subjects. Molecules 2018, 23, 983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, M.; Lee, S.G.; Davis, C.G.; Kenny, A.; Koo, S.I.; Chun, O.K. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J. Nutr. Biochem. 2012, 23, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Arsenio, L.; Caronna, S.; Dall’Aglio, E.; Frega, N.G.; Pacetti, D.; Boselli, E.; Tiano, L.; Principi, F.L.G. Effect of antioxidant-enriched foods on plasma Coenzyme Q 10 and total antioxidant capacity. Eur. J. Lipid Sci. Technol. 2008, 110, 990–996. [Google Scholar] [CrossRef]
- Rautiainen, S.; Serafini, M.; Morgenstern, R.; Prior, R.L.; Wolk, A. The validity and reproducibility of food-frequency questionnaire-based total antioxidant capacity estimates in Swedish women. Am. J. Clin. Nutr. 2008, 87, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, N.; Salvatore, S.; Valtueña, S.; Bedogni, G.; Porrini, M.; Pala, V.; Del Rio, D.; Sieri, S.; Miglio, C.; Krogh, V.; et al. Development and Validation of a Food Frequency Questionnaire for the Assessment of Dietary Total Antioxidant Capacity. J. Nutr. 2007, 137, 93–98. [Google Scholar] [CrossRef]
- Cao, G.; Booth, S.L.; Sadowski, J.A.; Prior, R.L. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am. J. Clin. Nutr. 1998, 68, 1081–1087. [Google Scholar] [CrossRef]
- Stringa, N.; Brahimaj, A.; Zaciragic, A.; Dehghan, A.; Ikram, M.A.; Hofman, A.; Muka, T.; Kiefte-de Jong, J.C.; Franco, O.H. Relation of antioxidant capacity of diet and markers of oxidative status with C-reactive protein and adipocytokines: A prospective study. Metabolism 2017, 71, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Detopoulou, P.; Panagiotakos, D.B.; Chrysohoou, C.; Fragopoulou, E.; Nomikos, T.; Antonopoulou, S.; Pitsavos, C.; Stefanadis, C. Dietary antioxidant capacity and concentration of adiponectin in apparently healthy adults: The ATTICA study. Eur. J. Clin. Nutr. 2010, 64, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Brighenti, F.; Valtueña, S.; Pellegrini, N.; Ardigò, D.; Del Rio, D.; Salvatore, S.; Piatti, P.; Serafini, M.; Zavaroni, I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br. J. Nutr. 2005, 93, 619–625. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Uenishi, K.; Yamasaki, M.; Hayabuchi, H.; Goda, T.; Oka, J.; Baba, K.; Ohki, K.; et al. Dietary total antioxidant capacity from different assays in relation to serum C-reactive protein among young Japanese women. Nutr. J. 2012, 11, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Valtueña, S.; Del Rio, D.; Pellegrini, N.; Ardigò, D.; Franzini, L.; Salvatore, S.; Piatti, P.M.; Riso, P.; Zavaroni, I.; Brighenti, F. The total antioxidant capacity of the diet is an independent predictor of plasma β-carotene. Eur. J. Clin. Nutr. 2007, 61, 69–76. [Google Scholar] [CrossRef]
- Svilaas, A.; Sakhi, A.K.; Andersen, L.F.; Svilaas, T.; Ström, E.C.; Jacobs, D.R.; Ose, L.; Blomhoff, R. Intakes of Antioxidants in Coffee, Wine, and Vegetables Are Correlated with Plasma Carotenoids in Humans. J. Nutr. 2004, 134, 562–567. [Google Scholar] [CrossRef]
- EPIC-Spain Group El estudio prospectivo europeo sobre dieta, cáncer y salud (EPIC) en España. Rev. Española Salud 2004, 78, 167–176. [CrossRef] [PubMed] [Green Version]
- Ferrari, C.K.B. Effects of xenobiotics on total antioxidant capacity. Interdiscip. Toxicol. 2012, 5, 117–122. [Google Scholar] [PubMed]
- Buckland, G.; González, C.A.; Agudo, A.; Vilardell, M.; Berenguer, A.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Basterretxea, M.; et al. Adherence to the mediterranean diet and risk of coronary heart disease in the spanish EPIC cohort study. Am. J. Epidemiol. 2009, 170, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- EPIC-Spain Group. Relative Validity and Reproducibility of a Diet History Questionnaire in Spain. I Foods. Int. J. Epidemiol. 1997, 26, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slimani, N.; Deharveng, G.; Unwin, I.; Southgate, D.A.T.; Vignat, J.; Skeie, G.; Salvini, S.; Parpinel, M.; Møller, A.; Ireland, J.; et al. The EPIC nutrient database project (ENDB): A first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur. J. Clin. Nutr. 2007, 61, 1037–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haytowitz, D.B.; Bhagwat, S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods; Release 2; Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center and Nutrient Data Laboratory: Beltsville, MD, USA, 2010. [Google Scholar]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans 1. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Haftenberger, M.; Schuit, A.J.; Tormo, M.J.; Boeing, H.; Wareham, N.; Bueno-de-Mesquita, H.B.; Kumle, M.; Hjartåker, A.; Chirlaque, M.D.; Ardanaz, E.; et al. Physical activity of subjects aged 50–64 years involved in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2002, 5, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggenete, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar]
- Lykkesfeldt, J. Ascorbate and dehydroascorbic acid as reliable biomarkers of oxidative stress: Analytical reproducibility and long-term stability of plasma samples subjected to acidic deproteinization. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2513–2516. [Google Scholar] [CrossRef] [Green Version]
- Battino, M.; Leone, L.; Bompadre, S. High-Performance Liquid Chromatography-EC Assay of Mitochondrial Coenzyme Q9, Coenzyme Q9H2, Coenzyme Q10, Coenzyme Q10H2, and Vitamin E with a Simplified On-Line Solid-Phase Extraction. Methods Enzymol. 2004, 378, 156–162. [Google Scholar]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef]
- McLachlan, G.J. Cluster analysis and related techniques in medical research. Stat. Methods Med. Res. 1992, 1, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.D. Linear Regression. Regres. Methods Biostat. 1977, 19, 69–131. [Google Scholar]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Khatibi, S.R.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose–response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019, 58, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Song, W.O.; Fernandez, M.L.; Bruno, R.S.; Koo, S.I.; Chun, O.K. Development and validation of an algorithm to establish a total antioxidant capacity database of the US diet. Int. J. Food Sci. Nutr. 2010, 61, 600–623. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Young, J.F.; Nielsen, S.E.; Haraldsdóttir, J.; Daneshvar, B.; Lauridsen, S.T.; Knuthsen, P.; Crozier, A.; Sandström, B.; Dragsted, L.O. Effect of fruit juice intake on urinary quercetin excretion and biomarkers of antioxidative status. Am. J. Clin. Nutr. 1999, 69, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Young, J.F.; Dragsted, L.O.; Daneshvar, B.; Lauridsen, S.T.; Hansen, M.; Sandström, B. The effect of grape-skin extract on oxidative status. Br. J. Nutr. 2000, 84, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.-H.; Meng, L.-P.; Ma, W.-W.; Li, S.; Feng, J.-F.; Yu, H.-L.; Xiao, R. The role of glutathione S-transferase M1 and T1 gene polymorphisms and fruit and vegetable consumption in antioxidant parameters in healthy subjects. Br. J. Nutr. 2012, 107, 928–933. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Zhang, L.; Ma, W.; Zhou, X.; Ji, J.; Li, N.; Xiao, R. Glutathione S-transferase M1 and T1 gene polymorphisms with consumption of high fruit-juice and vegetable diet affect antioxidant capacity in healthy adults. Nutrition 2013, 29, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Andrade, C.; Morales, F.J. Unraveling the Contribution of Melanoidins to the Antioxidant Activity of Coffee Brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
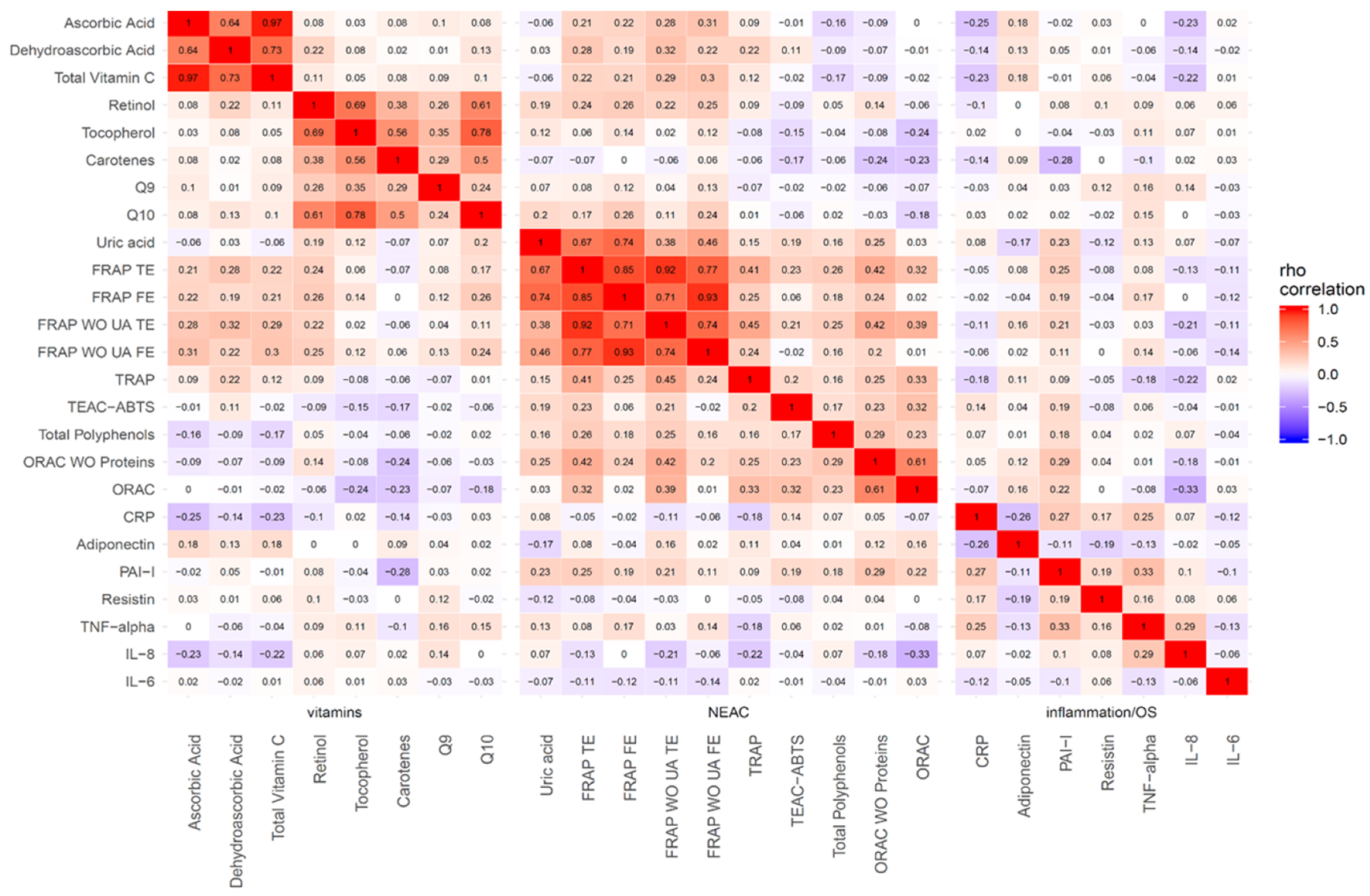

| Variables | All (n = 210) | Men (n = 63) | Women (n = 147) | p-Value 2 |
|---|---|---|---|---|
| Median [IQR 25–75] | Median [IQR 25–75] | Median [IQR 25–75] | ||
| Age | 48.8 [41.4;55.2] | 51.9 [46.6;57.1] | 46.3 [39.9;53.1] | 0.001 |
| BMI (kg/m2) | 27.2 [24.8;30.4] | 28 [25.9;30.7] | 26.4 [24.2;30.2] | 0.042 |
| Cigarettes/day among smokers | 10.0 [5.00;20.0] | 17.5 [7.00;20.0] | 9.00 [3.00;12.0] | 0.103 |
| N (%) | N (%) | N (%) | ||
| Abdominal obesity | 0.781 | |||
| Normal | 128 (60.9) | 37 (58.7) | 91 (61.9) | |
| Obese | 82 (39.1) | 26 (41.3) | 56 (38.1) | |
| Center | <0.001 | |||
| Granada | 105 (50.0) | 17 (27.0) | 88 (59.9) | |
| Gipuzkoa | 105 (50.0) | 46 (73.0) | 59 (40.1) | |
| Smoking status | ||||
| Never smoker | 130 (61.9) | 25 (39.7) | 105 (71.4) | <0.001 |
| Former smoker | 34 (16.2) | 19 (30.2) | 15 (10.2) | |
| Current smoker | 45 (21.4) | 19 (30.2) | 26 (17.7) | |
| Physical activity | ||||
| Inactive | 78 (37.1) | 5 (7.94) | 73 (49.7) | <0.001 |
| Moderately inactive | 68 (32.4) | 19 (30.2) | 49 (33.3) | |
| Moderately active | 41 (19.5) | 24 (38.7) | 17 (11.6) | |
| Active | 23 (11.0) | 15 (23.8) | 8 (5.44) | |
| Education Level | ||||
| None | 72 (34.6) | 13 (21.0) | 59 (40.4) | 0.088 |
| Primary school | 90 (43.3) | 34 (54.8) | 56 (38.4) | |
| Secondary school | 16 (7.70) | 5 (8.06) | 11 (7.53) | |
| Professional | 12 (5.80) | 2 (3.23) | 10 (6.85) | |
| University | 18 (8.70) | 8 (12.9) | 10 (6.85) | |
| Dietary Characteristics 1 | Median [IQR 25–75] | Median [IQR 25–75] | Median [IQR 25–75] | |
| Energy intake (kcal/day) | 1875 [1548;2310] | 2432 [2175;2934] | 1712 [1421;1999] | <0.001 |
| Fruits (g/day) | 254 [139;409] | 280 [142;436] | 250 [139;395] | 0.294 |
| Vegetables (g/day) | 206 [123;297] | 211 [118;313] | 206 [132;285] | 0.954 |
| Legumes (g/day) | 37.2 [23.3;63.4] | 48.0 [29.8;102] | 35.0 [22.6;51.2] | 0.001 |
| Cereals (g/day) | 202 [135;260] | 261 [202;326] | 173 [127;232] | <0.001 |
| Meat and meat products (g/day) | 101 [69.2;148] | 140 [102;163] | 89.6 [65.3;120] | <0.001 |
| Fish and seafood (g/day) | 54.3 [32.3;83.2] | 79.7 [49.3;120] | 49.3 [27.2;69.9] | <0.001 |
| Milk and dairy products (g/day) | 253 [161;376] | 246 [150;333] | 258 [166;399] | 0.232 |
| Red wine (g/day) | 0.00 [0.00;40.2] | 100 [0.00;192] | 0.00 [0.00;0.00] | <0.001 |
| Coffee (g/day) | 84.4 [3.36;152] | 98.3 [26.8;131] | 76.8 [2.93;174] | 0.901 |
| Tea (g/day) | 0.00 [0.00;0.00] | 0.00 [0.00;0.00] | 0.00 [0.00;0.00] | 0.684 |
| Flavonoids (mg/1000 Kcal/day) | 161 [111;227] | 170 [115;227] | 157 [107;225] | 0.385 |
| β-Carotene (µg/1000 Kcal/day) | 1092 [719;1574] | 922 [565;1436] | 1221 [836;1601] | 0.005 |
| Retinol (µg/ 1000 Kcal/day) | 145 [93.8;204] | 117 [88.5;183] | 149 [95.8;207] | 0.075 |
| α-Tocopherol (mg/1000 Kcal/day) | 5.7 [4.5;7.5] | 5.2 [4.5;7] | 6.1 [7.5;7.7] | 0.094 |
| Vitamin C (mg/1000 Kcal/day) | 66.6 [44.8;89.5] | 56.8 [40.4;71.6] | 71.6 [46.3;102] | <0.001 |
| Iron (mg/1000 Kcal/day) | 7.2 [6.3;8.3] | 7.32 [6.67;8.34] | 7.10 [6.06;8.21] | 0.153 |
| Alcohol (g/1000 Kcal/day) | 0.89 [0.01;5.9] | 8.67 [2.88;15.6] | 0.11 [0.00;1.99] | <0.001 |
| TRAP (µmol TE/day) | 8990 [3764;15,231] | 10,830 [5663;1,6160] | 8083 [3431;14,836] | 0.025 |
| TRAP without coffee (µmol TE/day) | 2771 [1876;4631] | 4584 [3103;7025] | 2285 [1748;3548] | <0.001 |
| FRAP (µmol Fe2+/day) | 22,388 [11,079;33,821] | 26,226 [17,529;36,363] | 19,713 [9421;33,458] | 0.009 |
| FRAP without coffee (µmol Fe2+/day) | 8765 [6560;13,636] | 14,221 [10,581;17,979] | 7720 [5948;11,355] | <0.001 |
| TEAC-ABTS (µmol TE/day) | 6855 [3625;10,336] | 8304 [5844;11,608] | 6130 [3176;10,050] | 0.003 |
| TEAC-ABTS without coffee (µmol TE/day) | 3083 [2321;4828] | 4791 [3417;6665] | 2739 [2037;4002] | <0.001 |
| ORAC (µmol TE/day) | 31,501 [15,818;48,097] | 33,193 [21,398;44,293] | 28,174 [14,444;49,880] | 0.286 |
| ORAC without coffee (µmol TE/day) | 12,042 [8597;16,299] | 14,300 [10,447;19,927] | 11,338 [8040;15,434] | <0.001 |
| Total Polyphenols without coffee (mgGAE/day) | 1519 [1108;2033] | 1760 [1386;2511] | 1433 [1056;1938] | <0.001 |
| Biomarkes | All | Men | Women | |||||
|---|---|---|---|---|---|---|---|---|
| N = 210 | N = 63 | N = 147 | ||||||
| Median | IQR (25–75) | N | Median | IQR (25–75) | Median | IQR (25–75) | p-Value 1 | |
| Ascorbic acid (µmol/L) | 27.8 | [20.9;46.3] | 210 | 28.2 | [23.9;54.0] | 27.8 | [20.4;42.7] | 0.201 |
| Dehydroascorbic acid (µmol/L) | 0.00 | [0.00;3.76] | 210 | 0.00 | [0.00;6.85] | 0.00 | [0.00;0.00] | 0.007 |
| Total vitamin C (µmol/L) | 29.3 | [21.4;52.5] | 210 | 31.6 | [24.3;64.3] | 28.9 | [20.4;44.5] | 0.113 |
| Retinol (µmol/L) | 2.22 | [1.85;2.73] | 210 | 2.31 | [1.94;2.81] | 2.16 | [1.79;2.66] | 0.066 |
| Tocopherol (µmol/L) | 28.4 | [21.6;37.3] | 210 | 27.3 | [20.7;37.0] | 29.4 | [21.7;37.5] | 0.410 |
| Carotenes (µmol/L) | 3.47 | [2.34;6.73] | 210 | 2.79 | [1.80;4.27] | 3.92 | [2.62;7.65] | <0.001 |
| Q9 (µmol/L) | 0.05 | [0.03;0.08] | 210 | 0.05 | [0.03;0.09] | 0.05 | [0.03;0.07] | 0.727 |
| Q10 (µmol/L) | 1.16 | [0.96;1.50] | 210 | 1.17 | [1.00;1.59] | 1.15 | [0.95;1.50] | 0.662 |
| Uric acid (mg/dl) | 3.73 | [3.05;4.48] | 210 | 4.71 | [3.96;5.41] | 3.45 | [2.92;4.03] | <0.001 |
| FRAP (µmol TE/L) | 457 | [403;519] | 210 | 528 | [471;560] | 428 | [393;476] | <0.001 |
| FRAP (µmol Fe2+/L) | 881 | [808;982] | 210 | 996 | [910;1079] | 853 | [785;924] | <0.001 |
| FRAP without uric ccid (µmol TE/L) | 314 | [267;355] | 210 | 345 | [312;387] | 296 | [257;341] | <0.001 |
| FRAP without uric acid (µmol Fe2+/L) | 634 | [574;699] | 210 | 692 | [630;754] | 616 | [556;678] | <0.001 |
| TRAP (µmol TE/L) | 976 | [884;1073] | 210 | 1034 | [907;1116] | 949 | [867;1047] | 0.001 |
| TEAC-ABTS (µmol TE/L) | 3041 | [2599;3677] | 210 | 3115 | [2508;3823] | 3008 | [2647;3384] | 0.642 |
| Total polyphenols (mg GAE/L) | 1207 | [1128;1276] | 210 | 1206 | [1126;1270] | 1207 | [1132;1277] | 0.850 |
| ORAC without proteins (µmol TE/L) | 1160 | [946;1399] | 210 | 1308 | [1098;1555] | 1124 | [908;1358] | <0.001 |
| ORAC (µmol TE/L) | 14,706 | [12,739;17,005] | 210 | 15,138 | [13,185;17,622] | 14,547 | [12,617;16,648] | 0.173 |
| CRP (mg/L) | 1.26 | [0.76;2.38] | 207 | 1.18 | [0.76;2.15] | 1.32 | [0.76;2.50] | 0.299 |
| Adiponectin (µg/mL) | 0.10 | [0.07;0.15] | 207 | 0,08 | [0.06;0.11] | 0.10 | [0.07;0.17] | 0.014 |
| PAI-I (ng/mL) | 20.0 | [14.6;27.0] | 210 | 22.9 | [17.9;29.8] | 19.4 | [14.3;25.2] | 0.008 |
| Resistin (ng/mL) | 14.2 | [11.5;18.2] | 210 | 13.3 | [10.5;16.1] | 14.4 | [12.0;18.8] | 0.024 |
| TNF-α (pg/mL) | 0.75 | [0.58;1.00] | 162 | 0.77 | [0.62;1.06] | 0.74 | [0.51;0.98] | 0.323 |
| IL-8 (pg/mL) | 1.08 | [0.68;1.70] | 146 | 1.09 | [0.68;1.68] | 1.06 | [0.69;1.71] | 0.850 |
| IL-6 (pg/mL) | 0.69 | [0.69;0.69] | 210 | 0.69 | [0.69;0.69] | 0.69 | [0.69;0.69] | 0.776 |
| Biomarkers | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β Coefficient | CI 95% | p-Value | R2 | β Coefficient | CI 95% | p-Value | R2 | |||
| Ascorbic acid (µmol/L) | −0.018 | −0.038 | 0.002 | 8.47 × 10−2 | 0.199 | −0.020 | −0.040 | 0.001 | 6.16 × 10−2 | 0.219 |
| Dehydroascorbic acid (µmol/L) | 0.000 | −0.001 | 0.001 | 9.33 × 10−1 | 0.187 | 0.000 | −0.001 | 0.001 | 9.71 × 10−1 | 0.205 |
| Total vitamin C (µmol/L) | −0.011 | −0.029 | 0.007 | 2.22 × 10−1 | 0.193 | −0.013 | −0.031 | 0.006 | 1.70 × 10−1 | 0.212 |
| Retinol (µmol/L) | −0.010 | −0.051 | 0.031 | 6.30 × 10−1 | 0.188 | −0.009 | −0.051 | 0.033 | 6.68 × 10−1 | 0.206 |
| α-Tocopherol (µmol/L) | −0.013 | −0.041 | 0.015 | 3.68 × 10−1 | 0.190 | −0.015 | −0.043 | 0.014 | 3.17 × 10−1 | 0.209 |
| Carotenes (µmol/L) | −0.002 | −0.016 | 0.012 | 8.23 × 10−1 | 0.187 | −0.003 | −0.017 | 0.012 | 6.90 × 10−1 | 0.206 |
| Q9 (µmol/L) | −0.010 | −0.024 | 0.004 | 1.80 × 10−1 | 0.194 | −0.011 | −0.026 | 0.003 | 1.27 × 10−1 | 0.214 |
| Q10 (µmol/L) | −0.001 | −0.027 | 0.025 | 9.55 × 10−1 | 0.187 | −0.002 | −0.029 | 0.025 | 9.1 × 10−1 | 0.205 |
| Uric acid (mg/dl) | 0.042 | 0.000 | 0.084 | 5.33 × 10−2 | 0.202 | 0.046 | 0.000 | 0.091 | 5.03 × 10−2 | 0.220 |
| FRAP (µmol TE/L) | 0.164 | 0.091 | 0.238 | 1.86 × 10−5 | 0.257 | 0.166 | 0.090 | 0.243 | 3.25 × 10−5 | 0.271 |
| FRAP (µmol Fe2+/L) | 0.110 | 0.030 | 0.190 | 7.88 × 10−3 | 0.215 | 0.102 | 0.019 | 0.186 | 1.73 × 10−2 | 0.227 |
| FRAP without uric ccid (µmol TE/L) | 0.143 | 0.079 | 0.207 | 1.84 × 10−5 | 0.257 | 0.139 | 0.074 | 0.205 | 4.54 × 10−5 | 0.269 |
| FRAP without uric acid (µmol Fe2+/L) | 0.083 | 0.010 | 0.155 | 2.59 × 10−2 | 0.206 | 0.072 | −0.002 | 0.147 | 5.85 × 10−2 | 0.219 |
| TEAC-ABTS (µmol TE/L) | 0.046 | 0.003 | 0.088 | 3.80 × 10−2 | 0.204 | 0.051 | 0.007 | 0.094 | 2.49 × 10−2 | 0.225 |
| Total polyphenols (mg GAE/L) | 0.144 | 0.018 | 0.269 | 2.58 × 10−2 | 0.206 | 0.146 | 0.012 | 0.280 | 3.45 × 10−2 | 0.223 |
| ORAC without proteins (µmol TE/L) | 0.033 | −0.008 | 0.075 | 1.18 × 10−1 | 0.197 | 0.039 | −0.004 | 0.081 | 7.78 × 10−2 | 0.217 |
| ORAC (µmol TE/L) | 0.077 | 0.021 | 0.133 | 7.45 × 10−3 | 0.215 | 0.079 | 0.022 | 0.135 | 7.29 × 10−3 | 0.233 |
| CRP (mg/L) | −0.004 | −0.016 | 0.009 | 5.78 × 10−1 | 0.187 | −0.004 | −0.018 | 0.010 | 5.43 × 10−1 | 0.205 |
| Adiponectin (µg/mL) | 0.006 | −0.008 | 0.020 | 3.95 × 10−1 | 0.195 | 0.007 | −0.008 | 0.021 | 3.69 × 10−1 | 0.216 |
| PAI-I (ng/mL) | 0.007 | −0.017 | 0.031 | 5.61 × 10−1 | 0.188 | 0.008 | −0.016 | 0.033 | 5.14 × 10−1 | 0.207 |
| Resistin (ng/mL) | −0.025 | −0.056 | 0.006 | 1.19 × 10−1 | 0.197 | −0.025 | −0.057 | 0.007 | 1.24 × 10−1 | 0.214 |
| TNF-α (pg/mL) | −0.008 | −0.035 | 0.019 | 5.55 × 10−1 | 0.202 | −0.008 | −0.036 | 0.019 | 5.55 × 10−1 | 0.219 |
| IL-8 (pg/mL) | −0.006 | −0.023 | 0.010 | 4.50 × 10−1 | 0.254 | −0.006 | −0.022 | 0.011 | 5.26 × 10−1 | 0.278 |
| IL-6 (pg/mL) | −0.001 | −0.017 | 0.015 | 8.93 × 10−1 | 0.187 | −0.002 | −0.018 | 0.015 | 8.19 × 10−1 | 0.205 |
| Biomarkers | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β Coefficient | CI 95% | p-Value | R2 | β Coeff | CI 95% | p-Value | R2 | |||
| Ascorbic Acid (µmol/L) | 0.027 | 0.004 | 0.051 | 2.38 × 10−2 | 0.247 | 0.027 | 0.003 | 0.050 | 2.81 × 10−2 | 0.292 |
| Dehydroascorbic acid (µmol/L) | 0.001 | 0.000 | 0.001 | 1.73 × 10−1 | 0.235 | 0.001 | 0.000 | 0.001 | 1.64 × 10−1 | 0.281 |
| Total vitamin C (µmol/L) | 0.017 | −0.004 | 0.038 | 1.07 × 10−1 | 0.238 | 0.017 | −0.004 | 0.038 | 1.21 × 10−1 | 0.283 |
| Retinol (µmol/L) | 0.081 | 0.035 | 0.127 | 7.18 × 10−4 | 0.270 | 0.080 | 0.033 | 0.127 | 9.92 × 10−4 | 0.313 |
| α-Tocopherol (µmol/L) | 0.050 | 0.018 | 0.082 | 2.27 × 10−3 | 0.263 | 0.050 | 0.018 | 0.082 | 2.77 × 10−3 | 0.306 |
| Carotenes (µmol/L) | 0.017 | 0.001 | 0.033 | 3.78 × 10−2 | 0.244 | 0.017 | 0.001 | 0.034 | 3.76 × 10−2 | 0.290 |
| Q9 (µmol/L) | 0.013 | −0.003 | 0.030 | 1.12 × 10−1 | 0.238 | 0.011 | −0.005 | 0.028 | 1.90 × 10−1 | 0.280 |
| Q10 (µmol/L) | 0.062 | 0.033 | 0.091 | 4.13 × 10−5 | 0.289 | 0.062 | 0.032 | 0.092 | 6.43 × 10−5 | 0.331 |
| Uric acid (mg/dl) | 0.245 | 0.209 | 0.282 | 2.02 × 10−29 | 0.585 | 0.252 | 0.213 | 0.291 | 1.06 × 10−27 | 0.603 |
| TRAP (µmol TE/L) | 0.149 | 0.040 | 0.257 | 7.88 × 10−3 | 0.254 | 0.133 | 0.024 | 0.242 | 1.73 × 10−2 | 0.295 |
| TEAC-ABTS (µmol TE/L) | −0.015 | −0.066 | 0.035 | 5.49 × 10−1 | 0.230 | −0.018 | −0.068 | 0.033 | 4.90 × 10−1 | 0.276 |
| Total polyphenols (mg GAE/L) | 0.262 | 0.119 | 0.406 | 4.20 × 10−4 | 0.274 | 0.246 | 0.095 | 0.397 | 1.65 × 10−3 | 0.310 |
| ORAC without proteins (µmol TE/L) | 0.033 | −0.016 | 0.082 | 1.85 × 10−1 | 0.235 | 0.031 | −0.019 | 0.079 | 2.24 × 10−1 | 0.280 |
| ORAC (µmol TE/L) | −0.037 | −0.103 | 0.029 | 2.72 × 10−1 | 0.233 | −0.043 | −0.109 | 0.023 | 2.02 × 10−1 | 0.280 |
| CRP (mg/L) | 0.002 | −0.013 | 0.016 | 8.37 × 10−1 | 0.229 | −0.004 | −0.019 | 0.012 | 6.44 × 10−1 | 0.277 |
| Adiponectin (µg/mL) | −0.005 | −0.021 | 0.011 | 5.29 × 10−1 | 0.231 | −0.002 | −0.018 | 0.014 | 8.11 × 10−1 | 0.276 |
| PAI-I (ng/mL) | 0.019 | −0.009 | 0.046 | 1.88 × 10−1 | 0.235 | 0.010 | −0.018 | 0.039 | 4.68 × 10−1 | 0.276 |
| Resistin (ng/mL) | 0.011 | −0.026 | 0.047 | 5.73 × 10−1 | 0.229 | 0.007 | −0.030 | 0.043 | 7.24 × 10−1 | 0.275 |
| TNF-α (pg/mL) | 0.022 | −0.009 | 0.053 | 1.68 × 10−1 | 0.207 | 0.016 | −0.015 | 0.047 | 3.14 × 10−1 | 0.252 |
| IL-8 (pg/mL) | −0.003 | −0.023 | 0.016 | 7.40 × 10−1 | 0.201 | −0.008 | −0.028 | 0.012 | 4.40 × 10−1 | 0.232 |
| IL-6 (pg/mL) | −0.016 | −0.035 | 0.002 | 8.73 × 10−2 | 0.239 | −0.016 | −0.035 | 0.002 | 8.97 × 10−2 | 0.285 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrión-García, C.J.; Guerra-Hernández, E.J.; García-Villanova, B.; Serafini, M.; Sánchez, M.-J.; Amiano, P.; Molina-Montes, E. Plasma Non-Enzymatic Antioxidant Capacity (NEAC) in Relation to Dietary NEAC, Nutrient Antioxidants and Inflammation-Related Biomarkers. Antioxidants 2020, 9, 301. https://doi.org/10.3390/antiox9040301
Carrión-García CJ, Guerra-Hernández EJ, García-Villanova B, Serafini M, Sánchez M-J, Amiano P, Molina-Montes E. Plasma Non-Enzymatic Antioxidant Capacity (NEAC) in Relation to Dietary NEAC, Nutrient Antioxidants and Inflammation-Related Biomarkers. Antioxidants. 2020; 9(4):301. https://doi.org/10.3390/antiox9040301
Chicago/Turabian StyleCarrión-García, Cayetano Javier, Eduardo Jesús Guerra-Hernández, Belén García-Villanova, Mauro Serafini, María-José Sánchez, Pilar Amiano, and Esther Molina-Montes. 2020. "Plasma Non-Enzymatic Antioxidant Capacity (NEAC) in Relation to Dietary NEAC, Nutrient Antioxidants and Inflammation-Related Biomarkers" Antioxidants 9, no. 4: 301. https://doi.org/10.3390/antiox9040301
APA StyleCarrión-García, C. J., Guerra-Hernández, E. J., García-Villanova, B., Serafini, M., Sánchez, M.-J., Amiano, P., & Molina-Montes, E. (2020). Plasma Non-Enzymatic Antioxidant Capacity (NEAC) in Relation to Dietary NEAC, Nutrient Antioxidants and Inflammation-Related Biomarkers. Antioxidants, 9(4), 301. https://doi.org/10.3390/antiox9040301







