Chemical and Sensory Profiling of Monovarietal Extra Virgin Olive Oils from the Italian Marche Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Sampling
2.3. Fatty Acid Composition
2.4. Quantification of Olive oil Polar Phenolic Compounds by HPLC-DAD-ESI/MS
2.5. Determination of α-Tocopherol
2.6. Volatile Substances
2.7. Squalene Analysis
2.8. Total Antioxidant Activity (TAA)
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
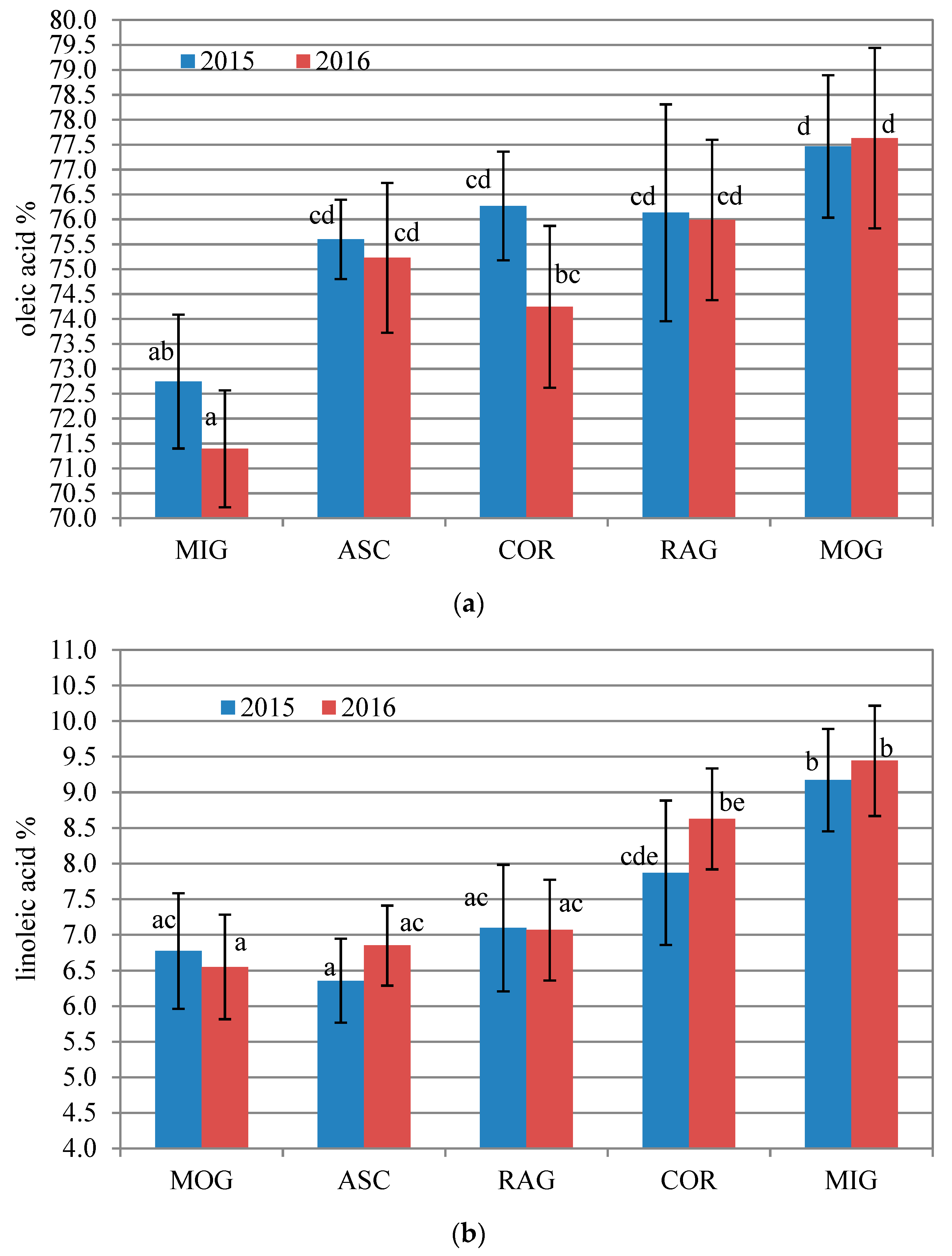
3.1. Fatty Acid Composition
3.2. Polar Phenolic Substances
3.3. α-Tocopherol
3.4. Volatile Substances
3.5. Squalene
3.6. Antioxidant Activity
3.7. Sensory Analysis
3.8. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caramia, G.; Gori, A.; Valli, E.; Cerretani, L. Virgin olive oil in preventive medicine: From legend to epigenetics. Eur. J. Lipid Sci. Technol. 2012, 114, 375–388. [Google Scholar] [CrossRef]
- Lopez-Miranda, J.; Perez-Jimenez, F.; Ros, E.; De Caterina, R.; Badimon, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Carreño, I.; Vergano, P.R. Geographical indications, “Food Fraud” and the Fight Against “Italian sounding” Products. Eur. J. Risk Reg. 2016, 7, 416–420. [Google Scholar] [CrossRef]
- Rotondi, A.; Alfei, B.; Pannelli, G.; Morrone, L.; Magli, M. Italian National Database of Monovarietal Extra Virgin Olive Oils. In The Mediterranean Genetic Code–Grapevine and Olive; Poljuha, D., Sladonja, B., Eds.; INTECH Open Access Publisher: Rijeka, Croatia, 2013; pp. 179–200. Available online: https://www.intechopen.com/books/the-mediterranean-genetic-code-grapevine-and-olive/italian-national-database-of-monovarietal-extra-virgin-olive-oils (accessed on 5 April 2020).
- European Union. Commission implementing regulation (EU) no 29/2012 of 13 January on marketing standards for olive oil (codification). Off. J. Eur. Union 2012, L12/14.
- Rotondi, A.; Alfei, B.; Magli, M.; Pannelli, G. Influence of genetic matrix and crop year on chemical and sensory profiles of Italian monovarietal extra-virgin olive oils. J. Sci. Food Agric. 2010, 90, 2641–2648. [Google Scholar] [CrossRef]
- Alfei, B. Oli monovarietali. 16° Rassegna Nazionale Oli Monovarietali. Olivo e Olio 2019, 3. Available online: http://www.olimonovarietali.it/repository/Catalogo%20oli%20monovarietali%202019.pdf/view (accessed on 16 April 2020).
- ASSAM Marche and CNR-IBIMET. Banca Dati Degli Oli Monovarietali Italiani. Available online: http://www.olimonovarietali.it/ (accessed on 4 September 2019).
- Cecchi, T.; Alfei, B. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME–GC–MS: Newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef]
- Fiori, F.; Boselli, E.; Falcone, P.M.; Balzano, M.; Frega, N.G. Effects of malaxation time on the quality of extra virgin olive oil from the ASC olive variety. Riv. Ital. Sostanze Gr. 2014, 91, 167–176. [Google Scholar]
- Dabbou, S.; Issaoui, M.; Servili, M.; Taticchi, A.; Sifi, S.; Montedoro, G.F.; Hammami, M. Characterisation of virgin olive oils from European olive cultivars introduced in Tunisia. Eur. J. Lipid Sci. Technol. 2009, 111, 292–401. [Google Scholar] [CrossRef]
- Caprioli, G.; Boarelli, M.C.; Ricciutelli, M.; Sagratini, G.; Fiorini, D. Micro-scaled Quantitative Method to Analyze Olive Oil Polyphenols. Food Anal. Methods 2019, 12, 1133–1139. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef]
- Pacetti, D.; Scortichini, S.; Boarelli, M.C.; Fiorini, D. Simple and rapid method to analyse squalene in olive oils and extra virgin olive oils. Food Control. 2019, 102, 240–244. [Google Scholar] [CrossRef]
- Pellegrini, N.; Visioli, F.; Buratti, S.; Brighenti, F. Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. J. Agric. Food Chem. 2001, 49, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission regulation (EEC) no 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Offic. J. Europ. Commun. 1991, 248, 1–83.
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Cecchi, T.; Passamonti, P.; Alfei, B.; Cecchi, P. Monovarietal Extra Virgin Olive Oils from the Marche Region, Italy: Analytical and Sensory Characterization. Int. J. Food Prop. 2011, 14, 483–495. [Google Scholar] [CrossRef]
- Alfei, B.; Fileni, L.; Santinelli, A.; Pannelli, G. Preliminari osservazioni sul comportamento produttivo delle principali varietà locali di olivo delle Marche. In Proceedings of the IV National Congress on Biodiversity, Alghero, Italy, 8–11 September 1998; pp. 253–258. [Google Scholar]
- Lavelli, V.; Fregapane, G.; Salvador, D.M. Effect of storage on secoiridoid and tocopherol contents and antioxidant activity of monovarietal extra virgin olive oils. J. Agric. Food Chem. 2006, 54, 3002–3007. [Google Scholar] [CrossRef]
- Chiavaro, E.; Vittadini, E.; Rodriguez-Estrada, M.T.; Cerretani, L.; Bonoli, M.; Bendini, A.; Lercker, G. Monovarietal extra virgin olive oils: Correlation between thermal properties and chemical composition. J. Agric. Food Chem. 2007, 55, 10779–10786. [Google Scholar] [CrossRef]
- European Commission. Commission regulation (EU) no 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 136, 1–40.
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic compounds and quality parameters of family farming versus protected designation of origin (PDO) extra-virgin olive oils. J. Food Compos. Anal. 2015, 43, 75–81. [Google Scholar] [CrossRef]
- Caporaso, N.; Savarese, M.; Paduano, A.; Guidone, G.; De Marco, E.; Sacchi, R. Nutritional quality assessment of extra virgin olive oil from the Italian retail market: Do natural antioxidants satisfy EFSA health claims? J. Food Compos. Anal. 2015, 40, 154–162. [Google Scholar] [CrossRef]
- Fiorini, D.; Boarelli, M.C.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; Gabbianelli, R.; Pacetti, D. Chemical and sensory differences between high price and low price extra virgin olive oils. Food Res. Int. 2018, 105, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; Garcia, A.; Rios, J.J.; Garcia, P.; Garrido, A. Use of 1-acetoxypinoresinol to authenticate Picual olive oils. Int. J. Food Sci. Technol. 2002, 37, 615–625. [Google Scholar] [CrossRef]
- Tura, D.; Gigliotti, C.; Pedo, S.; Failla, O.; Bassi, D.; Serraiocco, A. Influence of cultivar and site of cultivation on levels of lipophilic and hydrophilic antioxidants in virgin olive oils (Olea Europea L) and correlations with oxidative stability. Sci. Hortic. 2007, 112, 108–119. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Beltrán, G.; Ortega, D.; Fernández, A.; Jiménez, A.; Uceda, M. Characterisation of virgin olive oil of Italian olive cultivars: ‘Frantoio’ and ‘Leccino’, grown in Andalusia. Food Chem. 2005, 89, 387–391. [Google Scholar] [CrossRef]
- Haddada, F.M.; Krichène, D.; Manai, H.; Oueslati, I.; Daoud, D.; Zarrouk, M. Analytical evaluation of six virgin olive oils from Northern Tunisia. Eur. J. Lipid Sci. Technol. 2008, 110, 905–913. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Angerosa, F.; Camera, L.; D’Alessandro, N.; Mellerio, G. Characterization of Seven New Hydrocarbon Compounds Present in the Aroma of Virgin Olive Oils. J. Agric. Food Chem. 1998, 46, 648–653. [Google Scholar] [CrossRef]
- Alfonso, I.; Vacas, S.; Primo, J. Role of α-copaene in the susceptibility of olive fruits to Bactrocera oleae (Rossi). J. Agric. Food Chem. 2014, 62, 11976–11979. [Google Scholar] [CrossRef]
- Gómez-Coca, R.B.; Cruz-Hidalgo, R.; Fernandes, G.D.; Pérez-Camino, M.D.C.; Moreda, W. Analysis of methanol and ethanol in virgin olive oil. MethodsX 2014, 1, 207–211. [Google Scholar]
- Biedermann, M.; Bongartz, A.; Mariani, C.; Grob, K. Fatty acid methyl and ethyl esters as well as wax esters for evaluating the quality of olive oils. Eur. Food Res Technol. 2008, 288, 65–74. [Google Scholar] [CrossRef]
- Mariani, C.; Bellan, G. Sul possibile aumento degli alchil esteri negli oli extravergini di oliva. Riv. Ital. Sostanze Gr. 2001, 88, 3–10. [Google Scholar]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Beltran, G.; Bucheli, M.E.; Aguilera, M.P.; Belaj, A.; Jimenez, A. Squalene in virgin olive oil: Screening of variability in olive cultivars. Eur. J. Lipid Sci. Technol. 2016, 118, 1250–1253. [Google Scholar] [CrossRef]
- Forde, C.N.G.; Cox, A.; Williams, E.R.; Boss, P.K. Associations between the sensory attributes and volatile composition of Cabernet Sauvignon wines and the volatile composition of the grapes used for their production. J. Agric. Food Chem. 2011, 59, 2573–2583. [Google Scholar] [CrossRef] [PubMed]



| Hydroxytyrosol | Tyrosol | Vanillic Acid | p-Coumaric Acid | Ferulic Acid | Secoiridoid Derivatives | Pinoresinol | Acetoxy pinoresinol | Luteolin | Apigenin | Total Polar Phenolics | α-Tocopherol | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average (mg kg−1) ± SD | ||||||||||||
| ASC 2015 (n = 6) | 5.46 ab ± 2.14 | 6.00 ± 2.97 | 0.33 ± 0.16 | 0.10 ab ± 0.03 | 0.03 a ± 0.02 | 353.23 ± 108.89 | 12.49 a ± 5.55 | 5.98 a ± 7.54 | 2.30 ab ± 0.59 | 0.90 b ± 0.34 | 387.80 ab ± 182.13 | 270.91 adc ± 39.50 |
| COR 2015 (n = 7) | 4.06 a ± 1.62 | 4.67 ± 1.39 | 0.32 ± 0.17 | 0.11 a ± 0.04 | 0.03 a ± 0.03 | 340.19 ± 109.02 | 4.72 b ± 1.56 | 8.86 a ± 8.34 | 3.24 b ± 1.16 | 1.25 b ± 0.31 | 367.45 ab ± 113.52 | # 229.92 bc ± 16.48 |
| MIG 2015 (n = 7) | 8.75 b ± 5.16 | 9.24 ± 6.16 | 0.49 ± 0.23 | 0.09 ab ± 0.03 | 0.03 a ± 0.02 | 282.14 ± 113.72 | # 6.48 b ± 1.58 | 8.13 a ± 1.69 | 2.10 ab ± 0.47 | 0.49 a ± 0.15 | 317.94 ab ± 121.26 | # 295.94 a ± 25.69 |
| MOG 2015 (n = 9) | 2.38 a ± 1.53 | 5.11 ± 1.22 | 0.46 ± 0.21 | 0.12 a ± 0.04 | 0.08 b ± 0.02 | 248.22 ± 112.33 | 7.63 ab ± 2.33 | 5.31 a ± 4.32 | 1.49 a ± 0.95 | 0.94 b ± 0.30 | 271.73 a ± 112.89 | 192.35 b ± 42.15 |
| RAG 2015 (n = 7) | # 6.79 ab ± 2.79 | 6.86 ± 2.59 | 0.34 ± 0.14 | # 0.05 b ± 0.02 | 0.05 ab ± 0.02 | 429.75 ± 172.33 | # 3.83 b ± 0.54 | 35.47 b ± 10.34 | 3.08 b ± 1.47 | 1.25 b ± 0.18 | 487.48 b ± 175.89 | 215.98 bc ± 47.51 |
| ASC 2016 (n = 8) | 5.01 ± 2.86 | 6.24 ± 3.76 | 0.31 ± 0.19 | 0.12 ± 0.04 | 0.06 ± 0.02 | 334.77 ± 104.17 | 18.45 a ± 14.09 | 8.94 a ± 9.22 | 3.04 ab ± 0.99 | 0.92 ab ± 0.24 | 377.90 ± 103.23 | 356.68 bc ± 81.67 |
| COR 2016 (n = 7) | 5.32 ± 2.16 | 5.78 ± 2.78 | 0.35 ± 0.25 | 0.14 ± 0.06 | 0.05 ± 0.03 | 384.59 ± 127.68 | 6.27 b ± 1.25 | 5.52 a ± 4.65 | 3.61 ab ± 0.76 | 1.20 b ± 0.19 | 412.86 ± 133.15 | § 351.16 bc ± 50.76 |
| MIG 2016 (n = 8) | 6.37 ± 1.98 | 5.53 ± 2.41 | 0.52 ± 0.47 | 0.09 ± 0.05 | 0.05 ± 0.02 | 339.73 ± 105.57 | § 9.70 ab ± 1.74 | 10.30 a ± 5.03 | 2.51 ab ± 0.63 | 0.59 a ± 0.28 | 375.47 ± 105.31 | § 397.26 b ± 36.94 |
| MOG 2016 (n = 8) | 4.94 ± 5.46 | 7.47 ± 6.65 | 0.35 ± 0.16 | 0.18 ± 0.12 | 0.07 ± 0.04 | 335.27 ± 112.60 | 10.29 ab ± 5.77 | 9.03 a ± 6.41 | 2.06 a ± 0.75 | 0.92 ab ± 0.29 | 370.68 ± 119.33 | 259.01 a ± 44.19 |
| RAG 2016 (n = 12) | § 3.83 ± 2.08 | 6.20 ± 3.66 | 0.44 ± 0.26 | § 0.13 ± 0.09 | 0.07 ± 0.04 | 349.47 ± 131.68 | § 5.98 b ± 2.31 | 31.77 b ± 12.38 | 4.05 b ± 2.14 | 1.10 b ± 0.41 | 403.13 ± 132.91 | 286.66 ac ± 67.26 |
| ASC 2015/2016 (n = 14) | 5.21 ab ± 2.50 | 5.40 ± 2.00 | 0.32 ± 0.17 | 0.11 ± 0.04 | 0.05 a ± 0.02 | 342.68 ± 102.44 | 15.90 a ± 11.32 | 7.67 a ± 8.36 | 2.72 ab ± 0.90 | 0.91 b ± 0.27 | 376.97 ± 103.64 | 322.37 bc ± 79.12 |
| COR 2015/2016 (n = 14) | 4.69 ab ± 1.95 | 5.13 ± 1.98 | 0.34 ± 0.21 | 0.12 ± 0.05 | 0.04 a ± 0.03 | 362.39 ± 116.37 | 5.49 b ± 1.58 | 7.19 a ± 6.71 | 3.42 bc ± 0.96 | 1.23 c ± 0.25 | 390.16 ± 121.19 | 294.58 bc ± 72.97 |
| MIG 2015/2016 (n = 15) | 7.48 a ± 3.86 | 6.53 ± 3.17 | 0.51 ± 0.36 | 0.09 ± 0.04 | 0.04 a ± 0.02 | 312.85 ± 109.54 | 8.20 b ± 2.31 | 9.29 a ± 3.89 | 2.32 ab ± 0.58 | 0.54 a ± 0.22 | 348.63 ± 112.83 | 349.98 b ± 60.85 |
| MOG 2015/2016 (n = 17) | 3.58 b ± 3.99 | 6.13 ± 3.36 | 0.40 ± 0.19 | 0.15 ± 0.09 | 0.07 b ± 0.03 | 289.19 ± 117.74 | 8.88 b ± 4.37 | 7.06 a ± 5.57 | 1.76 a ± 0.88 | 0.93 b ± 0.29 | 318.30 ± 123.26 | 219.01 a ± 53.43 |
| RAG 2015/2016 (n = 19) | 4.92 ab ± 2.72 | 8.29 ± 6.26 | 0.40 ± 0.22 | 0.10 ± 0.08 | 0.06 ab ± 0.03 | 379.05 ± 148.59 | 5.19 b ± 2.12 | 33.14 b ± 11.52 | 3.70 c ± 1.94 | 1.16 bc ± 0.34 | 434.21 ± 151.18 | 260.62 ac ± 68.88 |
| Methanol | Ethanol | 3-Pentanone | 1-Penten-3-one | 4,8-Dimethyl-1,7-Nonadiene | 1-Penten-3-ol | (E)-2-Hexenal | Hexyl Acetate | Hexanal | Octanal | (Z)-3-Hexen-1-ol Acetate | (Z)-2-Penten-1-ol | 6-Methyl-5-Hepten-2-one | 1-Hexanol | (Z)-3-Hexen-1-ol | (E)-2-Hexen-1-ol | Nonanal | α-Copaene | 1-Octanol | α-Farnesene | Total Pentene Dimers | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area % ± SD | |||||||||||||||||||||
| ASC 2015 (n = 6) | #3.54ab ± 2.96 | #6.11 ± 6.46 | #1.68ab ± 1.25 | 3.83 ± 1.96 | 1.07 ± 0.70 | 1.91 b ± 1.61 | 49.84 a ± 17.41 | 0.67 ac ± 0.15 | 4.45 abc ± 3.78 | 0.68 ± 0.76 | 6.23 ± 1.63 | #0.18 ± 0.18 | nd | 2.53 ab ± 1.83 | #11.15 b ± 9.84 | 0.81 ± 0.53 | 2.92 ± 2.66 | 0.68 ab ± 0.75 | 0.31 ± 0.51 | 0.21 a ± 0.37 | 4.94 ab ± 4.49 |
| COR 201 (n = 7) | #3.23a ± 1.59 | 3.07 ± 2.72 | 0.75a ± 0.58 | 3.16 ± 1.87 | 1.10 ± 0.64 | 0.90 ab ± 0.42 | 64.96 ab ± 11.84 | 0.51 bc ± 0.49 | 2.38 c ± 1.50 | 0.28 ± 0.35 | #1.89 ± 1.25 | #0.32 ± 0.53 | nd | 1.63 a ± 1.94 | 3.04 a ± 2.08 | 1.83 ± 1.37 | 2.25 ± 3.18 | 0.25 b ± 0.24 | 0.08 ± 0.14 | 0.35 a ± 0.20 | 8.02 a ± 3.46 |
| MIG 2015 (n = 7) | #1.82ab ± 1.06 | 6.74 ± 5.02 | #2.43b ± 1.41 | 1.41 ± 1.11 | 1.23 ± 0.33 | 0.83 ab ± 0.34 | 47.60 a ± 22.76 | 1.12 a ± 0.44 | 1.97 c ± 1.20 | 0.32 ± 0.11 | 4.71 ± 2.47 | #0.10 ± 0.09 | 0.13 ± 0.15 | 5.36 b ± 4.27 | #4.72 ab ± 2.96 | 9.11 ± 1.74 | 1.28 ± 0.53 | 0.04 b ± 0.10 | nd | 0.98 b ± 0.58 | 8.09 a ± 2.22 |
| MOG 2015 (n = 9) | 1.75ab ± 1.16 | 3.04 ± 3.69 | #1.20ab ± 0.82 | 2.42 ± 1.17 | #0.60 ± 0.33 | 1.19 ab ± 0.68 | 64.68 ab ± 11.02 | 0.03 b ± 0.09 | 6.59 b ± 3.60 | 0.20 ± 0.19 | 0.27 ± 0.49 | #0.14 ± 0.13 | nd | 2.12 ab ± 1.15 | #7.25 a ± 3.75 | #2.28 ± 1.72 | 0.97 ± 0.84 | 1.00 a ± 0.56 | nd | 0.08 a ± 0.12 | 3.80 ab ± 1.66 |
| RAG 2015 (n = 7) | 0.60 b ± 0.38 | 1.87 ± 1.45 | 0.52a ± 0.52 | 1.63 ± 1.03 | #0.50 ± 0.18 | 0.57 a ± 0.30 | #82.91 b ± 4.60 | 0.09 b ± 0.13 | 1.84 c ± 0.50 | 0.20 ± 0.10 | 0.43 ± 0.34 | #0.10 ± 0.06 | nd | 1.41 a ± 0.97 | #0.84 a ± 0.18 | 2.27 ± 9.97 | 1.43 ± 1.28 | 0.16 b ± 0.14 | 0.06 ± 0.08 | 0.08 a ± 0.14 | #2.47 b ± 1.63 |
| ASC 2016 (n = 8) | §1.01 ± 1.46 | §0.43 ± 0.21 | §0.44 ± 0.64 | 3.16 ± 3.58 | 1.29 ± 1.66 | 0.94 ± 0.79 | 53.26 a ± 13.23 | 1.08 abc ± 0.83 | 4.03 ± 1.21 | 0.69 ± 0.83 | 4.94 ± 3.43 | §0.78 ± 0.45 | 0.10 ± 0.15 | 1.66 ± 0.86 | §4.61 c ± 1.04 | 3.92 ± 3.88 | 8.51 ± 8.43 | 0.14 a ± 0.33 | 0.69 ± 0.99 | 0.15 ± 0.17 | 8.14 ± 3.90 |
| COR 2016 (n = 7) | §1.43 ± 0.64 | 2.36 ± 2.76 | 0.57 ± 0.54 | 2.51 ± 1.31 | 1.42 ± 1.45 | 0.77 ± 0.39 | 58.58 ab ± 2.79 | 1.18 ac ± 0.86 | 3.21 ± 1.86 | 0.12 ± 0.28 | §6.22 ± 3.51 | §1.23 ± 0.50 | nd | 1.58 ± 0.81 | 3.32 bc ± 1.13 | 2.55 ± 1.62 | 2.72 ± 2.98 | 0.02 a ± 0.05 | 0.09 ± 0.21 | 0.17 ± 0.19 | 9.52 ± 3.70 |
| MIG 2016 (n = 8) | §0.62 ± 0.44 | 2.22 ± 2.50 | §0.38 ± 0.42 | 1.03 ± 0.80 | 1.25 ± 1.00 | 0.39 ± 0.37 | 52.36 a ± 15.19 | 1.54 a ± 0.75 | 3.63 ± 2.15 | 0.54 ± 1.00 | 6.48 ± 4.05 | §0.73 ± 0.45 | 0.13 ± 0.17 | 1.68 ± 0.93 | §2.63 ac ± 0.84 | 2.31 ± 1.67 | 8.32 ± 9.52 | nd | 0.28 ± 0.42 | 0.95 ± 0.73 | 12.75 ± 7.60 |
| MOG 2016 (n = 8) | 1.07 ± 0.61 | 1.48 ± 1.85 | §0.29 ± 0.37 | 1.67 ± 1.28 | §1.11 ± 1.28 | 0.48 ± 0.39 | 66.85 ab ± 7.14 | 0.03 d ± 0.09 | 3.84 ± 2.47 | nd | 0.33 ± 0.74 | §0.64 ± 0.48 | 0.24 ± 0.40 | 2.08 ± 1.95 | §3.05 cd ± 1.62 | §4.51 ± 4.60 | 2.57 ± 3.30 | 0.65 b ± 0.32 | nd | 0.85 ± 1.28 | 8.24 ± 5.88 |
| RAG 2016 (n = 12) | 0.66 ± 0.52 | 1.00 ± 0.84 | 0.42 ± 0.54 | 1.43 ± 0.71 | §2.17 ± 1.11 | 0.48 ± 0.14 | §72.11 b ± 10.35 | 0.34 bcd ± 0.25 | 2.61 ± 1.23 | 0.30 ± 0.68 | 0.69 ± 0.77 | §0.70 ± 0.35 | 0.03 ± 0.07 | 1.59 ± 1.52 | §1.15 a ± 0.55 | 3.54 ± 2.82 | 3.92 ± 8.92 | 0.01 a ± 0.05 | 0.21 ± 0.49 | 0.18 ± 0.20 | §6.46 ± 2.05 |
| ASC 2015/2016 (n = 14) | 2.10 ab ± 2.49 | 2.32 ± 4.38 | 0.91 ± 1.07 | 3.38 b ± 3.05 | 1.21 ± 1.33 | 1.32 b ± 1.21 | 51.88 b ± 14.01 | 0.95 bc ± 0.69 | 4.1 abc ± 2.21 | 0.69 b ± 0.77 | 5.37 a ± 2.93 | 0.55 ± 0.47 | 0.06 ± 0.13 | 1.99 ± 1.32 | 6.79 c ± 6.12 | 2.72 ± 3.37 | 6.36 ± 7.20 | 0.35 a ± 0.57 | 0.55 ± 0.84 | 0.17 ab ± 0.25 | 6.91 ab ± 4.27 |
| COR 2015/2016 (n = 14) | 2.33 a ± 1.49 | 2.74 ± 2.64 | 0.67 ± 0.55 | 2.86 ab ± 1.60 | 1.25 ± 1.05 | 0.84 ab ± 0.40 | 62.08 bc ± 9.17 | 0.82 cd ± 0.74 | 2.76 c ± 1.66 | 0.20 ab ± 0.32 | 3.89 abc ± 3.31 | 0.74 ± 0.69 | nd | 1.61 ± 1.47 | 3.17 ab ± 1.64 | 2.16 ± 1.47 | 2.67 ± 3.00 | 0.15 a ± 0.21 | 0.08 ± 0.17 | 0.27 ab ± 0.21 | 8.71 ab ± 3.50 |
| MIG 2015/2016 (n = 15) | 1.18 ab ± 0.98 | 3.96 ± 4.16 | 1.17 ± 1.36 | 1.17 a ± 0.91 | 1.24 ± 0.79 | 0.56 a ± 0.41 | 50.43 b ± 17.68 | 1.38 b ± 0.66 | 2.99 c ± 1.97 | 0.46 ab ± 0.78 | 5.80 a ± 3.52 | 0.48 ± 0.47 | 0.13 ± 0.15 | 3.21 ± 3.27 | 3.43 abc ± 2.11 | 4.93 ± 6.83 | 5.62 ± 8.10 | 0.02 a ± 0.06 | 0.17 ± 0.35 | 0.96 b ± 0.65 | 10.95 b ± 6.39 |
| MOG 2015/2016 (n = 17) | 1.43 ab ± 0.98 | 2.36 ± 3.05 | 0.78 ± 0.78 | 2.07 ab ± 1.24 | 0.84 ± 0.91 | 0.86 ab ± 0.66 | 65.69 ac ± 9.15 | 0.03 a ± 0.09 | 5.31 b ± 3.33 | 0.10 a ± 0.17 | 0.30 d ± 0.60 | 0.37 ± 0.42 | 0.11 ± 0.29 | 2.10 ± 1.51 | 5.29 bc ± 3.58 | 3.32 ± 3.44 | 1.72 ± 2.39 | 0.84 b ± 0.48 | nd | 0.44 a ± 0.93 | 5.87 a ± 4.63 |
| RAG 2015/2016 (n = 19) | 0.64 b ± 0.46 | 1.32 ± 1.15 | 0.46 ± 0.52 | 1.51 a ± 0.82 | 1.56 ± 1.20 | 0.51 a ± 0.21 | 76.09 a ± 9.98 | 0.25 ac ± 0.24 | 2.33 c ± 1.07 | 0.26 ab ± 0.54 | 0.60 bcd ± 0.65 | 0.48 ± 0.40 | 0.02 ± 0.06 | 1.52 ± 1.32 | 1.03 a ± 0.47 | 3.07 ± 2.50 | 3.00 ± 7.12 | 0.07 a ± 0.11 | 0.15 ± 0.39 | 0.14 a ± 0.18 | 4.99 a ± 2.71 |
| Sensory Analysis | Antioxidant Activity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fruity | Bitter | Pungent | Leaves/Grass | Almond | Artichoke | Tomato | Berries | Score | TAA (Average Trolox µm ± SD) | |
| Average ± SD | ||||||||||
| ASC 2015 (n = 6) | # 5.34 a ± 0.30 | 4.76 ± 0.41 | # 4.67 ab ± 0.30 | # 2.95 a ± 0.72 | 1.71 ± 1.02 | # 2.29 ± 0.97 | # 2.60 ± 0.47 | nd | 7.76 a ± 0.27 | 8.58 ± 1.81 |
| COR 2015 (n = 7) | # 4.44 b ± 0.45 | 4.55 ± 0.70 | # 4.65 ab ± 0.48 | 2.37 bd ± 0.53 | 2.28 ± 0.62 | 1.84 ± 0.90 | 0.27 ± 0.72 | 0.23 ± 0.61 | 7.26 ab ± 0.38 | 8.10 ± 1.74 |
| MIG 2015 (n = 7) | # 4.21 b ± 0.51 | 4.16 ± 0.52 | 3.78 a ± 0.62 | 1.18 b ± 0.86 | # 1.36 ± 0.79 | 0.57 ± 0.73 | nd | 1.95 ± 1.23 | 7.12 ab ± 0.66 | 7.81 ± 1.49 |
| MOG 2015 (n = 9) | 4.20 b ± 0.58 | 3.73 ± 0.87 | 4.05 ab ± 0.93 | 2.17 bd ± 0.93 | 1.82 ± 0.92 | 1.31 ± 0.99 | 0.19 ± 0.57 | 0.23 ± 0.70 | 7.06 b ± 0.50 | 7.50 ± 2.04 |
| RAG 2015 (n = 7) | # 4.79 ab ± 0.49 | # 4.93 ± 0.79 | # 4.83 b ± 0.48 | # 2.51 acd ± 0.79 | # 3.04 ± 0.44 | # 1.90 ± 1.05 | 0.21 ± 0.54 | nd | # 7.55 ab ± 0.27 | 9.59 ± 2.86 |
| ASC 2016 (n = 8) | § 4.06 ± 1.16 | 3.36 ± 1.54 | § 3.37 ± 1.15 | §1.47 ± 1.50 | 1.21 ± 0.98 | §0.76 ± 0.87 | §1.03 ± 1.45 | nd | 7.07 ± 0.85 | 5.84 ± 1.37 |
| COR 2016 (n = 7) | § 3.59 ± 0.55 | 3.76 ± 0.94 | § 3.74 ± 0.22 | 1.69 ± 1.21 | 1.59 ± 0.95 | 1.29 ± 1.21 | 0.16 ± 0.43 | nd | 7.13 ± 0.41 | 6.20 ± 2.33 |
| MIG 2016 (n = 8) | § 3.39 ± 0.42 | 3.66 ± 0.88 | 3.36 ± 0.66 | 0.53 ± 0.69 | §0.56 ± 0.50 | 0.24 ± 0.46 | nd | 1.21 ± 1.39 | 7.18 ± 0.52 | 5.47 ± 2.09 |
| MOG 2016 (n = 8) | 3.66 ± 0.83 | 3.36 ± 1.12 | 3.33 ± 1.12 | 1.25 ± 0.83 | 1.65 ± 0.76 | 1.07 ± 1.00 | nd | nd | 6.96 ± 0.54 | 6.03 ± 1.72 |
| RAG 2016 (n = 12) | § 3.30 ± 0.69 | § 2.74 ± 1.10 | § 3.13 ± 1.24 | § 0.94 ± 0.69 | § 1.73 ± 1.05 | § 0.71 ± 0.70 | nd | nd | § 6.89 ± 0.59 | 5.40 ± 1.94 |
| ASC 2015/2016 (n = 14) | 4.70 ± 0.73 | 4.06 ± 0.97 | 4.02 ± 0.72 | 2.10 a ± 1.41 | 1.46 ± 1.00 | 1.53 ± 0.92 | 1.81 ± 0.96 | nd | 7.41 ± 0.56 | 7.02 ± 2.06 |
| COR 2015/2016 (n = 14) | 4.01 ± 0.50 | 4.15 ± 0.82 | 4.19 ± 0.35 | 2.03 a ± 0.97 | 1.94 ± 0.78 | 1.57 ± 1.06 | 0.22 ± 0.58 | 0.12 ± 0.31 | 7.19 ± 0.39 | 7.15 ± 2.21 |
| MIG 2015/2016 (n = 15) | 3.80 ± 0.46 | 3.91 ± 0.70 | 3.57 ± 0.64 | 0.84 b ± 0.82 | 0.96 ± 0.65 | 0.40 ± 0.59 | nd | 0.98 ± 0.62 | 7.15 ± 0.59 | 6.56 ± 2.14 |
| MOG 2015/2016 (n = 17) | 3.93 ± 0.71 | 3.54 ± 1.00 | 3.69 ± 1.02 | 1.74 ab ± 0.98 | 1.74 ± 0.84 | 1.19 ± 1.00 | 0.09 ± 0.28 | 0.12 ± 0.35 | 7.01 ± 0.52 | 6.81 ± 1.99 |
| RAG 2015/2016 (n = 19) | 4.05 ± 0.59 | 3.84 ± 0.94 | 3.98 ± 0.86 | 1.68 ab ± 1.08 | 2.39 ± 0.74 | 1.31 ± 0.88 | 0.10 ± 0.27 | nd | 7.22 ± 0.43 | 6.94 ± 3.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacetti, D.; Boarelli, M.C.; Giovannetti, R.; Ferraro, S.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; et al. Chemical and Sensory Profiling of Monovarietal Extra Virgin Olive Oils from the Italian Marche Region. Antioxidants 2020, 9, 330. https://doi.org/10.3390/antiox9040330
Pacetti D, Boarelli MC, Giovannetti R, Ferraro S, Conti P, Alfei B, Caprioli G, Ricciutelli M, Sagratini G, Fedeli D, et al. Chemical and Sensory Profiling of Monovarietal Extra Virgin Olive Oils from the Italian Marche Region. Antioxidants. 2020; 9(4):330. https://doi.org/10.3390/antiox9040330
Chicago/Turabian StylePacetti, Deborah, Maria Chiara Boarelli, Rita Giovannetti, Stefano Ferraro, Paolo Conti, Barbara Alfei, Giovanni Caprioli, Massimo Ricciutelli, Gianni Sagratini, Donatella Fedeli, and et al. 2020. "Chemical and Sensory Profiling of Monovarietal Extra Virgin Olive Oils from the Italian Marche Region" Antioxidants 9, no. 4: 330. https://doi.org/10.3390/antiox9040330
APA StylePacetti, D., Boarelli, M. C., Giovannetti, R., Ferraro, S., Conti, P., Alfei, B., Caprioli, G., Ricciutelli, M., Sagratini, G., Fedeli, D., Gabbianelli, R., & Fiorini, D. (2020). Chemical and Sensory Profiling of Monovarietal Extra Virgin Olive Oils from the Italian Marche Region. Antioxidants, 9(4), 330. https://doi.org/10.3390/antiox9040330








