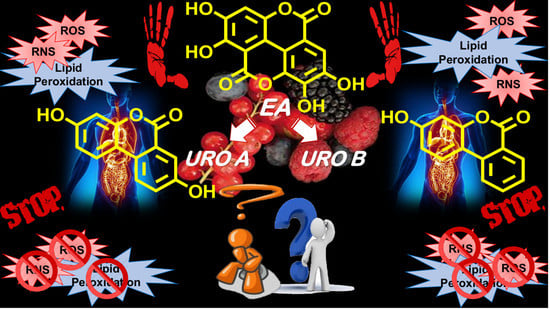
Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins?
Abstract
:1. Introduction
2. Oxidative Stress
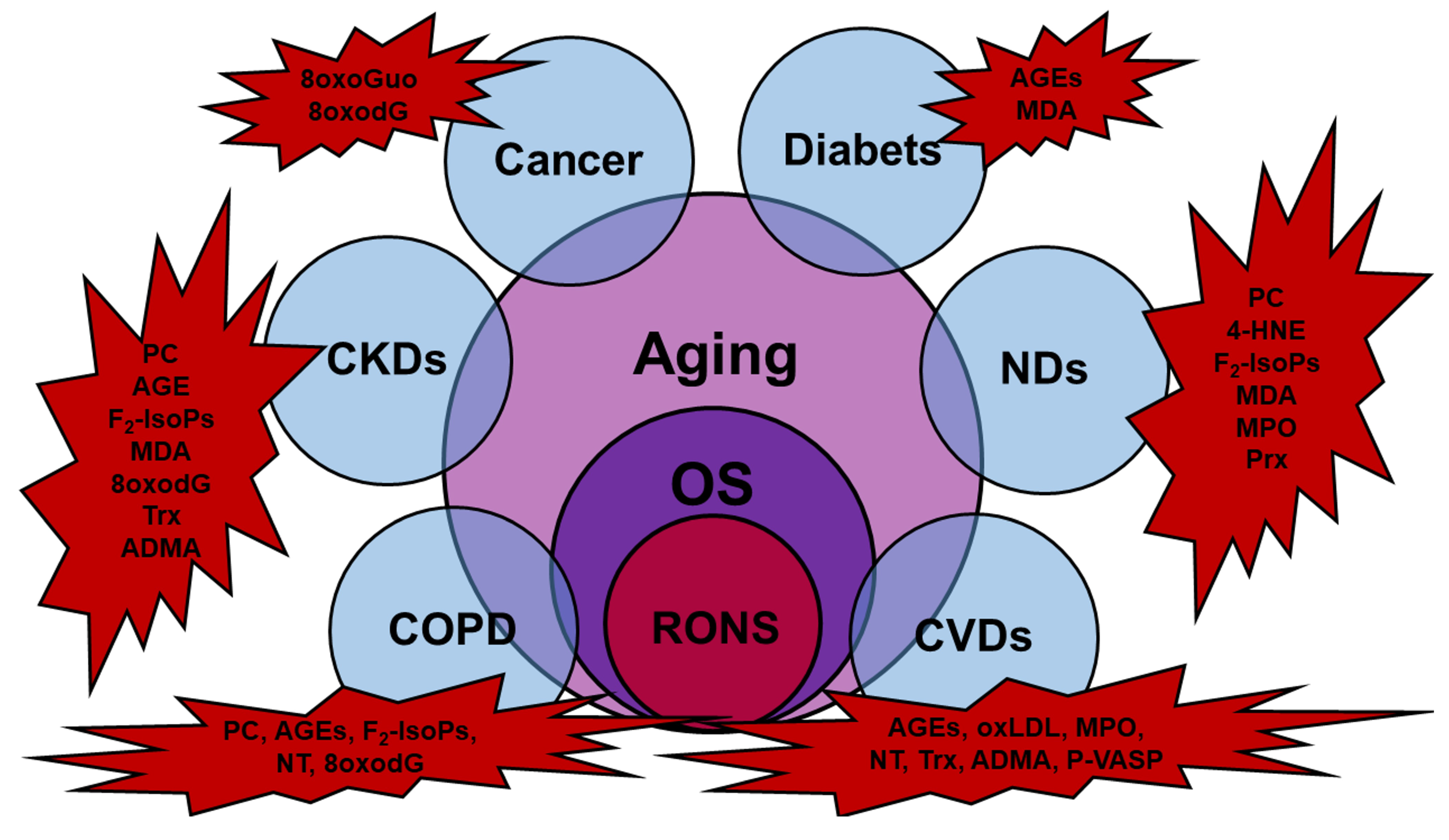
2.1. Cell Dysfunctions and Pathological Conditions Associated with Reactive Oxygen Species (ROS) and Nitrogen Species (RNS) Overproduction and OS
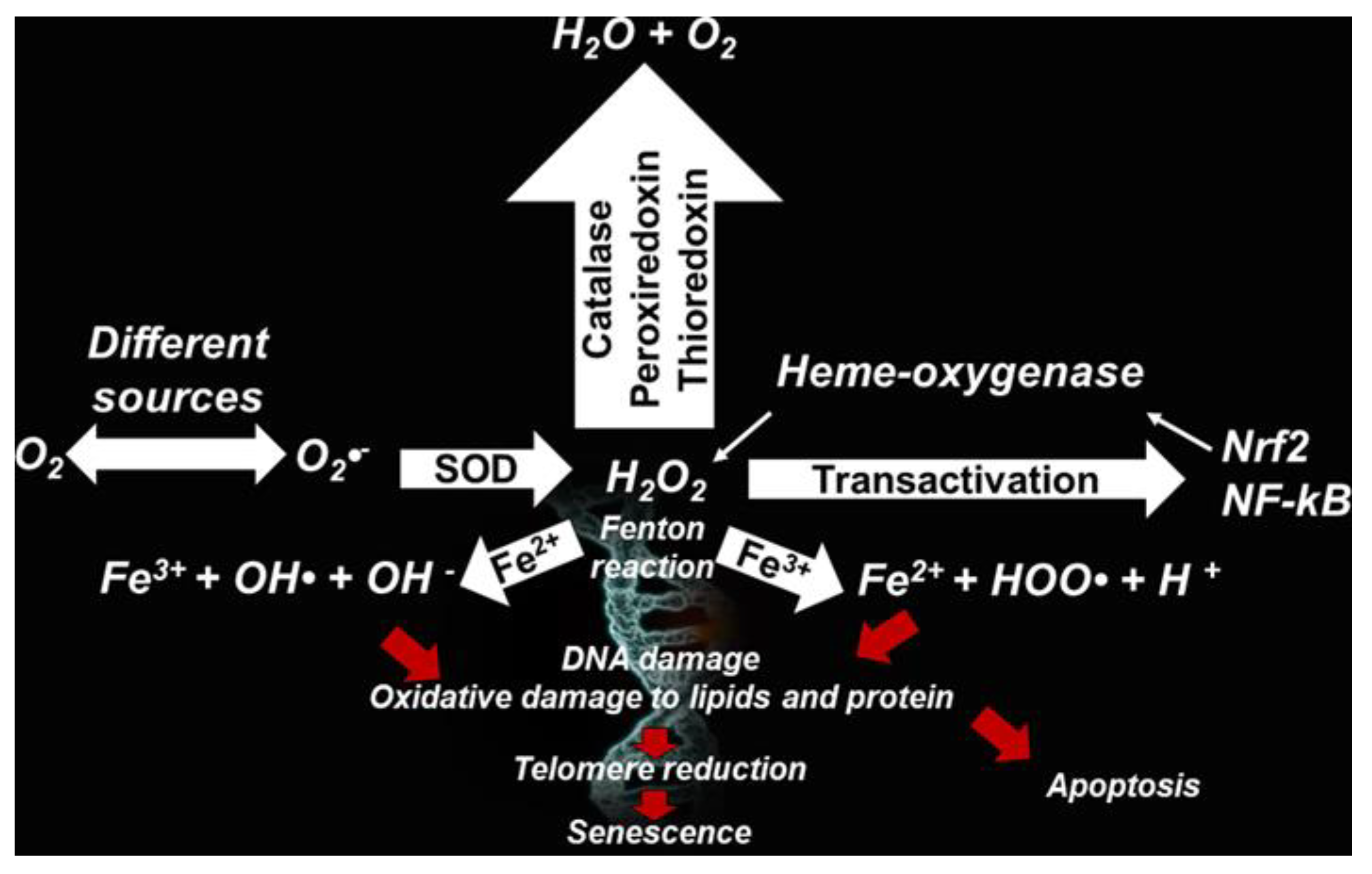
2.2. RONS Origin
3. Chemical Insights of EA
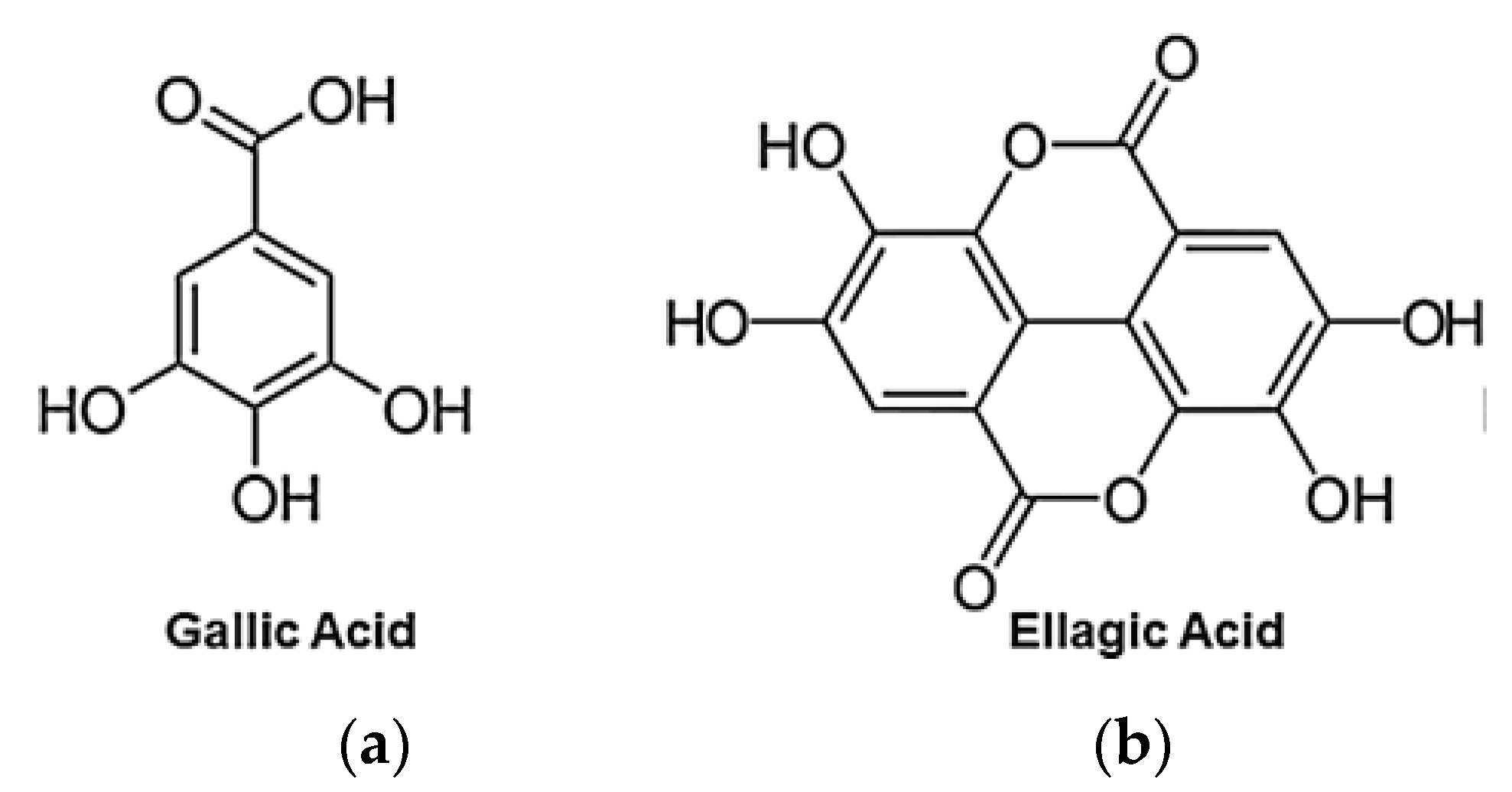
3.1. EA Chemical Structure and Physical Properties
3.2. EA In Vivo Formation and Metabolism
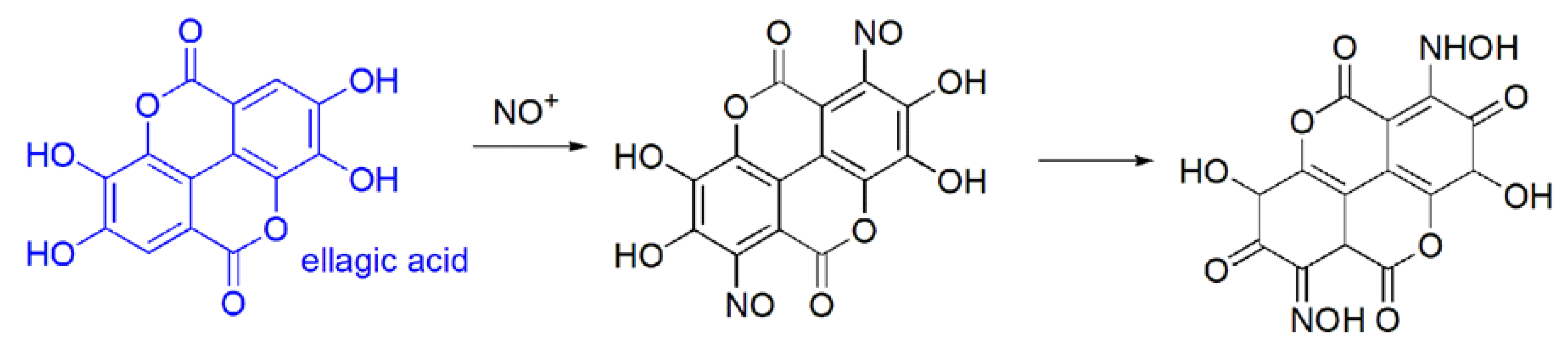
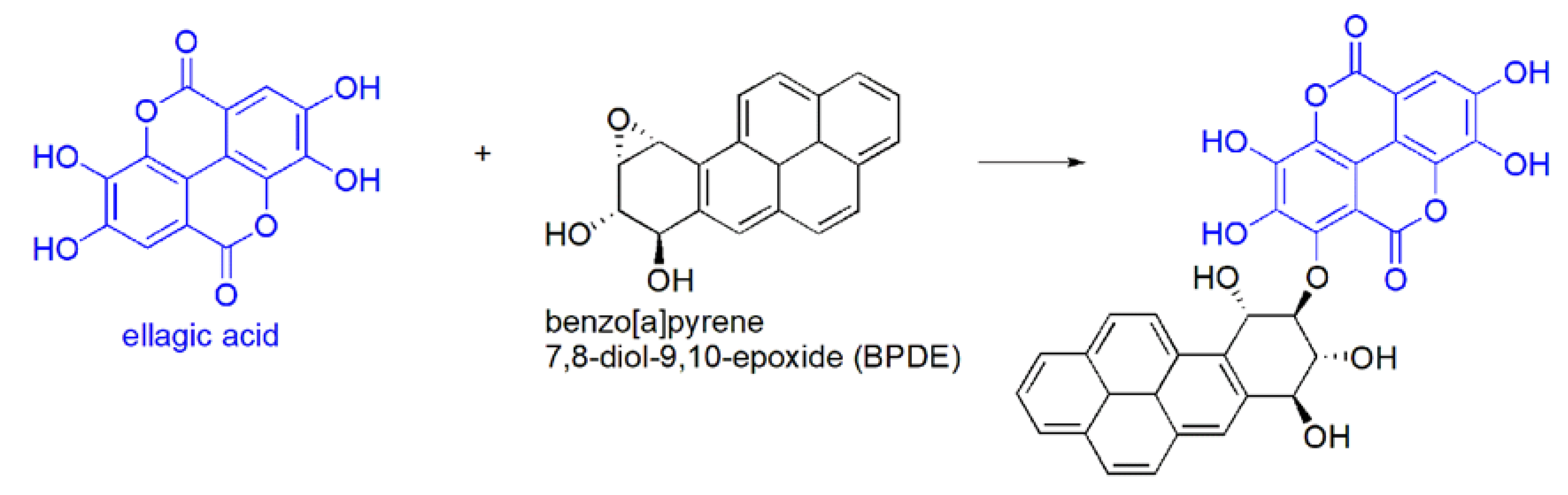
3.3. EA Chemical Reactivity
- (a)
- (b)
- in vivo oxidation reactions i.e.,
4. The Common Sources of EA
5. EA Antioxidant Power: The Proposed Mechanisms of Action
6. The Urolithins (UROs) System
6.1. Structure and Chemistry
6.2. Formation Pathway and Metabolism of UROs
6.3. Influence of Individual Metabotype on UROs Production
6.4. Not All Living Species Produce UROs
6.5. Mechanisms of Antioxidant Effects of UROs
7. EA or UROs: An Open Debate
Hazardous Implications Related to UROs Exposure
8. Authors Opinions, Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberan, F.A.; Espín, J.C.; García-Conesa, M.T. Bioavailability and Metabolism of Ellagic Acid and Ellagitannins. In Chemistry and Biology of Ellagitannins; Quideau, S., Ed.; World Scientific Publishing Co Pte Ltd.: Singapore, 2009; pp. 273–297. [Google Scholar]
- Beretta, G.; Rossoni, G.; Santagati, N.A.; Facino, R.M. Anti-Ischemic Activity and Endothelium-Dependent Vasorelaxant Effect of Hydrolysable Tannins from the Leaves of Rhus coriaria (Sumac) in Isolated Rabbit Heart and Thoracic Aorta. Planta Med. 2009, 75, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A Systematic Review of the Evidence Supporting a Causal Link Between Dietary Factors and Coronary Heart Disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, Y.; Ohie, T.; Yonekawa, Y.; Yonemoto, K.; Aizawa, H.; Mori, Y.; Watanabe, M.; Takeuchi, M.; Hasegawa, M.; Taguchi, C.; et al. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J. Agric. Food Chem. 2009, 57, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Ellagic Acid and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 473–479. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [Green Version]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Grilli, M.; Pittaluga, A.M.; Boggia, R. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. Eur. J. Med. Chem. 2019, 183, 111724. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chou, C.-W.; Kumar, K.J.; Senthil Fu, K.-T.; Wang, H.-M.; Hsu, L.-S.; Kuo, Y.-H.; Wu, C.-R.; Chen, S.-C.; Yang, H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Compl. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Heber, D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008, 269, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-γ pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef]
- Crescente, M.; Jessen, G.; Momi, S.; Höltje, H.-D.; Gresele, P.; Cerletti, C.; de Gaetano, G. Interactions of gallic acid, resveratrol, quercetin and aspirin at the platelet cyclooxygenase-1 level Functional and modelling studies. Thromb. Haemost. 2017, 102, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Trifirò, G.; Calogero, G.; Ippolito, F.M.; Cosentino, M.; Giuliani, R.; Conforti, A.; Venegoni, M.; Mazzaglia, G.; Caputi, A.P. Adverse drug events in emergency department population: A prospective Italian study. Pharmacoepidemiol. Drug Saf. 2005, 14, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Li Volti, G.; Cardile, V.; Abraham, N.G.; Sorrenti, V. Effects of Ellagic Acid on Angiogenic Factors in Prostate Cancer Cells. Cancers 2013, 5, 726–738. [Google Scholar] [CrossRef] [Green Version]
- Nejad, K.H.; Gharib-Naseri, M.K.; Sarkaki, A.; Dianat, M.; Badavi, M.; Farbood, Y. Effects of ellagic acid pretreatment on renal functions disturbances induced by global cerebral ischemic-reperfusion in rat. Iran J. Basic Med. Sci. 2017, 20, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Zafeer Mohd, F.; Waseem, M.; Anis, E.; Hossain, M.M.; Afzal, M. Ellagic acid mitigates arsenic-trioxide-induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. J. Biochem. Mol. Toxicol. 2018, 32, e22024. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Comprehensive characterization of the effects of ellagic acid and urolithins on colorectal cancer and key-associated molecular hallmarks: MicroRNA cell specific induction of CDKN1A (p21) as a common mechanism involved. Mol. Nutr. Food Res. 2016, 60, 701–716. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Roggeri, A.; Olivero, G.; Cisani, F.; Bonfiglio, T.; Summa, M.; Grilli, M.; Caviglioli, G.; Alfei, S.; et al. Neuroinflammation in Aged Brain: Impact of the Oral Administration of Ellagic Acid Microdispersion. Int. J. Mol. Sci. 2020, 21, 3631. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. A new flavone glucoside together with known ellagitannins and flavones with anti-diabetic and anti-obesity activities from the flowers of pomegranate (Punica granatum). Nat. Prod. Res. 2019, 33, 252–257. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Zazueta, C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R. The Effects of Ellagic Acid upon Brain Cells: A Mechanistic View and Future Directions. Neurochem. Res. 2016, 41, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell. Sign. 2007, 19, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging—Matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marengo, B.; Raffaghello, L.; Pistoia, V.; Cottalasso, D.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Reactive oxygen species: Biological stimuli of neuroblastoma cell response. Cancer Lett. 2005, 228, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef] [Green Version]
- Touqeer, A.; William, S.N.; Nabavi, S.F.; Ilkay, E.O.; Nady, B.; Eduardo, S.; Nabavi, S.M. Insights into effects of ellagic acid on the nervous system: A mini review. Cur. Pharm. Des. 2016, 22, 1350–1360. [Google Scholar]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Bruni, R.; Del Rio, D. Bioactivation of High-Molecular-Weight Polyphenols by the Gut Microbiome. In Diet-Microbe Interactions in the Gut; Tuohy, K., Del Rio, D., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 73–101. [Google Scholar]
- Sandhu, A.K.; Miller, M.G.; Thangthaeng, N.; Scott, T.M.; Shukitt-Hale, B.; Edirisinghe, I.; Burton-Freeman, B. Metabolic fate of strawberry polyphenols after chronic intake in healthy older adults. Food Funct. 2018, 9, 96–106. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Tranchant, C.; Shi, J.; Ye, X.; Xue, S.J. Ellagic acid in strawberry (Fragaria spp.): Biological, technological, stability, and human health aspects. Food Qual. Saf. 2017, 1, 227–252. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Muñoz, C.; Vaillant, F. Metabolic Fate of Ellagitannins: Implications for Health, and Research Perspectives for Innovative Functional Foods. Critic. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Lima, V.; Silva, A.; Sanches Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [Green Version]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2015, 2016, 6475624. [Google Scholar] [CrossRef] [Green Version]
- Stanisławska, I.J.; Piwowarski, J.P.; Granica, S.; Kiss, A.K. The effects of urolithins on the response of prostate cancer cells to non-steroidal antiandrogen bicalutamide. Phytomedicine 2018, 46, 176–183. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selma, M.V.; Tomás-Barberán, F.A.; Beltrán, D.; García-Villalba, R.; Espín, J.C. Gordonibacter urolithinfaciens sp. nov., a urolithin-producing bacterium isolated from the human gut. Int. J. Syst. Evol. Microbiol. 2014, 64, 2346–2352. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-de-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef] [Green Version]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.; Franco, M.C.; Estevez, A.G. Reactive nitrogen species in cellular signaling. Exp. Biol. Med. 2015, 240, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antiox. Red. Sign. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, E. Role of Protein Carbonylation in Skeletal Muscle Mass Loss Associated with Chronic Conditions. Proteomes 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.A.; Wouters, E.F.M.; Schalkwijk, C.G. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int. J. Biochem. Cell Biol. 2016, 81, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.D.; Noren Hooten, N.; Trzeciak, A.R.; Evans, M.K. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 2013, 134, 139–157. [Google Scholar] [CrossRef] [Green Version]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 4 August 2020).
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef]
- Behl, G.; Sharma, M.; Dahiya, S.; Chhikara, A.; Chopra, M. Synthesis, Characterization, and Evaluation of Radical Scavenging Ability of Ellagic Acid-Loaded Nanogels. J. Nanomat. 2011, 2011, 695138. [Google Scholar] [CrossRef]
- Huetz, P.; Mavaddat, N.; Mavri, J. Reaction between ellagic acid and an ultimate carcinogen. J. Chem. Inf. Model. 2005, 45, 1564–1570. [Google Scholar] [CrossRef]
- Ahire, V.; Kumar, A.; Mishra, K.P.; Kulkarni, G. Ellagic Acid Enhances Apoptotic Sensitivity of Breast Cancer Cells to γ-Radiation. Nutr. Cancer 2017, 69, 904–910. [Google Scholar] [CrossRef]
- Cheng, H.; Lu, C.; Tang, R.; Pan, Y.; Bao, S.; Qiu, Y.; Xie, M. Ellagic acid inhibits the proliferation of human pancreatic carcinoma PANC-1 cells in vitro and in vivo. Oncotarget 2017, 8, 12301–12310. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, G.; Bo, W.; Zhou, Y.; Dang, S.; Wei, J.; Li, X.; Liu, M. Multiple effects of ellagic acid on human colorectal carcinoma cells identified by gene expression profile analysis. Int. J. Oncol. 2017, 50, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Fu, W.; Wang, J.; Kang, D.; Xu, L.; Zhao, Y.; Liu, A.L.; Du, G.H. Identification of Estrogen Receptor α Antagonists from Natural Products via In Vitro and In Silico Approaches. Oxid. Med. Cell Longev. 2018, 2018, 6040149. [Google Scholar] [CrossRef] [Green Version]
- Kolšek, K.; Mavri, J.; Sollner Dolenc, M.; Gobec, S.; Turk, S. Endocrine Disruptome—An Open Source Prediction Tool for Assessing Endocrine Disruption Potential through Nuclear Receptor Binding. J. Chem. Inf. Model. 2014, 54, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Muñoz, J.L.; Garcia-Molina, F.; Garcia-Molina, M.; Tudela, J.; García-Cánovas, F.; Rodriguez-Lopez, J.N. Ellagic acid: Characterization as substrate of polyphenol oxidase. IUBMB Life 2009, 61, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Morina, F.; Takahama, U.; Yamauchi, R.; Hirota, S.; Veljovic-Jovanovic, S. Quercetin 7-O-glucoside suppresses nitrite-induced formation of dinitrosocatechins and their quinones in catechin/nitrite systems under stomach simulating conditions. Food Funct. 2015, 6, 218–228. [Google Scholar] [CrossRef]
- Siraj, M.A.; Shilpi, J.A.; Hossain, M.G.; Uddin, S.J.; Islam, M.K.; Jahan, I.A.; Hossain, H. Anti-Inflammatory and Antioxidant Activity of Acalypha hispida Leaf and Analysis of its Major Bioactive Polyphenols by HPLC. Adv. Pharm. Bull. 2016, 6, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, H.; Sakagami, H.; Hatano, T. Three new flavonoids, proanthocyanidin, and accompanying phenolic constituents from Feijoa sellowiana. Biosci. Biotechnol. Biochem. 2018, 82, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-García, V.; Trindade, C.; Lima, E.; Guecheva, T.N.; Villela, I.; Martinez-Lopez, W.; Corrêa, D.S.; Ferraz, A.d.B.F.; Moura, S.; Sosa, M.Q.; et al. Chemical characterization and cytotoxic, genotoxic, and mutagenic properties of Baccharis trinervis (Lam, Persoon) from Colombia and Brazil. J. Ethnopharmacol. 2018, 213, 210–220. [Google Scholar] [CrossRef]
- Yang, R.; Guan, Y.; Zhou, J.; Sun, B.; Wang, Z.; Chen, H.; He, Z.; Jia, A. Phytochemicals from Camellia nitidissima Chi Flowers Reduce the Pyocyanin Production and Motility of Pseudomonas aeruginosa PAO1. Front. Microbiol. 2018, 8, 2640. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.F.; Espindola, P.P.d.T.; Torquato, H.F.V.; Vital, W.D.; Justo, G.Z.; Silva, D.B.; Carollo, C.A.; de Picoli Souza, K.; Paredes-Gamero, E.J.; dos Santos, E.L. Leaf and Root Extracts from Campomanesia adamantium (Myrtaceae) Promote Apoptotic Death of Leukemic Cells via Activation of Intracellular Calcium and Caspase-3. Front. Pharmacol. 2017, 8, 466. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.-P.; Gu, X.-L.; Chen, J.-X.; Yang, J.; Tan, S.-Y.; Duan, W.-J. Chemical constituents from Canarium album Raeusch and their anti-influenza A virus activities. J. Nat. Med. 2018, 72, 808–815. [Google Scholar] [CrossRef]
- Hafsa, J.; Hammi, K.M.; Khedher, M.R.B.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H. Inhibition of protein glycation, antioxidant and antiproliferative activities of Carpobrotus edulis extracts. Biomed. Pharmacother. 2016, 84, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, P.T.; Xuan, T.D.; Tu Anh, T.T.; Mai Van, T.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed Suppressing Potential and Isolation of Potent Plant Growth Inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, E.; Nohooji, M.G.; Habibi, M.; Ebrahimi, M.; Mehrafarin, A.; Khalighi-Sigaroodi, F. Antioxidant potential assessment of phenolic and flavonoid rich fractions of Clematis orientalis and Clematis ispahanica (Ranunculaceae). Nat. Prod. Res. 2018, 32, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Shendge, A.K.; Basu, T.; Chaudhuri, D.; Panja, S.; Mandal, N. In vitro Antioxidant and Antiproliferative Activities of Various Solvent Fractions from Clerodendrum viscosum Leaves. Pharmacogn. Mag. 2017, 13, S344–S353. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhang, Y.; Dong, L.; Gao, Q.; Yin, L.; Quan, H.; Chen, R.; Fu, X.; Lin, D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 2018, 213, 280–301. [Google Scholar] [CrossRef]
- Cho, C.H.; Jang, H.; Lee, M.; Kang, H.; Heo, H.J.; Kim, D.-O. Sea Buckthorn (Hippophae rhamnoides L.) Leaf Extracts Protect Neuronal PC-12 Cells from Oxidative Stress. J. Microbiol. Biotechnol. 2017, 27, 1257–1265. [Google Scholar] [CrossRef]
- Vieira, G.S.; Marques, A.S.F.; Machado, M.T.C.; Silva, V.M.; Hubinger, M.D. Determination of anthocyanins and non-anthocyanin polyphenols by ultra performance liquid chromatography/electrospray ionization mass spectrometry (UPLC/ESI–MS) in jussara (Euterpe edulis) extracts. J. Food Sci. Technol. 2017, 54, 2135–2144. [Google Scholar] [CrossRef]
- Falcão, T.R.; de Araújo, A.A.; Soares, L.A.L.; de Moraes Ramos, R.T.; Bezerra, I.C.F.; Ferreira, M.R.A.; de Souza Neto, M.A.; Melo, M.C.N.; de Araújo, R.F.; de Aguiar Guerra, A.C.V.; et al. Crude extract and fractions from Eugenia uniflora Linn leaves showed anti-inflammatory, antioxidant, and antibacterial activities. BMC Complem. Altern. Med. 2018, 18, 84. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-S.; Jung, S.-H.; Kim, J.S. Polyphenols from Euphorbia pekinensis Inhibit AGEs Formation In Vitro and Vessel Dilation in Larval Zebrafish In Vivo. Planta Med. 2018, 84, 176–181. [Google Scholar] [CrossRef]
- Ton That, Q.; Nguyen Thien, T.V.; Dang, H.P.; Le Hoan, N.; Vo, L.K.T.; Nguyen, M.H.D.; Ngu, N.T.; Nguyen, T.S.; Hansen, P.E. Chemical constituents of Geum urbanum L. roots. Nat. Prod. Res. 2018, 32, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Pacheco, A.; Escalona Arranz, J.C.; Beaven, M.; Peres-Roses, R.; Gámez, Y.M.; Camacho-Pozo, M.I.; Maury, G.L.; de Macedo, M.B.; Cos, P.; Tavares, J.F.; et al. Bioassay-guided In vitro Study of the Antimicrobial and Cytotoxic Properties of the Leaves from Excoecaria Lucida Sw. Pharmacogn. Res. 2017, 9, 396–400. [Google Scholar] [CrossRef]
- Vu, D.C.; Vo, P.H.; Coggeshall, M.V.; Lin, C.-H. Identification and Characterization of Phenolic Compounds in Black Walnut Kernels. J. Agric. Food Chem. 2018, 66, 4503–4511. [Google Scholar] [CrossRef]
- Pereira, L.O.M.; Vilegas, W.; Tangerina, M.M.P.; Arunachalam, K.; Balogun, S.O.; Orlandi-Mattos, P.E.; Colodel, E.M.; Martins, D.T.d.O. Lafoensia pacari A. St.-Hil.: Wound healing activity and mechanism of action of standardized hydroethanolic leaves extract. J. Ethnopharmacol. 2018, 219, 337–350. [Google Scholar] [CrossRef]
- De Oliveira, L.M.; Porte, A.; de Oliveira Godoy, R.L.; da Costa Souza, M.; Pacheco, S.; de Araujo Santiago, M.C.P.; Gouvêa, A.C.M.S.; da Silva de Mattos do Nascimento, L.; Galhardo Borguini, R. Chemical characterization of Myrciaria floribunda (H. West ex Willd) fruit. Food Chem. 2018, 248, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Díaz-de-Cerio, E.; Arráez-Román, D.; Segura-Carretero, A.; Ferranti, P.; Nicoletti, R.; Perrotta, G.M.; Gómez-Caravaca, A.M. Establishment of pressurized-liquid extraction by response surface methodology approach coupled to HPLC-DAD-TOF-MS for the determination of phenolic compounds of myrtle leaves. Ana. Bioanal. Chem. 2018, 410, 3547–3557. [Google Scholar] [CrossRef]
- Hernández, C.; Ascacio-Valdés, J.; De la Garza, H.; Wong-Paz, J.; Aguilar, C.N.; Martínez-Ávila, G.C.; Castro-López, C.; Aguilera-Carbó, A. Polyphenolic content, in vitro antioxidant activity and chemical composition of extract from Nephelium lappaceum L. (Mexican rambutan) husk. Asian Pac. J. Trop. Med. 2017, 10, 1201–1205. [Google Scholar] [CrossRef]
- Ifeanacho, M.O.; Ikewuchi, C.C.; Ikewuchi, J.C. Investigation of the profile of phenolic compounds in the leaves and stems of Pandiaka heudelotii using gas chromatography coupled with flame ionization detector. Food Sci. Nutr. 2017, 5, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Flavonoids and Ellagitannins Characterization, Antioxidant and Cytotoxic Activities of Phyllanthus acuminatus Vahl. Plants 2017, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Yuan, B.; Xiao, H.; Zhao, L.; Wu, X.; Rakariyatham, K.; Zhong, L.; Han, Y.; Muinde Kimatu, B.; Yang, W. Polyphenols-rich extract from Pleurotus eryngii with growth inhibitory of HCT116 colon cancer cells and anti-inflammatory function in RAW264.7 cells. Food Funct. 2018, 9, 1601–1611. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Duarte, P.A.; Lapa, J.; Miguel, M.A.L.; Monteiro, M. Jabuticaba (Myrciaria jaboticaba) juice obtained by steam-extraction: Phenolic compound profile, antioxidant capacity, microbiological stability, and sensory acceptability. J. Food Sci. Technol. 2018, 55, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.d.A.; Stringheta, P.C.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Flavonols and ellagic acid derivatives in peels of different species of jabuticaba (Plinia spp.) identified by HPLC-DAD-ESI/MSn. Food Chem. 2018, 252, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Pitz, H.d.S.; Voytena, A.P.L.; Trevisan, A.C.D.; Ribeiro-Do-Valle, R.M.; Maraschin, M. Jaboticaba (Plinia peruviana) extract nanoemulsions: Development, stability, and in vitro antioxidant activity. Drug Develop. Ind. Pharm. 2018, 44, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Imura, K.; Akagi, Y.; Muraoka, O.; Ninomiya, K. Ellagic acid glycosides with hepatoprotective activity from traditional Tibetan medicine Potentilla anserina. J. Nat. Med. 2018, 72, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sobral-Souza, C.E.; Silva, A.R.P.; Leite, N.F.; Costa, J.G.M.; Menezes, I.R.A.; Cunha, F.A.B.; Rolim, L.A.; Coutinho, H.D.M. LC–MS analysis and cytoprotective effect against the mercurium and aluminium toxicity by bioactive products of Psidium brownianum Mart. ex DC. J. Hazard. Mater. 2019, 370, 54–62. [Google Scholar] [CrossRef]
- Odubanjo, V.O.; Ibukun, E.O.; Oboh, G.; Adefegha, S.A. Aqueous extracts of two tropical ethnobotanicals (Tetrapleura tetraptera and Quassia undulata) improved spatial and non-spatial working memories in scopolamine-induced amnesic rats: Influence of neuronal cholinergic and antioxidant systems. Biomed. Pharmacother. 2018, 99, 198–204. [Google Scholar] [CrossRef]
- Ghadage, D.M.; Kshirsagar, P.R.; Pai, S.R.; Chavan, J.J. Extraction efficiency, phytochemical profiles and antioxidative properties of different parts of Saptarangi (Salacia chinensis L.)—An important underutilized plant. Biochem. Biophys. Rep. 2017, 12, 79–90. [Google Scholar] [CrossRef]
- Pinto, J.; Spínola, V.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Molina-García, L.; Castilho, P.C. Polyphenolic profile and antioxidant activities of Madeiran elderberry (Sambucus lanceolata) as affected by simulated in vitro digestion. Food Res. Int. 2017, 100, 404–410. [Google Scholar] [CrossRef]
- Im, S.H.; Wang, Z.; Lim, S.S.; Lee, O.-H.; Kang, I.-J. Bioactivity-guided isolation and identification of anti-adipogenic compounds from Sanguisorba officinalis. Pharm. Biol. 2017, 55, 2057–2064. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- De Britto Policarpi, P.; Turcatto, L.; Demoliner, F.; Ferrari, R.A.; Bascuñan, V.L.A.F.; Ramos, J.C.; Jachmanián, I.; Vitali, L.; Micke, G.A.; Block, J.M. Nutritional potential, chemical profile and antioxidant activity of Chichá (Sterculia striata) nuts and its by-products. Food Res. Int. 2018, 106, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, S.; Chandran, R.; Thankarajan, S.; Abrahamse, H.; Thangaraj, P. Phytochemical composition, antioxidant and anti-bacterial activity of Syzygium calophyllifolium Walp. fruit. J. Food Sci. Technol. 2018, 55, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Gajera, H.P.; Gevariya, S.N.; Hirpara, D.G.; Patel, S.V.; Golakiya, B.A. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black jamun (Syzygium cumini L.) landraces. J. Food Sci. Technol. 2017, 54, 3180–3191. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.; Karwasra, R.; Gupta, Y.K.; Ray, S.B.; Singh, S. Terminalia chebula supplementation attenuates cisplatin-induced nephrotoxicity in Wistar rats through modulation of apoptotic pathway. Nat. Prod. Res. 2019, 33, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.-H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and Quantification of Phenolic Compounds in Berries of Fragaria and Rubus Species (Family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant Activity and Phenolic Content of Oregon Caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Burren, B.; Tinggi, U.; Sultanbawa, Y. Organic acids in Kakadu plum (Terminalia ferdinandiana): The good (ellagic), the bad (oxalic) and the uncertain (ascorbic). Food Res. Int. 2016, 89, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, F.; Nasri, M.H.F.; Farhangfar, H.; Modaresi, J. Nutritive value and polyphenol content of pomegranate seed pulp ensiled with different tannin-inactivating agents. Anim. Feed Sci. Technol. 2015, 207, 262–266. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green Extraction from Pomegranate Marcs for the Production of Functional Foods and Cosmetics. Pharmaceuticals 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Kumpulainen, J. Determination of Free and Total Phenolic Acids in Plant-Derived Foods by HPLC with Diode-Array Detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Kärenlampi, S.O.; Mykkänen, H.M.; Heinonen, I.M.; Törrönen, A.R. Ellagic acid content in berries: Influence of domestic processing and storage. Eur. Food Res. Technol. 2000, 212, 75–80. [Google Scholar] [CrossRef]
- Da Silva Pinto, M.; Lajolo, F.M.; Genovese, M.I. Bioactive Compounds and Antioxidant Capacity of Strawberry Jams. Plant Food Hum. Nutr. 2007, 62, 127–131. [Google Scholar] [CrossRef]
- Clifford, M.N.; Scalbert, A. Ellagitannins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Glabasnia, A.; Hofmann, T. Sensory-Directed Identification of Taste-Active Ellagitannins in American (Quercus alba L.) and European Oak Wood (Quercus robur L.) and Quantitative Analysis in Bourbon Whiskey and Oak-Matured Red Wines. J. Agric. Food Chem. 2006, 54, 3380–3390. [Google Scholar] [CrossRef]
- Bushman, B.S.; Phillips, B.; Isbell, T.; Ou, B.; Crane, J.M.; Knapp, S.J. Chemical Composition of Caneberry (Rubus spp.) Seeds and Oils and Their Antioxidant Potential. J. Agric. Food Chem. 2004, 52, 7982–7987. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.-Y.; Barlow, P.J. Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem. 2006, 97, 524–530. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Res. Int. 2014, 66, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Konczak, I.; Maillot, F.; Dalar, A. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chem. 2014, 151, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef]
- Santos Sánchez, N.; Salas-Coronado, R.; Villanueva, C.; Hernandez-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Intechopen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Klein, E.; Lukeš, V.; Ilčin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2015, 58, 107–116. [Google Scholar] [CrossRef]
- Mazzone, G.; Galano, A.; Alvarez-Idaboy, J.R.; Russo, N. Coumarin–Chalcone Hybrids as Peroxyl Radical Scavengers: Kinetics and Mechanisms. J. Chem. Inf. Model. 2016, 56, 662–670. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Hydroxyl radical and hypochlorous acid scavenging activity of small Centaury (Centaurium erythraea) infusion. A comparative study with green tea (Camellia sinensis). Phytomedicine 2003, 10, 517–522. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidative Properties of Cardoon (Cynara cardunculus L.) Infusion Against Superoxide Radical, Hydroxyl Radical, and Hypochlorous Acid. J. Agric. Food Chem. 2002, 50, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.d.L. Antioxidant Activity of Hypericum androsaemum Infusion: Scavenging Activity against Superoxide Radical, Hydroxyl Radical and Hypochlorous Acid. Biol. Pharm. Bull. 2002, 25, 1320–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.d.L. Studies on the Antioxidant Activity of Lippia citriodora Infusion: Scavenging Effect on Superoxide Radical, Hydroxyl Radical and Hypochlorous Acid. Biol. Pharm. Bull. 2002, 25, 1324–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galano, A.; Francisco Marquez, M.; Pérez-González, A. Ellagic Acid: An Unusually Versatile Protector against Oxidative Stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Diduk, R.; Galano, A.; Tan, D.X.; Reiter, R.J. N-Acetylserotonin and 6-Hydroxymelatonin against Oxidative Stress: Implications for the Overall Protection Exerted by Melatonin. J. Phys. Chem. B 2015, 119, 8535–8543. [Google Scholar] [CrossRef] [Green Version]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Jilma-Stohlawetz, P.; Rios, J.; Hingorani, L.; Derendorf, H. Absorption, Metabolism, and Antioxidant Effects of Pomegranate (Punica granatum L.) Polyphenols after Ingestion of a Standardized Extract in Healthy Human Volunteers. J. Agric. Food Chem. 2006, 54, 8956–8961. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Stefańska, J.; Kiss, A.K. Differences in Metabolism of Ellagitannins by Human Gut Microbiota ex Vivo Cultures. J. Nat. Prod. 2016, 79, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Truchado, P.; Ito, H.; Espín, J.C.; Tomás-Barberán, F.A. UV and MS Identification of Urolithins and Nasutins, the Bioavailable Metabolites of Ellagitannins and Ellagic Acid in Different Mammals. J. Agric. Food Chem. 2011, 59, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Milala, J.; Kosmala, M.; Karlińska, E.; Juśkiewicz, J.; Zduńczyk, Z.; Fotschki, B. Ellagitannins from Strawberries with Different Degrees of Polymerization Showed Different Metabolism through Gastrointestinal Tract of Rats. J. Agric. Food Chem. 2017, 65, 10738–10748. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Hernández, L.; Pérez, A.; Vaillant, F. Diversity of urinary excretion patterns of main ellagitannins’ colonic metabolites after ingestion of tropical highland blackberry (Rubus adenotrichus) juice. Food Res. Int. 2014, 55, 161–169. [Google Scholar] [CrossRef]
- Kallio, T.; Kallio, J.; Jaakkola, M.; Mäki, M.; Kilpeläinen, P.; Virtanen, V. Urolithins Display both Antioxidant and Pro-oxidant Activities Depending on Assay System and Conditions. J. Agric. Food Chem. 2013, 61, 10720–10729. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; López, V. The Metabolite Urolithin-A Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of Antioxidant and Chemopreventive Ellagitannins from Strawberries, Raspberries, Walnuts, and Oak-Aged Wine in Humans: Identification of Biomarkers and Individual Variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [Green Version]
- Yordi, E.; Molina Pérez, E.; Matos, M.J.; Uriarte, E. Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; InTech: Rijeka, Croatia, 2012; pp. 23–48. [Google Scholar]

| Components Influenced by RONS | Effects |
|---|---|
| Regulation of mTORC1 | Impairments in cell growth and metabolism |
| Production of IL-1α | Pro-inflammatory state ↑NFκB ↑Epithelial–mesenchymal transition ↑Tumor metastatic progression |
| Induction of MMPs expression | Cancer, Alzheimer’s, atherosclerosis, osteoarthritis, lung emphysema |
| ↓FOXO proteins activity | ↓IGF-1-mediated protection from OS |
| ↓Sarco/endoplasmic reticulum Ca2+-ATPase activity | Cardiac senescence |
| ↓Sirtuins activity | ↑RONS by SOD inhibition Pro-inflammatory state, tumorigenic effect ↑TNFα and NFκB ↑Proto-oncogene c-Jun and c-Myc |
| Regulation of p16INK4a/pRB and proteins p53/p21 | Senescence |
| Endogenous Sources | Exogenous Sources | Reactive Species | |
|---|---|---|---|
| Enzymatic | Non-Enzymatic | ||
| NOX | Respiratory chain | Air | O2•− H2O2 •OH O•OH ONOO• NO2• CO3•− NO• ONOOCO2− NO2+ ONOOH N2O3 ONOO− ONOOCO2− |
| MPO | Glucose auto-oxidation | Water pollution | |
| Lipoxygenase | NAD• | Tobacco | |
| Angiotensin II | Semiquinone radicals | Alcohol | |
| NOS | Radical pyridinium | Heavy/transition metals | |
| Xanthene oxidase | Hemoproteins | Drugs | |
| Cyclooxygenase | Industrial solvents | ||
| FpH• | Cooking | ||
| Radiation | |||
| Radical | Source | Function |
|---|---|---|
| O2•− | Enzymatic process Autoxidation reactions Non-enzymatic electron transfer reactions | Reducing agent of iron complexes such as cytochrome-c Oxidizing agent to oxidize ascorbic acid and α-tocopherol |
| HO2• | Protonation of O2•− | HOO• initiates fatty acid peroxidation |
| HO• | H2O2 generates HO• through the metal-catalyzed Fenton reaction and the Haber Weiss recombination (HWR) | HO• reacts with both organic and inorganic molecules (DNA, proteins, lipids, carbohydrates) |
| NO• | L-arginine (substrate) NADPH (electron source) Nitric oxide-synthase | Intracellular second messenger Stimulates guanylate cyclase and protein kinases Causes smooth muscle relaxation in blood vessels |
| NO2• | Protonation of ONOO− Homolytic fragmentation of ONOOCO2− | Acts on the antioxidant mechanism Decreases ascorbate and α-tocopherol in plasma |
| ONOO• | Reaction of O2 with NO• | Oxidizes and nitrates methionine and L-tyrosine residues in proteins Oxidizes DNA to form nitroguanine |
| CO3•− | (SOD)-Cu2+-•OH reacts with HCO3- to generate CO3•− | Oxidizes proteins and nucleic acids |
| Reactive specie | Source | Function |
| ONOOCO2− | Reaction of ONOO− with CO2 | Promotes nitration of tyrosine of the oxyhemoglobin via free radicals |
| Cellular Macromolecules | Reactions | OS Biomarkers | Ref |
|---|---|---|---|
| Proteins | RNS with free or within polypeptide sequences L-tyrosine | Nitrotyrosine (NT) | [53] |
| Fenton reaction of oxidants with L-lysine, L-arginine, L-proline, L-threonine | PC | [54] | |
| Proteins and lipids | Michael-addition of aldehydic lipid oxidation products to L-lysine, L-cysteine, L-histidine | PC | [54] |
| Proteins and lipids | Complex oxidative process | oxLDL | [55] |
| Proteins and carbohydrates | Glyco-oxidation between L-lysine amino groups and L-arginine carbonyl groups linked to carbohydrates | AGEs (N-ε-carboxymethyl-lysine pentosidine glucosepane) | [56] |
| Lipids | Hydroxyl and peroxyl radicals-mediated lipid peroxidation of poly-unsaturated fatty acids (linoleic, arachidonic acids) | 4-HNE MDA F2-IsoPs | [53] |
| DNA | Mutagenic oxidation | 2-hydroxy adenine 8-oxoadenine 5-hydroxycytosine Cytosine glycol Thymine Glycol 8-oxoGuo 8-oxodG | [57] |
| Endogenous Molecules | Actions | Exogenous Molecules | Effect | |
|---|---|---|---|---|
| Not enzymatic | Vitamin E Vitamin C carotenes Ferritin Ceruloplasmin selenium GSH manganese Ubiquinone zinc Flavonoids coenzyme Q Melatonin Bilirubin taurine cysteine Albumin Uric acid | Interact with RONS and terminate the free radical chain reactions | Vitamin C | ↓O2• ↓•OH |
| Vitamin E | ↓Lipid peroxidation | |||
| Resveratrol Phenolic acids Flavonoids | ↓O2• ↓•OH ↓Lipid peroxidation | |||
| Oil lecithin | ↓O2• ↓•OH ↓Lipid peroxidation | |||
| Selenium Zinc | Antioxidant | |||
| Acetylcysteine | Antioxidant | |||
| Enzymatic | SOD | Converts O2 to H2O2 ↓hydroxyl radical production | ||
| Catalase (CAT) | Decomposes H2O2 to H2O+O2 ↓hydroxyl radical production | |||
| GSH-Px | Converts peroxides and hydroxyl radicals into nontoxic forms by the oxidation of reduced glutathione (GSH) into glutathione disulphide | |||
| GR | Converts glutathione disulphide to GSH | |||
| GSTs | Catalyze the conjugation of GSH to xenobiotic substrate detoxification | |||
| G6PD | Catalyzes the dehydrogenation of glucose-6-phosphate to 6-phosphoglucono-Δ-lactone | |||
| Nrf2 | Regulates the expression of antioxidant proteins | |||
| ARE | Encodes for detoxification enzymes and cytoprotective proteins | |||
| NQO1 | Catalyzes the two-electron reduction of quinones and quinonoid compounds to hydroquinones | |||
| MSR | Carries out the enzymatic reduction of the oxidized form of methionine to methionine | |||
| Physicochemical Identifiers | Descriptive Data |
|---|---|
| Chemical Name 1 | Ellagic Acid |
| CAS number | 476-66-4 |
| Molecular formula | C14H6O8 |
| Molecular weight | 302.194 g/mol |
| Hydrogen bond donor count | 4 |
| Hydrogen bond acceptor count | 8 |
| Covalently bonded unit count | 1 |
| Form/color | Cream colored needles from pyridine Yellow powder |
| Melting point | >360 °C |
| Density | 1.667 at 18 °C |
| Dissociation constants | pKa1 = 6.69 (phenol) pKa2 = 7.45 (phenol) pKa3 = 9.61 (phenol) pKa4 = 11.50 (phenol) |
| Solubility | Slightly soluble in alcohol and water Insoluble in ether Soluble in alkalis and pyridine |
| Vapor pressure | 2.81×10−15 mm Hg at 25 °C |
| Spectral properties | UV max (ethanol): 366, 255 nm |
| Plant Species | Family | Ref. |
|---|---|---|
| Acalypha hispida Burm.f. | Euphorbiaceae | [69] |
| Acca sellowiana (O.Berg) Burret 1 | Myrtaceae | [70] |
| Baccharis inamoena Gardner 1 | Compositae | [71] |
| Camellia nitidissima C.W.Chi 1 | Theaceae | [72] |
| Campomanesia adamantium (Cambess.) O.Berg | Myrtaceae | [73] |
| Canarium album (Lour.) DC. 1 | Burseraceae | [74] |
| Carpobrotus edulis (L.) N. E.Br. | Aizoaceae | [75] |
| Castanea crenata Sieb. and Zucc. | Fagaceae | [76] |
| Clematis ispahanica Boiss. | Ranunculaceae | [77] |
| Clematis orientalis L. | Ranunculaceae | [77] |
| Clerodendrum infortunatum L. 1 | Lamiaceae | [78] |
| Cornus officinalis Siebold and Zucc. | Cornaceae | [79] |
| Elaeagnus rhamnoides (L.) A.Nelson 1 | Elaeagnaceae | [80] |
| Euterpe edulis Mart. | Arecaceae | [81] |
| Eugenia uniflora L. | Myrtaceae | [82] |
| Euphorbia pekinensis Rupr. | Euphorbiaceae | [83] |
| Geum urbanum L. | Rosaceae | [84] |
| Gymnanthes lucida Sw. 1 | Euphorbiaceae | [85] |
| Juglans regia L. | Juglandaceae | [86] |
| Lafoensia pacari A. St.-Hil. | Lythraceae | [87] |
| Myrciaria floribunda (H.West exWilld.) O.Berg | Myrtaceae | [88] |
| Myrtus communis L. | Myrtaceae | [89] |
| Nephelium lappaceum L. | Sapindaceae | [90] |
| Pandiaka angustifolia (Vahl) Hepper | Amaranthaceae | [91] |
| Phyllanthus acuminatus Vahl | Phyllanthaceae | [92] |
| Pleurotus eryngii (DC. ex Fr.) Quel | Pleurotaceae | [93] |
| Plinia cauliflora (Mart.) Kausel 1 | Myrtaceae | [94] |
| Plinia coronata (Mattos) Mattos 1 | Myrtaceae | [95] |
| Plinia peruviana (Poir.) Govaerts | Myrtaceae | [96] |
| Potentilla anserina L. | Rosaceae | [97] |
| Psidium brownianum | Myrtaceae | [98] |
| Quassia undulata (Guill. and Perr.) D.Dietr. | Simaroubaceae | [99] |
| Tetrapleura tetraptera (Schum. and Thonn.) Taub. | Leguminosae | [99] |
| Salacia chinensis L. | Celastraceae | [100] |
| Sambucus lanceolata R.Br. | Adoxaceae | [101] |
| Sanguisorba officinalis L. | Rosaceae | [102] |
| Sedum roseum (L.) Scop. 1 | Crassulaceae | [103] |
| Sterculia striataA. St.-Hil. and Naudin | Malvaceae | [104] |
| Syzygium calophyllifolium (Wight) Walp. | Myrtaceae | [105] |
| Syzygium cumini (L.) Skeels | Myrtaceae | [106] |
| Terminalia chebula Retz. | Combretaceae | [107] |
| Food/Fruits | EA Content | Ref. |
|---|---|---|
| Red raspberry (Ottawa) | 70.8 ± 2.8 mg/100 g | [108] |
| Blackberries | 150.0 ± 12.0 mg/100 g | [109] |
| Cranberry | 12.0 ± 0.4 mg/100 g | [109] |
| Blackberries | 150.0 ± 12.0 mg/100 g | [109] |
| Arctic bramble | 390 mg/100 g | [110] |
| Raspberry (wild) | 270 mg/100 g | [110] |
| Yellow raspberry | 1900 mg/100 g | [110] |
| Black raspberries | 90 mg/100 g | [111] |
| Boysenberries | 70 mg/100 g | [111] |
| Evergreen blackberries | 60 mg/100 g | [111] |
| Marionberries | 73 mg/100 g | [111] |
| Cloudberry | 1090–1423 mg/100 g | [108] |
| 360 mg/100 g | [110] | |
| 315.1 mg/100 g | [112] | |
| Raspberry | 1692–1794 mg/100 g | [108] |
| 150.0 ± 10.0 mg/100 g | [109] | |
| 263.7 mg/100 g | [112] | |
| Rose hip | 109.6 mg/100 g | [112] |
| Sea buckthorn | 1.0 mg/100 g | [112] |
| Strawberry, Honeoye | 77.6 mg/100 g | [112] |
| Strawberry, Polka | 68.3 mg/100 g | [112] |
| Kakadu | ||
| Puree–2014 (month 0) | 1496 ± 76 mg/100 g | [113] |
| Puree–2014 (month 3) | 1165 ± 24 mg/100 g | [113] |
| Whole fruit–2014 (month 0) | 1726 ± 334 mg/100 g | [113] |
| Whole fruit–2014 (month 3) | 1214 ± 192 mg/100 g | [113] |
| Average whole fruit (indiv.) | 976 ± 223 mg/100 g | [113] |
| Average leaves (indiv.) | 5848 ± 1046 mg/100 g | [113] |
| Pomegranate seeds pulp | 980–2960 mg/100 g | [114] |
| Pomegranate dry peels | 8300–26,300 mg/100 g (pressurized water extraction) | [115] |
| 137–6310 mg/100 g (different solvents) | [116] | |
| Pomegranate whole fruit | 26–2497 mg/100 g (different solvents) | [116] |
| Pomegranate marcs 1 | 216–352 mg/100 g (different methods) | [117] |
| Red raspberry | 103.0 ± 3.3 mg/100 g | [109] |
| 47 mg/100 g | [111] | |
| 160 mg/100 g | [110] | |
| Strawberries | 81–184 mg/100 g | [108] |
| 63.0 ± 9.0 mg/100 g | [109] | |
| 65–85 mg/100 g | [110] | |
| 31 mg/100 g | [118] | |
| Strawberry, Jonsok | 79.9 mg/100 g | [112] |
| 40.3 ± 7.5 mg/100 g | [119] | |
| Strawberry jam | 24.5 mg/100 g | [112] |
| 17–29.5 mg/100 g | [120] | |
| Nuts | ||
| Pecan | 330.3 mg/100 g | [109] |
| Walnuts | 590.1 mg/100 g | [109] |
| Beverages | ||
| Cognac | 31–55 mg/L | [121] |
| Oak-age red wine | 0.0094 mg/L | [122] |
| Whiskey | 1.2 mg/L | [122] |
| Seeds | ||
| Marionvblackberry | 3200 mg/100 g | [123] |
| Red raspberries | 870 mg/100 g | [123] |
| Black raspberries | 670 mg/100 g | [123] |
| Evergreen blackberry | 2100 mg/100 g | [123] |
| Boysenberries | 3000 mg/100 g | [123] |
| Longan | 160 mg/100 g | [124] |
| Mango | 120 mg/100 g | [124] |
| Action Mechanism | Chemical Equation | Features | Natural Compounds [128] |
|---|---|---|---|
| Type I | |||
| HAT | HnAntiox + •R → Hn−1Antiox• + HR | A key reaction mechanism | Polyphenols EA |
| PCET | HnAntiox + •R → Hn−1Antiox• + H+ + • → HR | Exactly the same products as HAT | Flavonoids Quinone-hydroquinone |
| RAF | HnAntiox + •R → [HnAntiox-R]• | Presence of multiple bonds peculiar of electrophilic radicals | Carotenoids Gentisic acid Rebamipide Hydroxybenzyl alcohols |
| SET | HnAntiox + •R → HnAntiox+• + R− | Primary pathway | EA Curcumin Carotenoids Catechins Edaravone Resveratrol |
| HnAntiox + •R → HnAntiox+ −• + R+ | Secondary pathway | Xanthones Carotenoids Trolox Caffeic acid Genistein | |
| SPLET | HnAntiox → Hn−1Antiox− + H+ Hn−1Antiox− + •R → Hn−1Antiox• + R− | Crucial mechanism in the scavenging activity in polar environments | Trolox Curcumin Vitamin E Quercetin Epicatechin Piceatannol Resveratrol Kaempferol Esculetin Fraxetin Morin Hydroxybenzoic Dihydroxybenzoic Flavonoids Isoflavonoids Xanthones Procyanidins Edaravone GA Erodiol |
| SEPT | (1) HnAntiox + •R → Hn−1Antiox•+ + R− (2) Hn−1Antiox•+ → Hn−1Antiox• + H+ | A two-step mechanism involving electron transfer and deprotonation as in SPLET but in a different order | Vitamin E Galvinoxyl α-tocopherol Baicalein Astaxanthin Quercetin |
| SPLHAT | (1) HnAntiox → Hn−1Antiox− + H+ (2) Hn−1Antiox− + •R → Hn−2Antiox•− + HR | Deprotonation of the antioxidant and an H transfer reaction | EA Anthocyanidins GA Esculetin α-Mangostin Propyl gallate |
| Property Name | URO A | URO B |
|---|---|---|
| Molecular weight | 228.2 g/mol | 212.2 g/mol |
| XLogP3-AA | 2.3 | 2.7 |
| Hydrogen bond donor count | 2 | 1 |
| Hydrogen bond acceptor count | 4 | 3 |
| Rotatable bond count | 0 | 0 |
| Exact mass | 228.0 g/mol | 212.0 g/mol |
| Monoisotopic mass | 228.0 g/mol | 212.0 g/mol |
| Topological polar surface area | 66.8 Ų | 46.5 Ų |
| Heavy atom count | 17 | 16 |
| Formal charge | 0 | 0 |
| Complexity | 317 | 289 |
| Isotope atom amount | 0 | 0 |
| Defined atom stereocenter count | 0 | 0 |
| Undefined atom stereocenter count | 0 | 0 |
| Defined bond stereocenter count | 0 | 0 |
| Undefined bond stereocenter count | 0 | 0 |
| Covalently bonded unit count | 1 | 1 |
| Compound is canonicalized | Yes | Yes |
| Mammalian | Source | URO Type |
|---|---|---|
| Rat (Rattus norvegicus) 1 | Pomegranate husk 1 | A, B, C 1 |
| Rat (Rattus norvegicus) 1 | Ellagic acid 1 | A 1 |
| Rat (Rattus norvegicus) 1 | Oak-flavored milk 1 | A, B, C 1 |
| Rat (Rattus norvegicus) 1 | Pomegranate extract 1 | A, M-6, M-7 1 |
| Rat (Rattus norvegicus) 1 | Geraniin (Geranium thunbergii) 1 | M-5 1 |
| Mouse (Mus musculos) 1 | Pomegranate extract 1 | A 1 |
| Mouse (Mus musculos) 1 | Pomegranate husk 1 | A 1 |
| Baver (Castor canadensis) 1 | Wood 1 | A, B 1 |
| Complex toothed squirrel (Trogopterus xanthipes) 1 | Unknown 1 | A 1 |
| Sheep (Ovis Aries) 1 | Trifoleum Subterraneum1 | A, B 1 |
| Sheep (Ovis Aries) | Quebracho 1 | A 1 |
| Cattle (Bos primigenius) 1 | Young oak leaves 1 | A, Iso A, B |
| Pig (Sus scrofa domesticus) 1 | Acorns 1 | A, C, D, B |
| Humans (Homo Sapiens) 1 | Pomegranate juice 1 | A, C, Iso A, B |
| Humans (Homo Sapiens) 1 | Pomegranate extract 1 | A, B, C |
| Humans (Homo Sapiens) 1 | Walnuts 1 | A, B, C |
| Humans (Homo Sapiens) 1 | Strawberry 1 | A, C, Iso A, B |
| Humans (Homo Sapiens) 1 | Raspberry 1 | A, C, Iso A, B |
| Humans (Homo Sapiens) 2 | Blackberry 2 | A, C 2 |
| Humans (Homo Sapiens) 3 | Cloudberry 3 | A 3 |
| Humans (Homo Sapiens) 1 | Oak-aged red wine 1 | A 1 |
| Humans (Homo Sapiens) 1 | Tea 1 | A 1 |
| Humans (Homo Sapiens) 1 | Nuts 1 | A, Iso A, B 1 |
| Models N° Tests for Animal Type | Biological Fluid | Tissue | UROs Type in Biological Fluids | UROs Type in Tissues |
|---|---|---|---|---|
| Animals 1 Tests = 20 | Urine | Kidney | A, A-glur, A-3-glur, B, C, B-sulfate, B-glur, C, D-derivatives, Iso A-3-glur, Iso A-9-glur, Iso B-glur, A-sulfate, Iso A-sulfate, A-glur-sulfate, Iso A-sulfo-glur, A-diglur, Iso A-diglur, nasutin-A-glur, Isonasutin-A-glur, trihydroxy-urolithin-diglur, dihydroxy-urolithin derivative | M-7, A-glur, A, B, A-sulfate |
| Feces | Liver | A, Iso A, B, M-6, C, Nasutin, | A, M7, A-glur, A-sulfate, | |
| Plasma | Intestinal lumen | A, B, C, 8-methy-C, C, D-derivatives, A-glur, B-glur, nasutin-A-glur, trihydroxy-urolithin-diglur, trihydroxy-urolithin derivative, A-sulfate, Iso A-glur, Iso A-sulfate, B-sulfate, Iso A-solfo-glur | A, C, D, A-sulfate | |
| Blood | Intestinal tissue | A, C, D, A-glur, A-sulfate | ||
| Bile | Hurt | A, C, D, A-glur, nasutin-A-glur, Isonasutin-A-glur | B | |
| Ruminal fluid | Brain | A, Iso A, B, C, A-glur. | A, Methyl-A | |
| Beaver excretions | Lung | nasutin-A-glur | A, A-sulfate, A-glur, | |
| Prostate | A, A-sulfate, Methyl-A, A-glur | |||
| Colon | A, A-sulfate, Methyl-A, A-glur | |||
| Cecum | A, Nasutin | |||
| Stomach | Nasutin | |||
| Humans 2 Tests = 27 3 | Urine | Prostate | A, B, C, M-5, D, B-sulfate, A-glur, Iso A, Iso A-glur, B-glur, A-3-glur, A-8-glur, trihydroxyurolithin, A-sulfate, Methyl-A, A-sulfo-glur, D-methyletherglur, A-diglur, Dimethy-C, C-glur, D-glur, C-methylethersulfoglur, Methyl-C-glur, Methyl-D-glur, C-sulfate, | A, A-glur, B-glur, |
| Feces | Normal and malignant colon tissue | A, B, C, D, E, M-5, M-6, M-7, Iso A | A-glur, D, Iso A-glur, A-sulfate, C, B-glur, A, Iso A, B-sulfate, B, M-5, M-6 | |
| Plasma | A, B, D, A-sulfate, B-sulfate, trihydroxyurolithin, A-glur, Iso A-glur, B-glur, C, Methyl-A, C-glur, Methyl-C-methyletherglur, C-diglur, D-glur, A-sulfo-glur, C-sulfate, IsoA-sulfate, |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Marengo, B.; Zuccari, G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants 2020, 9, 707. https://doi.org/10.3390/antiox9080707
Alfei S, Marengo B, Zuccari G. Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants. 2020; 9(8):707. https://doi.org/10.3390/antiox9080707
Chicago/Turabian StyleAlfei, Silvana, Barbara Marengo, and Guendalina Zuccari. 2020. "Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins?" Antioxidants 9, no. 8: 707. https://doi.org/10.3390/antiox9080707
APA StyleAlfei, S., Marengo, B., & Zuccari, G. (2020). Oxidative Stress, Antioxidant Capabilities, and Bioavailability: Ellagic Acid or Urolithins? Antioxidants, 9(8), 707. https://doi.org/10.3390/antiox9080707