COVID-19 Seroprevalence in Canada Modelling Waning and Boosting COVID-19 Immunity in Canada a Canadian Immunization Research Network Study
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Model Fit
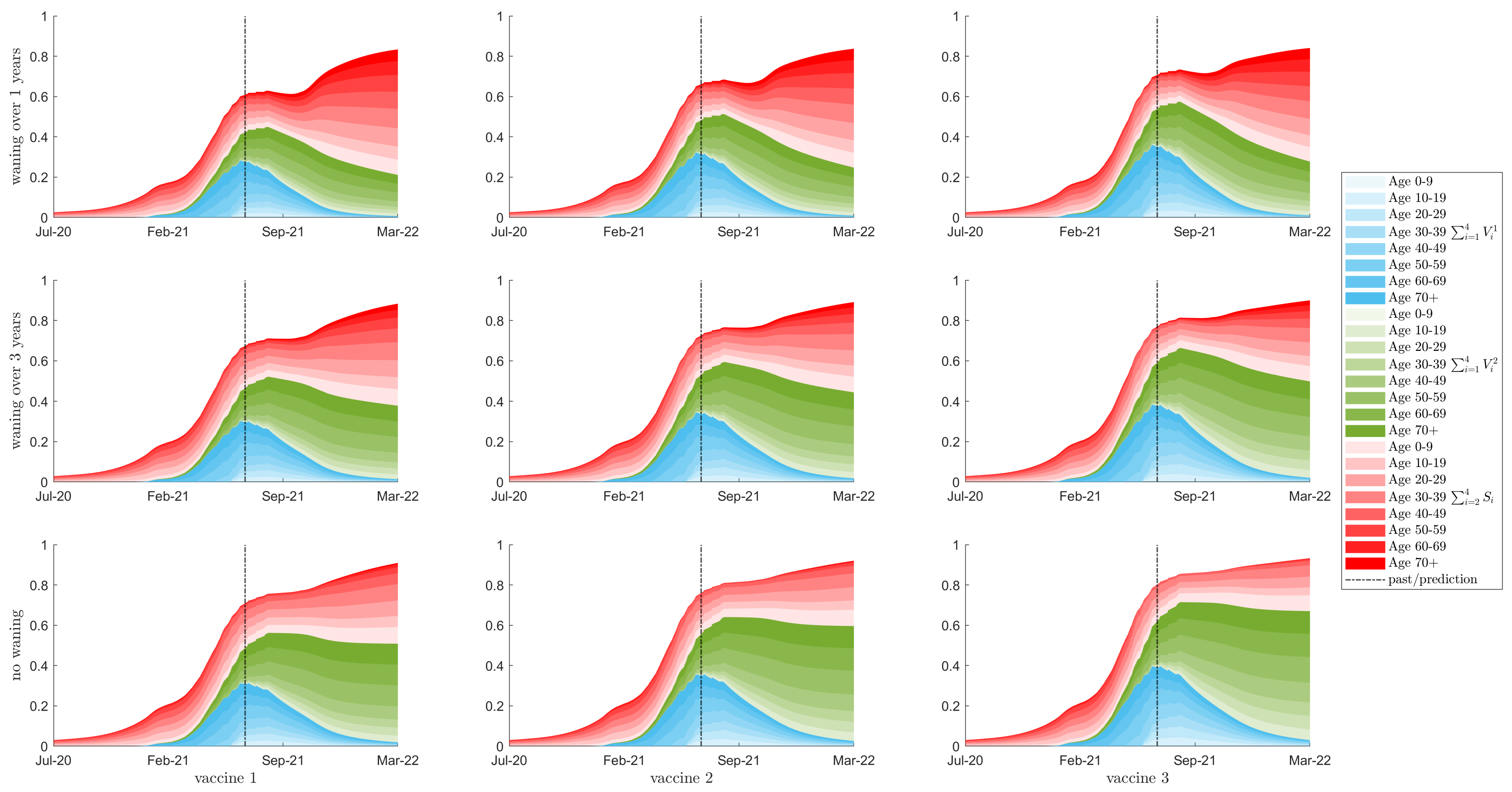
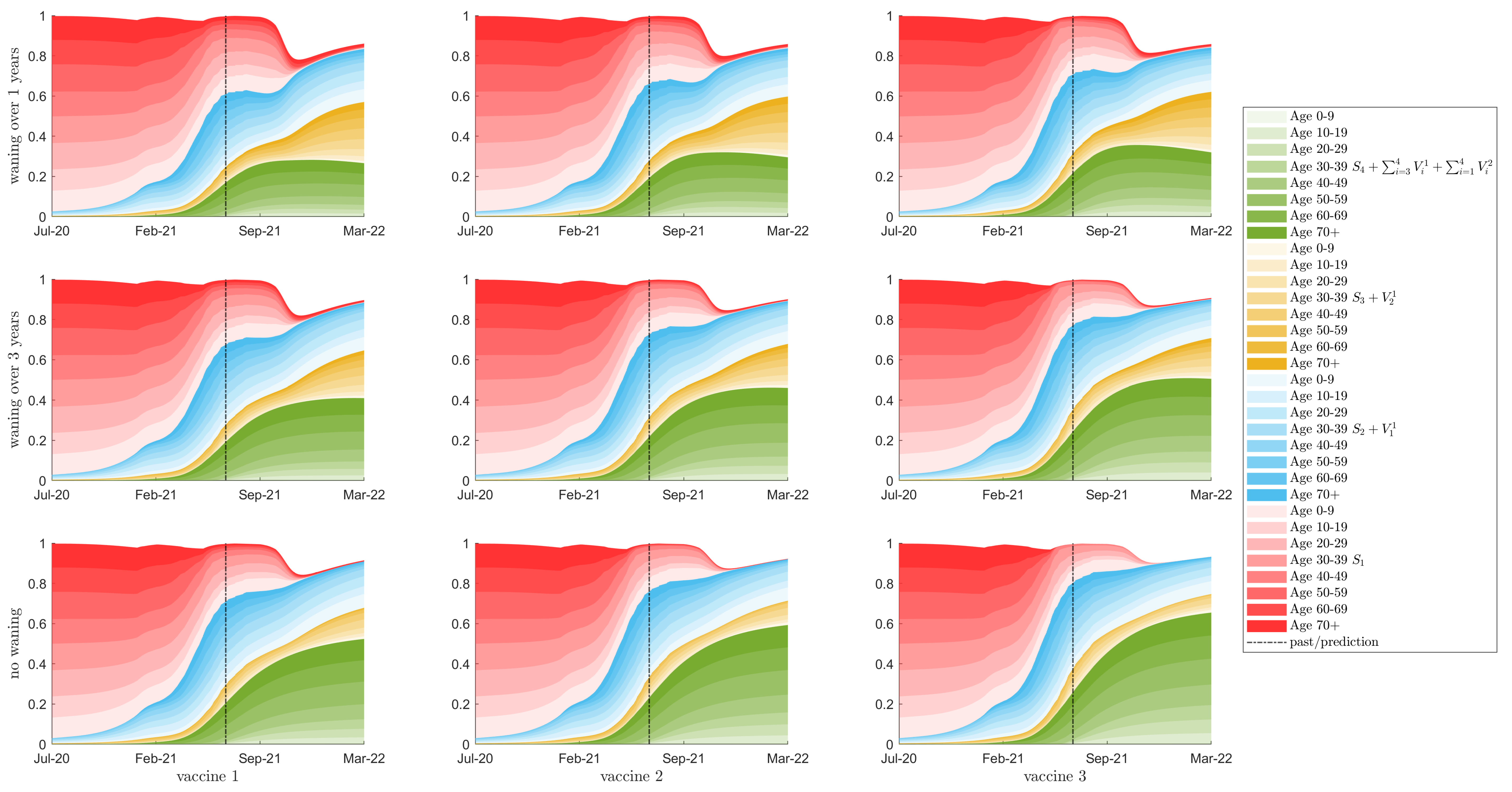
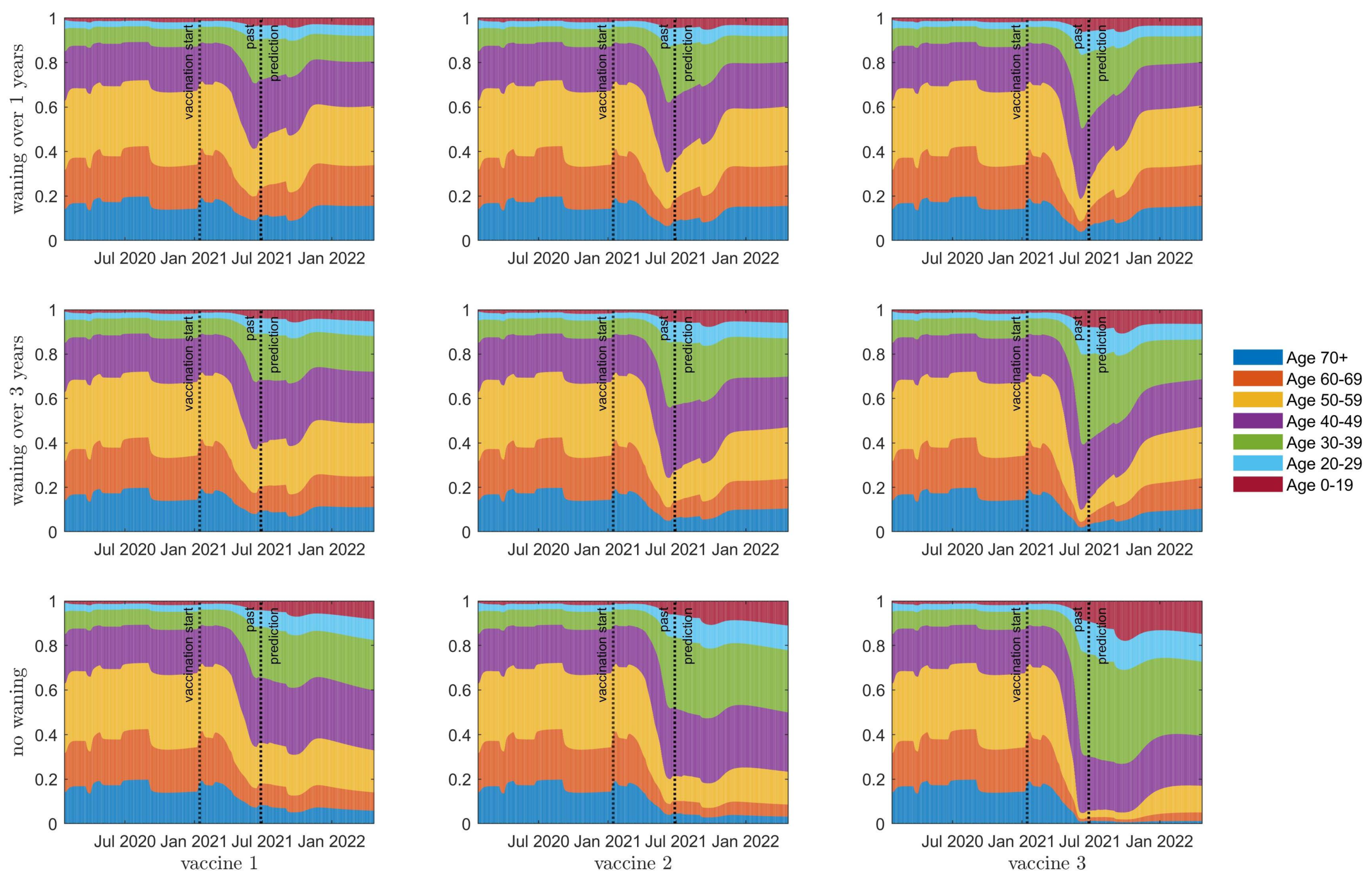
3.2. Seroprevalence
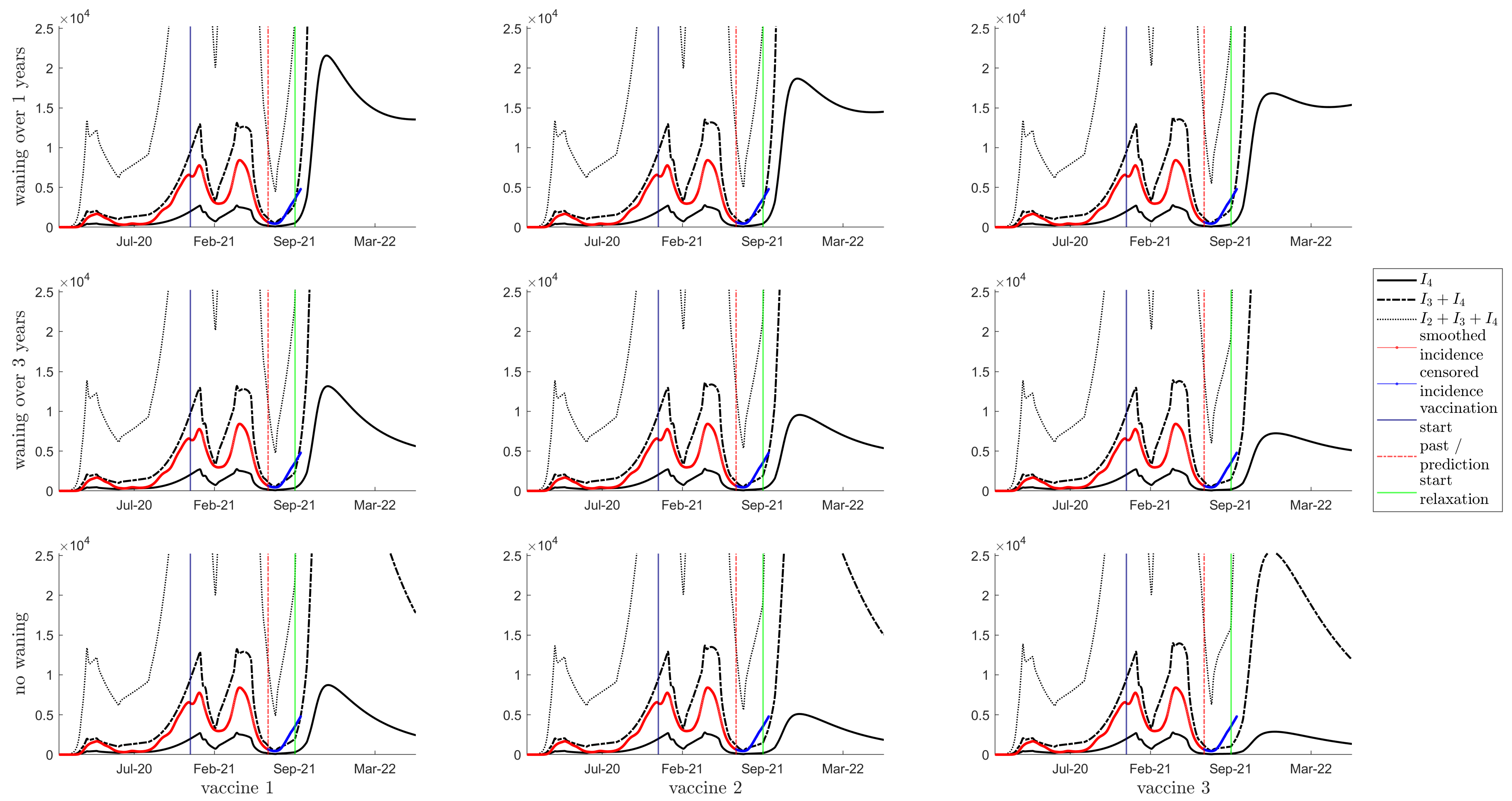
3.3. Herd Immunity
3.4. Resurgence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
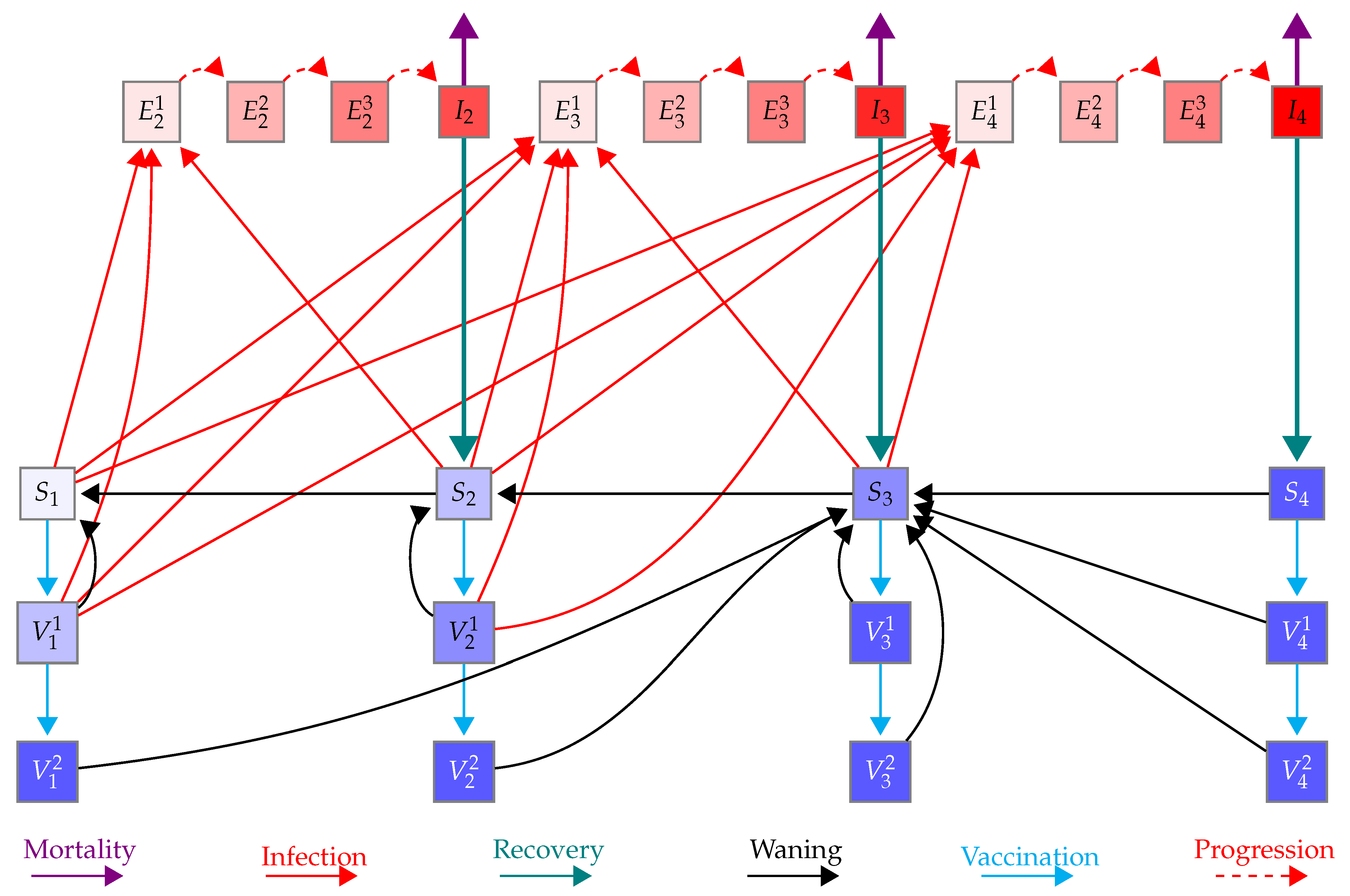
Appendix A.1. Model Equations
Appendix A.2. Reproduction Number
Appendix B. Parameters
| Parameter | Definition |
|---|---|
| susceptibility of individuals from (i immunity status, n age group) | |
| infectivity of infected individuals from (j immunity status, m age group) | |
| recovery rate of infected individuals from (j immunity status, m age group) | |
| rates of progress through the pre-infectious period of infeciton (i immunity status, n age group, k stage) | |
| disease-induced mortality rate of infected individuals from (j immunity status, m age group) | |
| waning rate of immunity of individuals from (i immunity status, n age group) | |
| proportion of going to upon infection with and ( immunity status, n age group) | |
| vaccine efficacy (i immunity status, n age group) | |
| vaccination rate for first and second dose | |
| per capita activity counts of individuals in age group n | |
| mixing matrix between individuals in age group a and age group n |
| Age Group | |||
|---|---|---|---|
| 0–4 | 0.979187625 | 0.019532353 | 0.001280022 |
| 5–9 | 0.970340674 | 0.02795418 | 0.001705146 |
| 10–14 | 0.971354725 | 0.026962603 | 0.001682673 |
| 15–19 | 0.963790465 | 0.033836424 | 0.002373111 |
| 20–24 | 0.94736408 | 0.048618385 | 0.004017534 |
| 25–29 | 0.923743993 | 0.069289774 | 0.006966234 |
| 30–34 | 0.897203802 | 0.09151371 | 0.011282488 |
| 35–39 | 0.865605311 | 0.116760519 | 0.01763417 |
| 40–44 | 0.806984827 | 0.162542801 | 0.030472373 |
| 45–49 | 0.756587998 | 0.197861718 | 0.045550284 |
| 50–54 | 0.690245134 | 0.241501371 | 0.068253495 |
| 55–59 | 0.600190415 | 0.296345592 | 0.103463993 |
| 60–64 | 0.503245046 | 0.346804634 | 0.14995032 |
| 65–69 | 0.409505408 | 0.383960071 | 0.206534521 |
| 70–74 | 0.324664092 | 0.404030163 | 0.271305745 |
| 75+ | 0.215150255 | 0.373779207 | 0.411070537 |
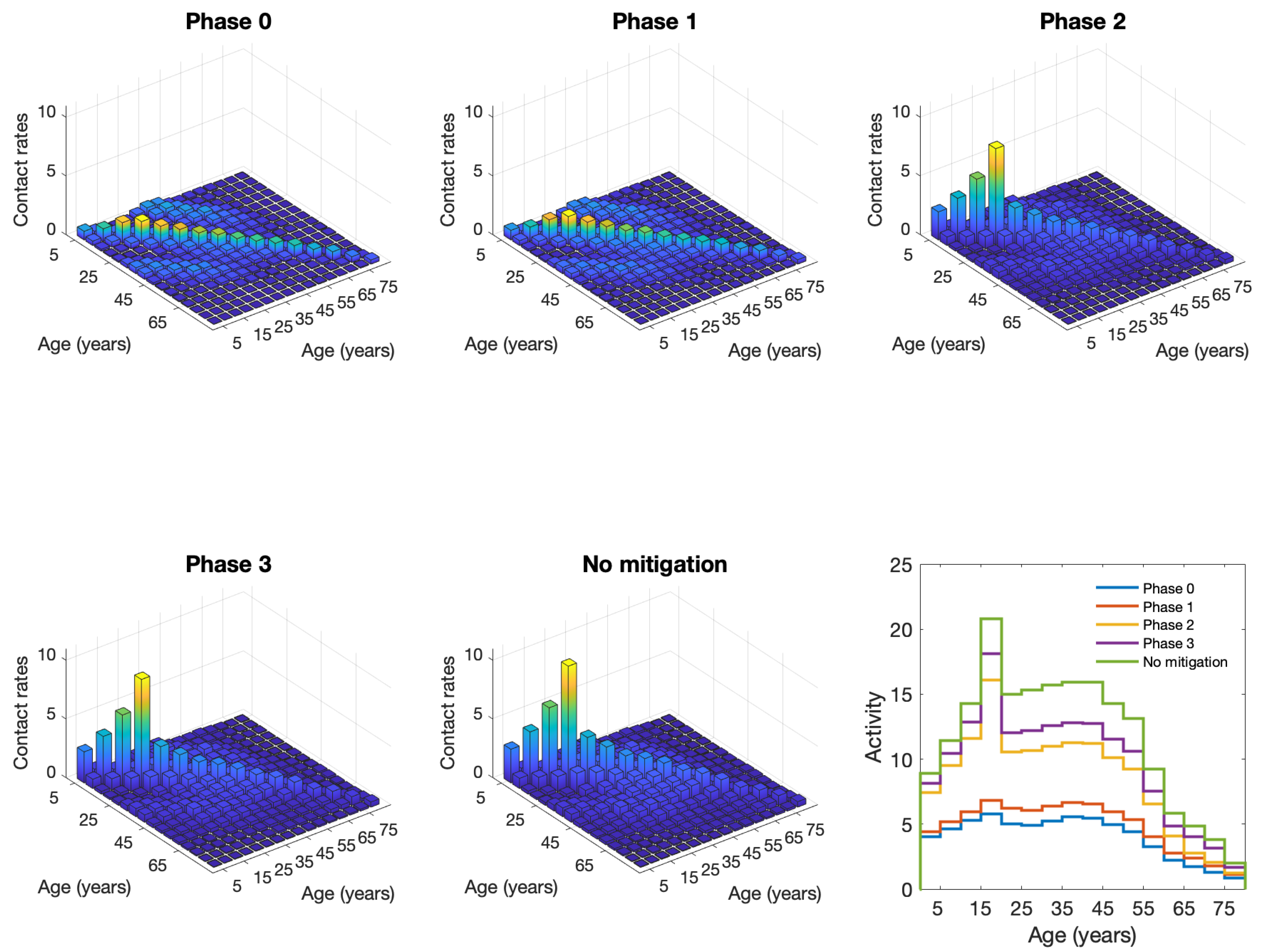
| Phase | School Contacts | Other Contacts | Work Contacts |
|---|---|---|---|
| 0 | 95% | 90% under 65, 95% over 65 | 75% under 65, 95% over |
| 1 | 95% | 75% | 70% under 65, 95% over |
| 2 | 95% | 40% under 65, 65% over | 70% under 65, 95% over |
| 3 | 15% under 20, 25% over | 40% under 65, 65% over | 35% under 65, 95% over |
| no mitigation | 0% | 0% | 0% |
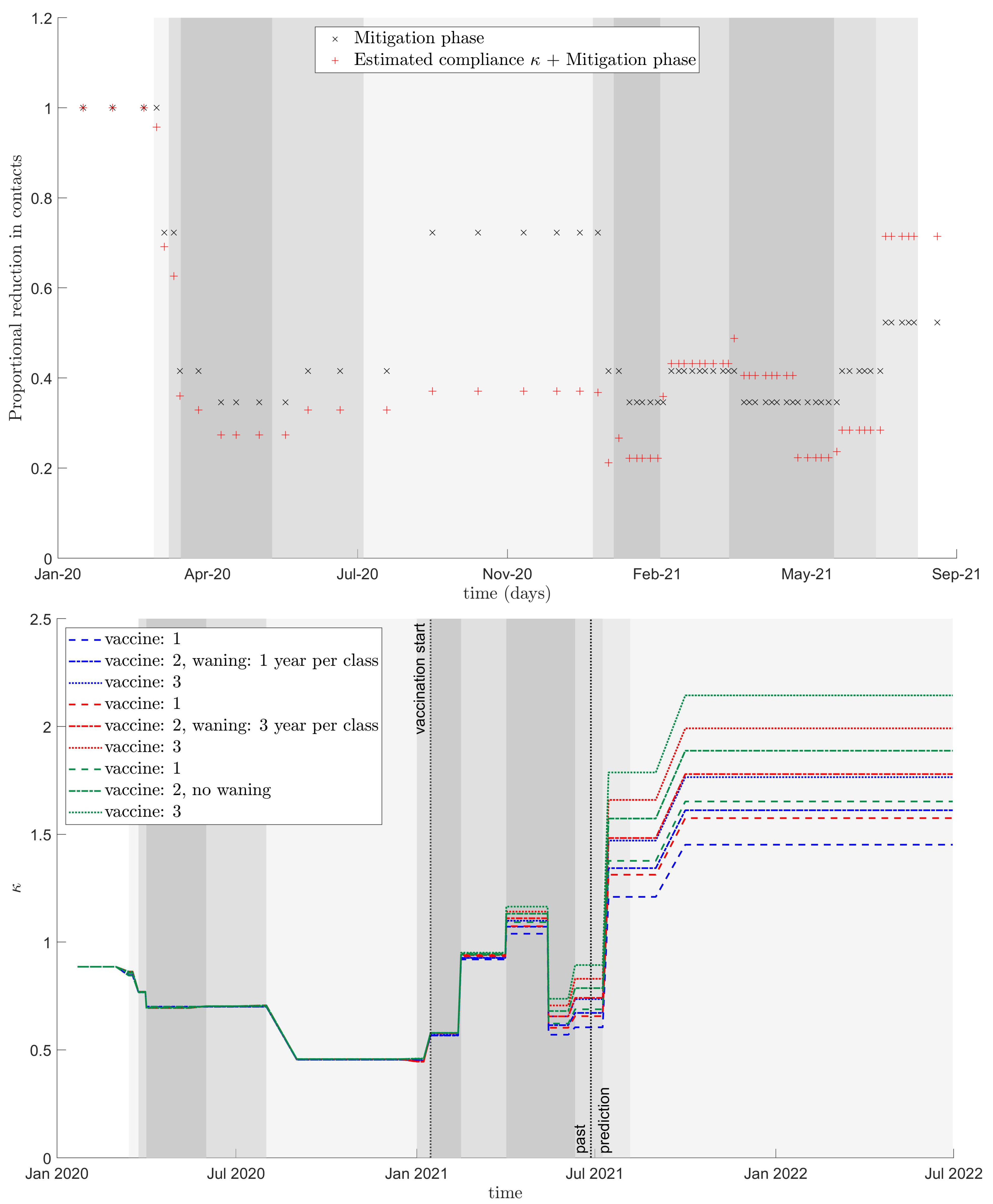
| Start | End | Phase |
|---|---|---|
| 2020-02-05 | 2020-03-18 | no mitigation |
| 2020-03-18 | 2020-03-31 | 3 |
| 2020-03-31 | 2020-04-25 | 1 |
| 2020-04-25 | 2020-06-19 | 0 |
| 2020-06-19 | 2020-09-01 | 1 |
| 2020-09-01 | 2021-01-08 | 3 |
| 2021-01-08 | 2021-01-22 | 1 |
| 2021-01-22 | 2021-02-19 | 0 |
| 2021-02-19 | 2021-04-09 | 1 |
| 2021-04-09 | 2021-05-14 | 0 |
| 2021-05-14 | 2021-07-15 | 1 |
| 2021-07-15 | 2021-09-01 | 2 |
| 2021-09-01 | 2021-12-31 | 3 |
| 2021-09-01 | 2021-12-31 | 3 |
Appendix C. Model Fitting
References
- Lewin, A.; Therrien, R.; De Serres, G.; Grégoire, Y.; Perreault, J.; Drouin, M.; Fournier, M.J.; Tremblay, T.; Beaudoin, J.; Beaudoin-Bussières, G.; et al. SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: A nested case-control study. Can. J. Public Health 2021, 112, 576–586. [Google Scholar] [CrossRef]
- Saeed, S.; Drews, S.J.; Pambrun, C.; Yi, Q.L.; Osmond, L.; O’Brien, S.F. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion 2021, 61, 862–872. [Google Scholar] [CrossRef]
- Serotracker. Available online: https://serotracker.com/en/Explore (accessed on 12 July 2021).
- CITF. Task Force Funded Research; Technical Report. 2021. Available online: https://www.covid19immunitytaskforce.ca/task-force-research/ (accessed on 12 July 2021).
- CITF. Immunity Monitoring Report: Cumulative SARS-CoV-2 Seropositivity in Canada. Technical Report. 2021. Available online: https://www.covid19immunitytaskforce.ca (accessed on 12 July 2021).
- Childs, L.; Dick, D.W.; Feng, Z.; Heffernan, J.M.; Li, J.; Röst, G. Modeling waning and boosting of COVID-19 in Canada with vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Berry, I.; Soucy, J.P.R.; Tuite, A.; Fisman, D. Open access epidemiologic data and an interactive dashboard to monitor the COVID-19 outbreak in Canada. Can. Med. Assoc. J. 2020, 192, E420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Advisory Council on Immunzation, Canada. High Consequence Infectious Diseases Working Group on COVID-19 Vaccines: Modelling Scenarios and Assumptions. Available online: https://www.medrxiv.org/content/10.1101/2021.05.18.21257426v1.full.pdf (accessed on 6 November 2020).
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob. Health 2020, 8, E1003–E1017. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024–1042. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B. 1.1. 7 and B. 1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- Nasreen, S.; He, S.; Chung, H.; Brown, K.A.; Gubbay, J.B.; Buchan, S.A.; Wilson, S.E.; Sundaram, M.E.; Fell, D.B.; Chen, B.; et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. medRxiv 2021. [Google Scholar] [CrossRef]
- Emary, K.R.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B. 1.1. 7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y.; et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236. [Google Scholar] [CrossRef]
- Public Health England. Vaccines Highly Effective against B.1.617.2 Variant after 2 Doses; Technical Report 2021. Available online: https://www.gov.uk/government/news/vaccines-highly-effective-against-b-1-617-2-variant-after-2-doses (accessed on 23 November 2021).
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Collin, A.; Hejblum, B.P.; Vignals, C.; Lehot, L.; Thiébaut, R.; Moireau, P.; Prague, M. Using Population Based Kalman Estimator to Model COVID-19 Epidemic in France: Estimating the Effects of Non-Pharmaceutical Interventions on the Dynamics of Epidemic. medRxiv 2021. [Google Scholar] [CrossRef]
- Knpil, D.H.; Rost, G. Modelling the strategies for age specific vaccination scheduling during influenza pandemic outbreaks. Math. Biosci. Eng. 2010, 8, 123–139. [Google Scholar]
- Glasser, J.; Feng, Z.; Moylan, A.; Del Valle, S.; Castillo-Chavez, C. Mixing in age-structured population models of infectious diseases. Math. Biosci. 2012, 235, 1–7. [Google Scholar] [CrossRef]
- Feng, Z.; Hill, A.N.; Smith, P.J.; Glasser, J.W. An elaboration of theory about preventing outbreaks in homogeneous populations to include heterogeneity or preferential mixing. J. Theor. Biol. 2015, 386, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Han, Q.; Qiu, Z.; Hill, A.N.; Glasser, J.W. Computation of R in age-structured epidemiological models with maternal and temporary immunity. Discret. Contin. Dyn. Syst. Ser. B 2016, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- Glasser, J.W.; Feng, Z.; Omer, S.B.; Smith, P.J.; Rodewald, L.E. The effect of heterogeneity in uptake of the measles, mumps, and rubella vaccine on the potential for outbreaks of measles: A modelling study. Lancet Infect. Dis. 2016, 16, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Hill, A.N.; Curns, A.T.; Glasser, J.W. Evaluating targeted interventions via meta-population models with multi-level mixing. Math. Biosci. 2017, 287, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Poghotanyan, G.; Feng, Z.; Glasser, J.W.; Hill, A.N. Constrained minimization problems for the reproduction number in meta-population models. J. Math. Biol. 2018, 77, 1795–1831. [Google Scholar] [CrossRef]
- Feng, Z.; Feng, Y.; Glasser, J.W. Influence of demographically-realistic mortality schedules on vaccination strategies in age-structured models. Theor. Popul. Biol. 2020, 132, 24–32. [Google Scholar] [CrossRef]
- Carlsson, R.M.; Childs, L.M.; Feng, Z.; Glasser, J.W.; Heffernan, J.M.; Li, J.; Röst, G. Modeling the waning and boosting of immunity from infection or vaccination. J. Theor. Biol. 2020, 497, 110265. [Google Scholar] [CrossRef]
- Heffernan, J.M.; Keeling, M.J. Implications of vaccination and waning immunity. Proc. R. Soc. B Biol. Sci. 2009, 276, 2071–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhatwal, J.; Mueller, P.C.P.; Ayer, T.; Adee, M.G.; Dalgic, O.; Ladd, M.A.; Linas, B. Changing Dynamics of COVID-19 in the US with the Emergence of the Delta Variant: Projections of the COVID-19 Simulator. medRxiv 2021. [Google Scholar] [CrossRef]
- Public Health Agency of Canada/National Microbiology Lab. COVID-19: PHAC Modelling Group Report. Public Health Agency of Canada/National Microbiology Lab. Available online: https://nccid.ca/wp-content/uploads/sites/2/2021/01/Modelling-Group-report-2020_01_14_Final.pdf (accessed on 25 February 2021).
- Heffernan, J.M.; Smith, R.J.; Wahl, L.M. Perspectives on the basic reproductive ratio. J. R. Soc. Interface 2005, 2, 281–293. [Google Scholar] [CrossRef]
- Van den Driessche, P.; Watmough, J. Further notes on the basic reproduction number. In Mathematical Epidemiology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 159–178. [Google Scholar]
- Diekmann, O.; Heesterbeek, J.; Roberts, M.G. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface 2010, 7, 873–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, J.; Mishra, S. Estimating effective reproduction number using generation time versus serial interval, with application to COVID-19 in the greater Toronto area, Canada. Infect. Dis. Model. 2020, 5, 889–896. [Google Scholar] [CrossRef]
| Against Infection | Against Severe Disease | ||
|---|---|---|---|
| Two Doses | First Dose | ||
| Vaccine 1 | 70% | 50% | 75% |
| Vaccine 2 | 80% | 70% | 80% |
| Vaccine 3 | 90% | 70% | 92% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dick, D.W.; Childs, L.; Feng, Z.; Li, J.; Röst, G.; Buckeridge, D.L.; Ogden, N.H.; Heffernan, J.M. COVID-19 Seroprevalence in Canada Modelling Waning and Boosting COVID-19 Immunity in Canada a Canadian Immunization Research Network Study. Vaccines 2022, 10, 17. https://doi.org/10.3390/vaccines10010017
Dick DW, Childs L, Feng Z, Li J, Röst G, Buckeridge DL, Ogden NH, Heffernan JM. COVID-19 Seroprevalence in Canada Modelling Waning and Boosting COVID-19 Immunity in Canada a Canadian Immunization Research Network Study. Vaccines. 2022; 10(1):17. https://doi.org/10.3390/vaccines10010017
Chicago/Turabian StyleDick, David W., Lauren Childs, Zhilan Feng, Jing Li, Gergely Röst, David L. Buckeridge, Nick H. Ogden, and Jane M. Heffernan. 2022. "COVID-19 Seroprevalence in Canada Modelling Waning and Boosting COVID-19 Immunity in Canada a Canadian Immunization Research Network Study" Vaccines 10, no. 1: 17. https://doi.org/10.3390/vaccines10010017
APA StyleDick, D. W., Childs, L., Feng, Z., Li, J., Röst, G., Buckeridge, D. L., Ogden, N. H., & Heffernan, J. M. (2022). COVID-19 Seroprevalence in Canada Modelling Waning and Boosting COVID-19 Immunity in Canada a Canadian Immunization Research Network Study. Vaccines, 10(1), 17. https://doi.org/10.3390/vaccines10010017






