Multi-Targeted Approaches and Drug Repurposing Reveal Possible SARS-CoV-2 Inhibitors
Abstract
:1. Introduction
2. Methodology
2.1. Protein Structure Modelling and Preparation
2.2. Nutraceuticals (Ligands) Preparation
2.3. Docking Studies
2.4. Docking Studies Using Glide
2.5. Docking Studies Using GOLD
2.6. Docking Studies Using AutoDock Vina
2.7. Interaction Analyses
2.8. Normal Mode Analyses
3. Results and Discussion
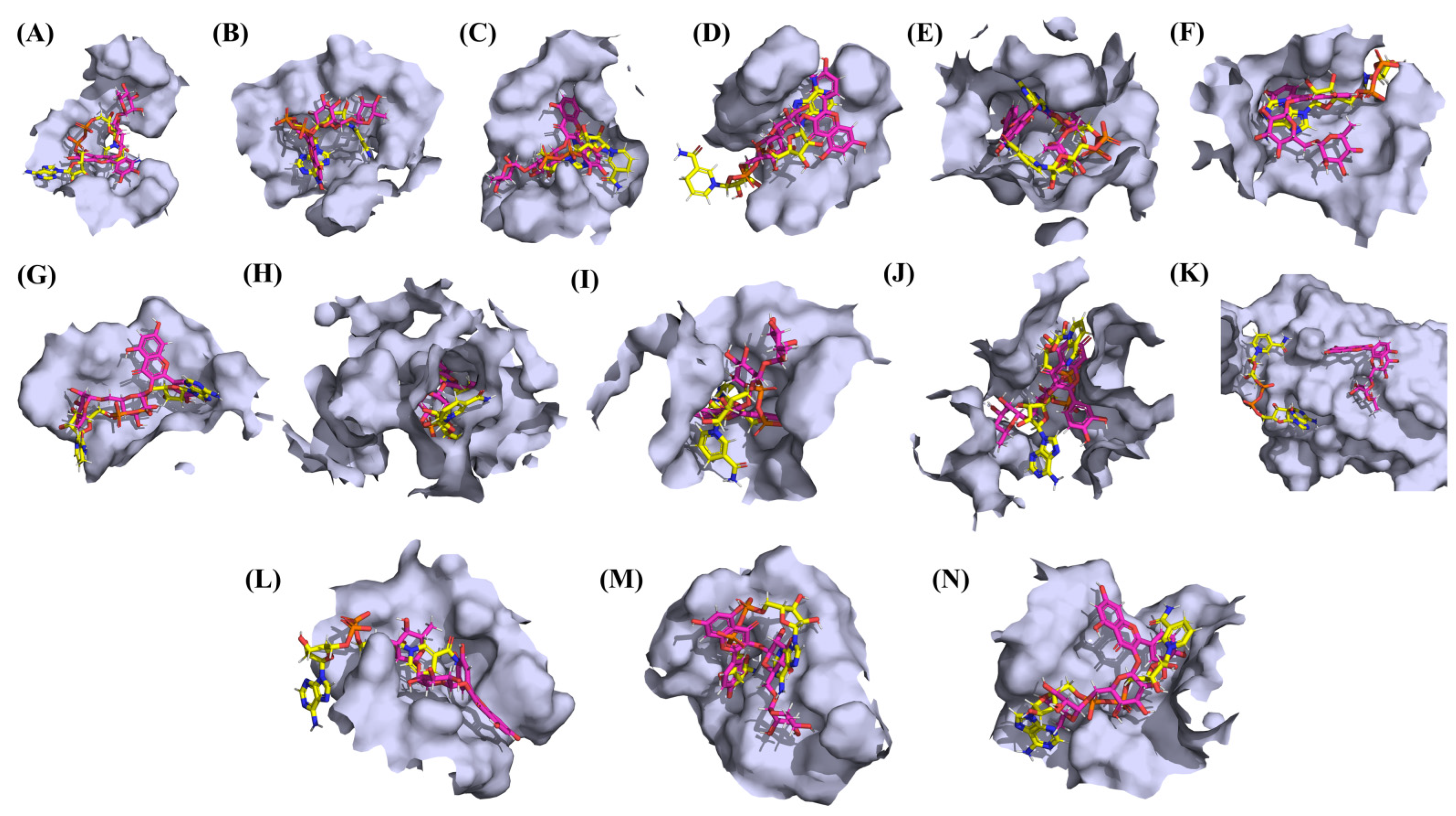
3.1. Molecular Docking Analyses
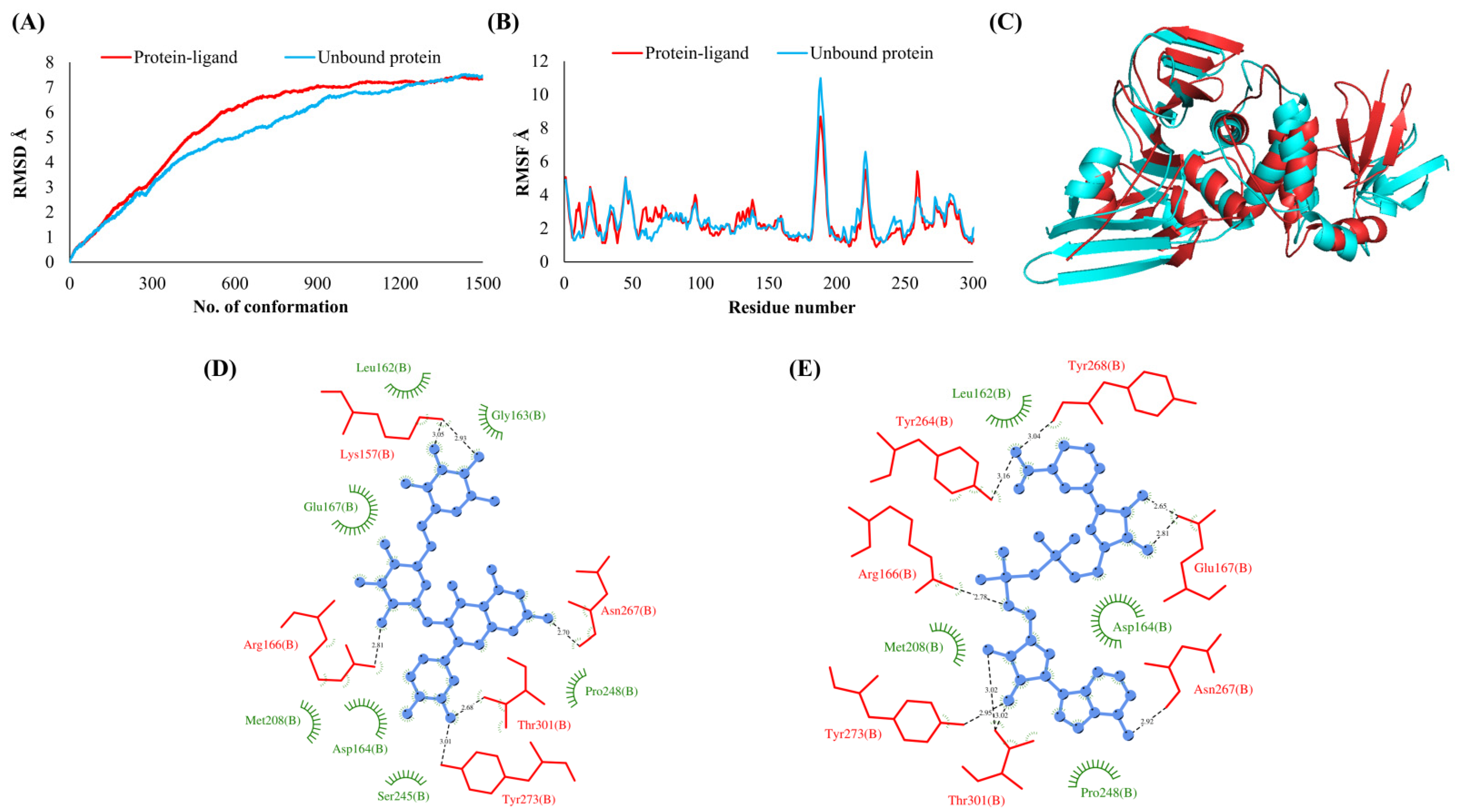
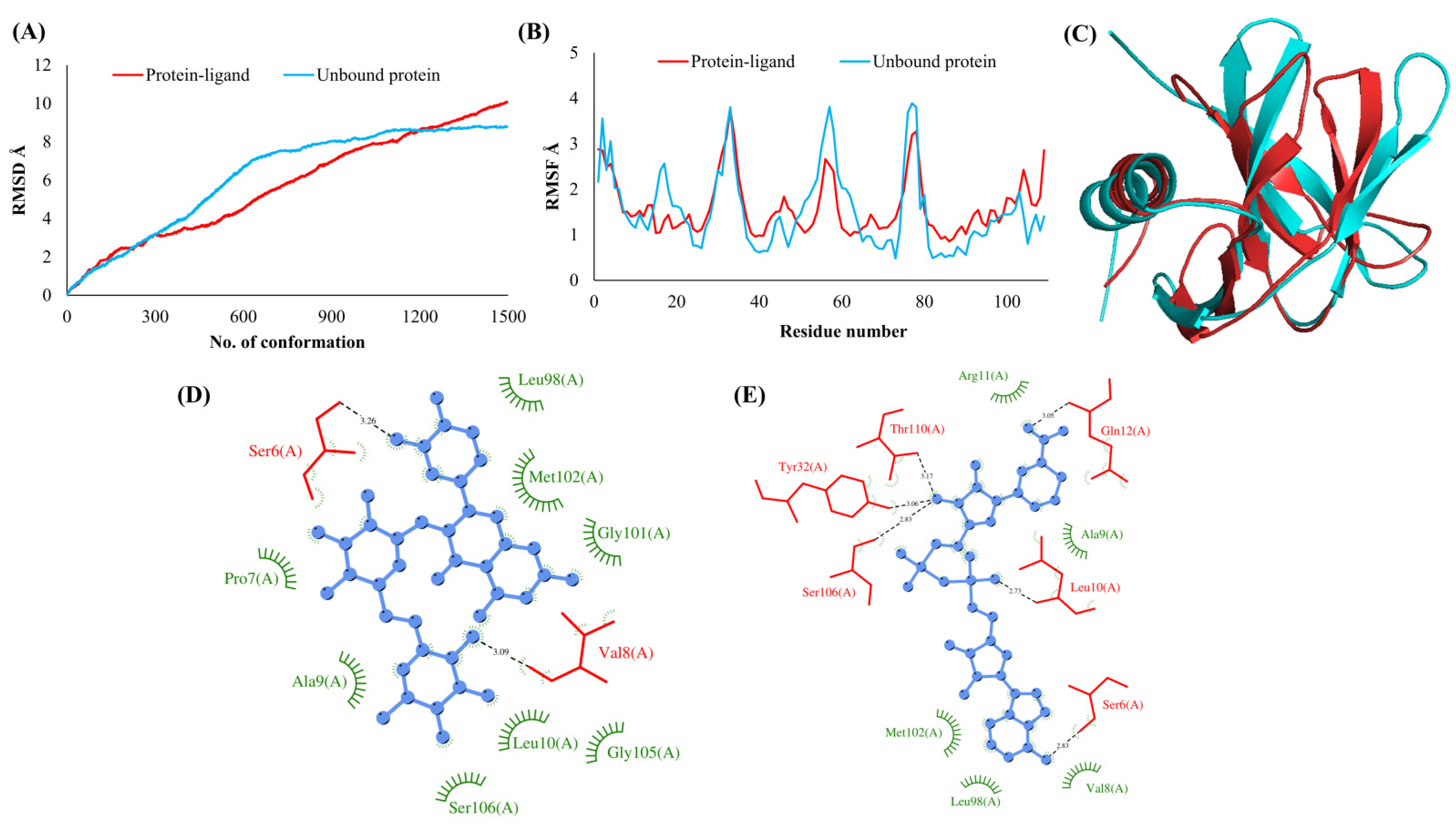
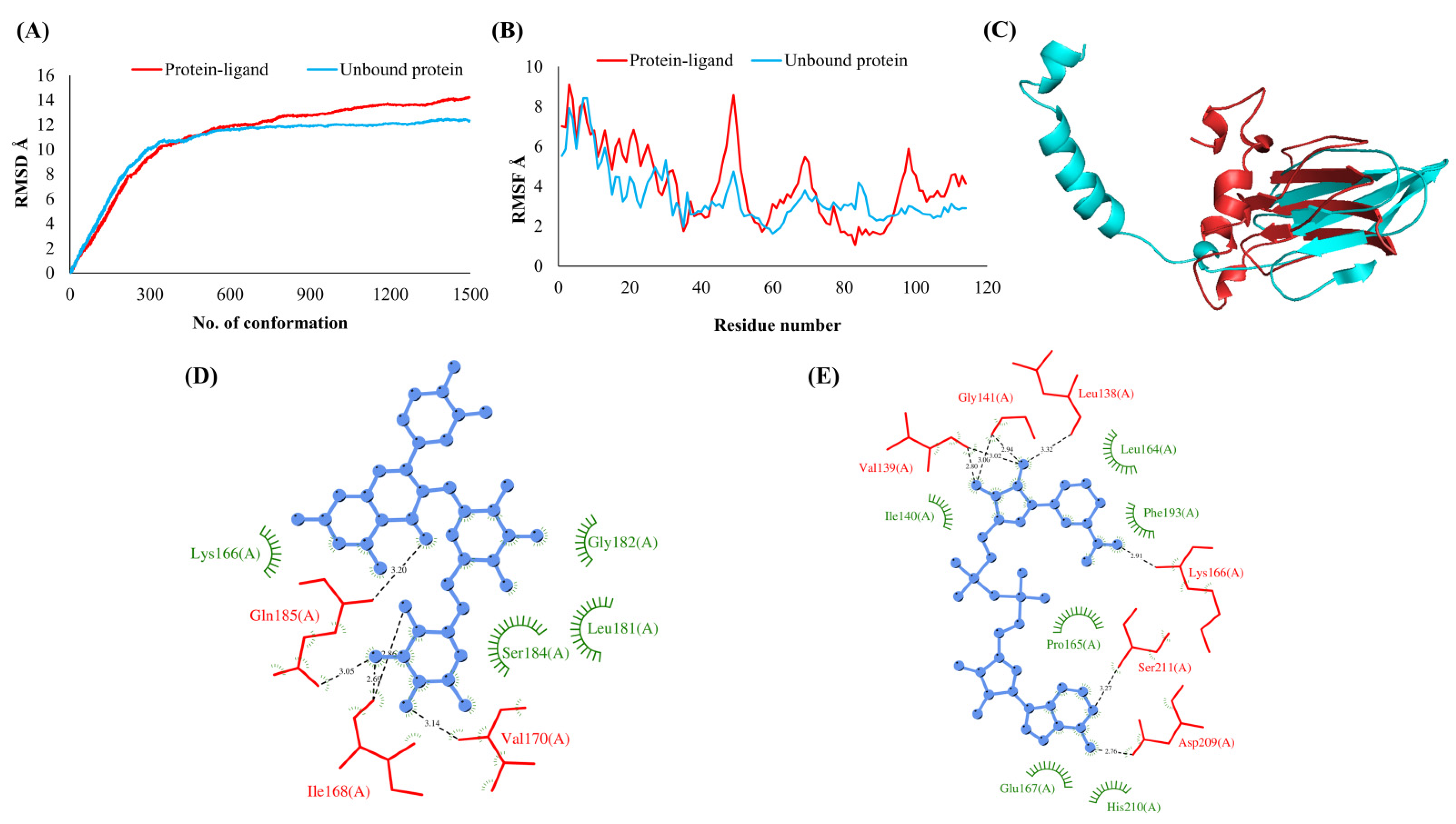
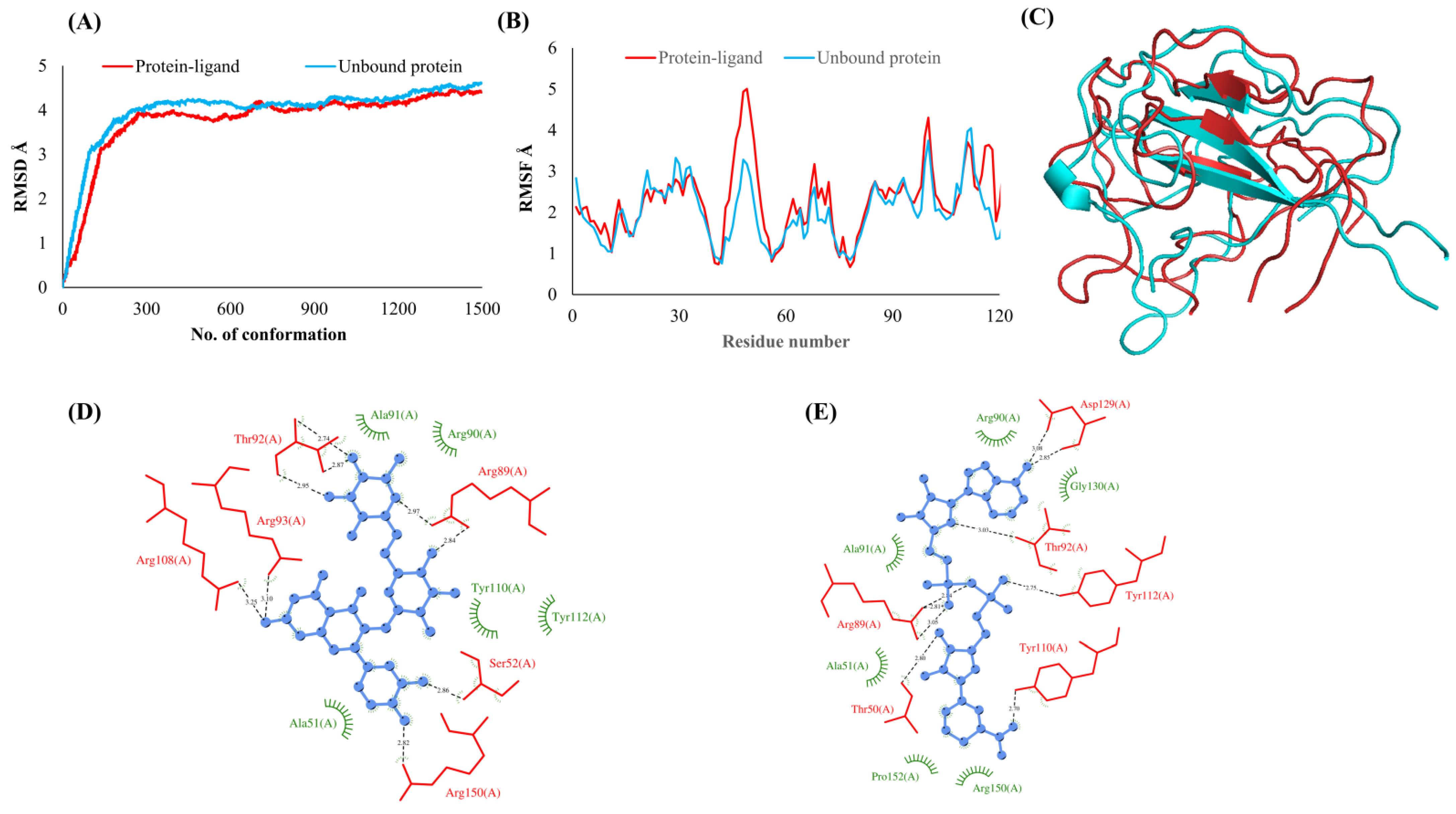
3.2. Simulations Analyses
3.3. Protein-Ligand Interaction Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report—87; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Gupta, V.; Haider, S.; Verma, M.; Singhvi, N.; Ponnusamy, K.; Malik, M.Z.; Verma, H.; Kumar, R.; Sood, U.; Hira, P.; et al. Comparative Genomics and Integrated Network Approach Unveiled Undirected Phylogeny Patterns, Co-mutational Hot Spots, Functional Cross Talk, and Regulatory Interactions in SARS-CoV-2. mSystems 2021, 6, e00030-21. [Google Scholar] [CrossRef] [PubMed]
- Mishra, C.B.; Pandey, P.; Sharma, R.D.; Malik, M.Z.; Mongre, R.K.; Lynn, A.M.; Prasad, R.; Jeon, R.; Prakash, A. Identifying the natural polyphenol catechin as a multi-targeted agent against SARS-CoV-2 for the plausible therapy of COVID-19: An integrated computational approach. Brief. Bioinform. 2021, 22, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kai Kupferschmidt, J.C. WHO launches global megatrial of the four most promising coronavirus treatments. Sci. Mag. 2020. Available online: https://www.science.org/content/article/who-launches-global-megatrial-four-most-promising-coronavirus-treatments (accessed on 12 November 2021). [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; O’Meara, M.J.; Guo, J.Z.; Swaney, D.L.; Tummino, T.A.; Hüttenhain, R.; et al. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020, 54, 159–163. [Google Scholar] [CrossRef]
- Banaganapalli, B.; Al-Rayes, N.; Awan, Z.A.; Alsulaimany, F.A.; Alamri, A.S.; Elango, R.; Malik, M.Z.; Shaik, N.A. Multilevel systems biology analysis of lung transcriptomics data identifies key miRNAs and potential miRNA target genes for SARS-CoV-2 infection. Comput. Biol. Med. 2021, 135, 104570. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. -Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Kruger, D.M.; Ahmed, A.; Gohlke, H. NMSim web server: Integrated approach for normal mode-based geometric simulations of biologically relevant conformational transitions in proteins. Nucleic Acids Res. 2012, 40, W310–W316. [Google Scholar] [CrossRef]
- Petrone, P.; Pande, V.S. Can conformational change be described by only a few normal modes? Biophys. J. 2006, 90, 1583–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueda, M.; Bottegoni, G.; Abagyan, R. Consistent improvement of cross-docking results using binding site ensembles generated with elastic network normal modes. J. Chem. Inf. Model. 2009, 49, 716–725. [Google Scholar] [CrossRef]
- Dietzen, M.; Zotenko, E.; Hildebrandt, A.; Lengauer, T. On the applicability of elastic network normal modes in small-molecule docking. J. Chem. Inf. Model. 2012, 52, 844–856. [Google Scholar] [CrossRef]
- Tama, F.; Sanejouand, Y.H. Conformational change of proteins arising from normal mode calculations. Protein Eng. 2001, 14, 1–6. [Google Scholar] [CrossRef]
- Singh, R.K.S.; Malik, M.Z.; Singh, R.K.B. Diversity of SARS-CoV-2 isolates driven by pressure and health index. Epidemiol. Infect. 2021, 149, e38. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J 2015, 14, 59–63. [Google Scholar] [PubMed]
- Galsnapp, A.; Schaefer, E. Nicotinamide-Adenine Dinucleotide (NADH) in Parkinson’s Disease and Alzheimer’s Disease. Int. J. Pharm. Compd. 2000, 4, 276–279. [Google Scholar]
- Castro-Marrero, J.; Cordero, M.D.; Segundo, M.J.; Sáez-Francàs, N.; Calvo, N.; Román-Malo, L.; Aliste, L.; de Sevilla, T.F.; Alegre, J. Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid. Redox Signal. 2015, 22, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Rahman, F.; Tabrez, S.; Ali, R.; Alqahtani, A.S.; Ahmed, M.Z.; Rub, A. Molecular docking analysis of rutin reveals possible inhibition of SARS-CoV-2 vital proteins. J. Tradit. Complement. Med. 2021, 11, 173–179. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, L.; Zhang, X.; Zhang, Q.; Yang, Z.; Liu, Y.; Liu, W. Discovery of Potential Flavonoid Inhibitors against COVID-19 3CL Proteinase Based on Virtual Screening Strategy. Front. Mol. Biosci. 2020, 7, 556481. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.C., Jr.; Ji, H.F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med. Infect. Dis. 2020, 35, 101646. [Google Scholar] [CrossRef]
- White, M.A.; Lin, W.; Cheng, X. Discovery of COVID-19 Inhibitors Targeting the SARS-CoV2 Nsp13 Helicase. bioRxiv 2020. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- He, F.; Yu, C.; Liu, T.; Jia, H. Ginsenoside Rg1 as an Effective Regulator of Mesenchymal Stem Cells. Front. Pharmacol. 2019, 10, 1565. [Google Scholar] [CrossRef] [Green Version]











| Drug | Description | Activity against ORFs |
|---|---|---|
| Rutin | An existing USFDA-approved drug used for strengthening weakened capillaries. Additionally, it has a powerful antioxidant with potential biological effect in reducing post-thrombotic syndrome, veins insufficiency or endothelial dysfunction. | NSP1, NSP3, NSP5, NSP9, NSP12, NSP13, NSP15, ORF3a, Spike, Envelope, Membrane, ORF6, ORF7a, Nucleocapsid |
| NADH | NADH plays essential metabolic roles and has been used to combat chronic fatigue syndrome. It is also being explored to be used against dementia and improving mental health. | NSP1, NSP3, NSP5, NSP9, NSP12, NSP13, NSP15, ORF3a, Spike, Envelope, Membrane, ORF6, ORF7a, Nucleocapsid |
| Ginsenoside Rg1, protopanaxatriol | Ginsenoside is a major component of the root and stem of ginseng plant. It possesses a broad spectrum of pharmacological properties such as neuroprotection, anti-inflammation, anti-aging, anti-fatigue and memory-enhancing properties. | NSP5, Nucleocapsid, NSP1, Envelope, NSP12, ORF5, ORF7a, NSP3, NSP9, NSP15 |
| Protein | Drug Name | Glide Score (kcal/mol) | GOLD Score | AutoDock Score (kcal/mol) | |
|---|---|---|---|---|---|
| 1 | Spike (S) | NADH | −11.31 | 80.76 | −8.4 |
| Rutin | −9.94 | 95.52 | −6.7 | ||
| 2 | Main protease (NSP5) | Ginsenoside Rb1 | −9.37 | 132.14 | −19.9 |
| Rutin | −9.58 | 96.35 | −7.4 | ||
| NADH | −8.65 | 85.20 | −8.5 | ||
| Ginsenoside Rg1 | −8.16 | 110.03 | −10.7 | ||
| 3 | Nucleocapsid (N) | Rutin | −9.34 | 87.13 | −5.6 |
| Ginsenoside C | −8.82 | 122.35 | −12.0 | ||
| NADH | −8.13 | 70.55 | −8.6 | ||
| Ginsenoside Rg1 | −5.17 | 110.79 | −10.7 | ||
| 4 | ORF6 | Ginsenoside C | −7.51 | 109.10 | −13.5 |
| Spermine | −7.21 | 43.27 | −6.8 | ||
| Rutin | −6.59 | 87.57 | −5.7 | ||
| NADH | −4.98 | 60.11 | −8.6 | ||
| 5 | Leader protein (NSP1) | Ginsenoside C | −9.30 | 107.85 | −11.6 |
| NADH | −7.38 | 65.42 | −7.7 | ||
| Rutin | −7.09 | 80.41 | −4.7 | ||
| Ginsenoside Rg1 | −5.81 | 85.13 | −9.4 | ||
| 6 | Envelope (E) | Ginsenoside Rg1 | −8.30 | 94.13 | −11.5 |
| α−tocopherol succinate | −8.11 | 39.97 | −16.7 | ||
| Rutin | −3.86 | 94.13 | −11.5 | ||
| NADH | −3.08 | 68.11 | −9.5 | ||
| 7 | RNA−dependent RNA polymerase (NSP12) | Ginsenoside Rb1 | −11.00 | 123.74 | −19.6 |
| Rutin | −10.98 | 113.81 | −7.0 | ||
| Ginsenoside Rg1 | −10.14 | 143.41 | −12.2 | ||
| NADH | −9.00 | 77.89 | −9.5 | ||
| 8 | ORF 3a | Rutin | −11.47 | 93.67 | −7.5 |
| Ornithine | −9.10 | 39.31 | −6.3 | ||
| NADH | −3.34 | 91.53 | −10.0 | ||
| 9 | Membrane (M) | Ginsenoside Rg1 | −9.35 | 81.77 | −9.4 |
| Rutin | −7.62 | 77.90 | −5.5 | ||
| NADH | −5.40 | 58.68 | −8.0 | ||
| 10 | ORF 7a | Ginsenoside Rb1 | −8.70 | 166.98 | −16.8 |
| Ginsenoside Rg1 | −7.00 | 135.96 | −10.7 | ||
| NADH | −6.83 | 78.90 | −7.6 | ||
| Rutin | −4.96 | 126.00 | −5.0 | ||
| 11 | Papain-like protease (NSP3) | Ginsenoside Rg1 | −10.37 | 103.78 | −10.2 |
| Rutin | −8.22 | 91.30 | −6.7 | ||
| NADH | −6.63 | 80.28 | −8.0 | ||
| 12 | Helicase (NSP13) | Glutathione | −10.31 | 60.00 | −6.4 |
| NADH | −9.54 | 87.21 | −10.5 | ||
| Rutin | −7.85 | 85.62 | −7.1 | ||
| 13 | RNA binding protein (Orf1ab, nsp9) | Ginsenoside Rb1 | −7.92 | 102.08 | −20.1 |
| Ginsenoside Rg1 | −6.84 | 91.46 | −9.6 | ||
| Rutin | −6.56 | 70.25 | −5.1 | ||
| NADH | −5.66 | 55.60 | −7.9 | ||
| 14 | Endoribonuclease (NSP15) | Ginsenoside Rb1 | −13.50 | 110.76 | −24.4 |
| Ginsenoside Rg1 | −11.00 | 111.16 | −12.5 | ||
| NADH | −9.95 | 78.94 | −9.2 | ||
| Rutin | −9.60 | 100.05 | −7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, K.M.; Farah, M.A.; Hor, Y.-Y. Multi-Targeted Approaches and Drug Repurposing Reveal Possible SARS-CoV-2 Inhibitors. Vaccines 2022, 10, 24. https://doi.org/10.3390/vaccines10010024
Alanazi KM, Farah MA, Hor Y-Y. Multi-Targeted Approaches and Drug Repurposing Reveal Possible SARS-CoV-2 Inhibitors. Vaccines. 2022; 10(1):24. https://doi.org/10.3390/vaccines10010024
Chicago/Turabian StyleAlanazi, Khalid Mashay, Mohammad Abul Farah, and Yan-Yan Hor. 2022. "Multi-Targeted Approaches and Drug Repurposing Reveal Possible SARS-CoV-2 Inhibitors" Vaccines 10, no. 1: 24. https://doi.org/10.3390/vaccines10010024
APA StyleAlanazi, K. M., Farah, M. A., & Hor, Y.-Y. (2022). Multi-Targeted Approaches and Drug Repurposing Reveal Possible SARS-CoV-2 Inhibitors. Vaccines, 10(1), 24. https://doi.org/10.3390/vaccines10010024






