Overexpression of Interleukin-33 in Recombinant Rabies Virus Enhances Innate and Humoral Immune Responses through Activation of Dendritic Cell-Germinal Center Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, Antibodies, and Animals
2.2. Construction and Rescue of rRABV Expressing Murine IL-33
2.3. Virus Titration
2.4. Cell Viability Assay
2.5. IL-33 Concentration Determination through ELISA
2.6. Mouse Immunization and Challenge Test
2.7. Pathogenicity Determination
2.8. Flow Cytometry Assay
2.9. Immunofluorescence Assay
2.10. Virus-Neutralizing Antibody (VNA) Test
2.11. RABV-Specific Antibody Isotype Test
2.12. Statistical Analysis
3. Results
3.1. Construction and Expression of rLBNSE-IL33
3.2. rRABVs Pathogenicity in Mice
3.3. Overexpression of IL-33 in rRABV Promotes Activation of Dendritic Cells (DCs)
3.4. Overexpression of IL-33 in rRABV Facilitates Growth and Development of Draining LNs
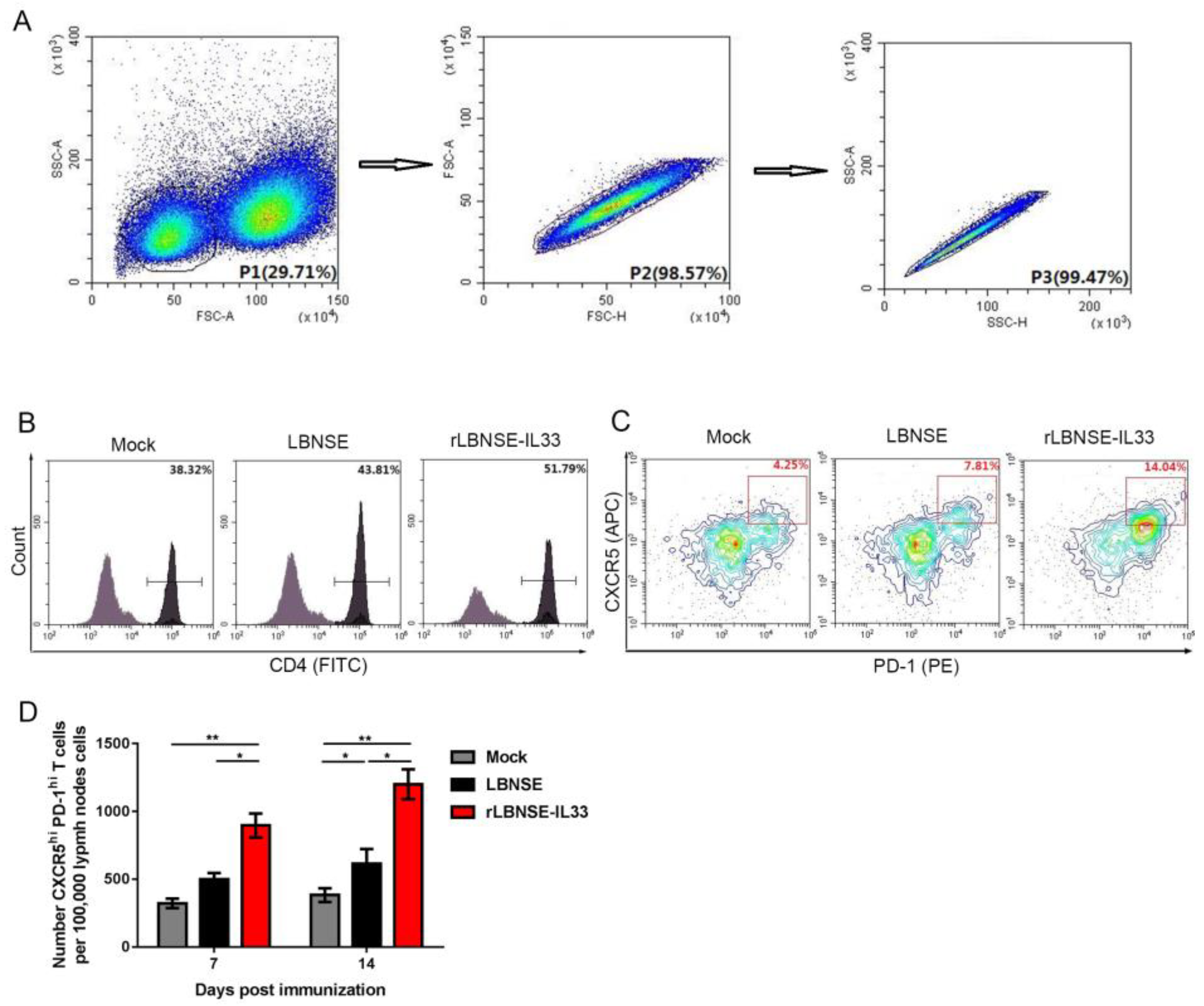
3.5. Overexpression of IL-33 in rRABV Induces Generation of Tfh Cells
3.6. Overexpression of IL-33 in rRABV Enhances Expansion of GC B Cells
3.7. Overexpression of IL-33 in rRABV Promotes Formation of GCs
3.8. Overexpression of IL-33 in rRABV Increases Number of Plasma Cells (PCs)
3.9. Overexpression of IL-33 in rRABV Improves Antibody Responses and Protection against Rabies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Albertini, A.A.V.; Ruigrok, R.W.H.; Blondel, D. Rabies virus transcription and replication. Adv. Virus Res. 2011, 79, 1–22. [Google Scholar]
- Fahrion, A.S.; Mikhailov, A.; Abela-Ridder, B.; Giacinti, J.; Harries, J. Human rabies transmitted by dogs: Current status of global data, 2015. Wkly Epidemiol. Rec. 2016, 91, 13–20. [Google Scholar]
- Lankester, F.; Hampson, K.; Lembo, T.; Palmer, G.; Taylor, L.; Cleaveland, S. Implementing Pasteur’s vision for rabies elimination. Science 2014, 345, 1562–1564. [Google Scholar] [CrossRef]
- Fooks, A.R.; Banyard, A.C.; Horton, D.L.; Johnson, N.; Mcelhinney, L.M.; Jackson, A.C. Current status of rabies and prospects for elimination. Lancet 2014, 384, 1389–1399. [Google Scholar] [CrossRef]
- Franka, R.; Smith, T.G.; Dyer, J.L.; Wu, X.F.; Niezgoda, M.; Rupprecht, C.E. Current and future tools for global canine rabies elimination. Antiviral Res. 2013, 100, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefrançais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, D.; Ye, C.; Geng, Z.; Chen, S.; Fan, J.; Qin, L.; Long, A.; Wang, L.; Zhang, Z.; Zhang, Y.; et al. Exogenous Il-33 restores dendritic cell activation and maturation in established cancer. J. Immunol. 2017, 198, 1365–1375. [Google Scholar] [CrossRef] [Green Version]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.O.; Wise, M.C.; Walters, J.N.; Reuschel, E.L.; Choi, M.J.; Obeng-Adjei, N.; Yan, J.; Morrow, M.P.; Weiner, D.B. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014, 74, 1789–1800. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, W.V.; Fröhlich, A.; Senn, K.; Kallert, S.; Fernandez, M.; Johnson, S.; Kreutzfeldt, M.; Hegazy, A.; Schrick, C.; Fallon, P.G.; et al. The alarmin interleukin-33 drives protective antiviral CD8+T cell responses. Science 2012, 335, 984–989. [Google Scholar] [CrossRef]
- Zhao, P.W.; Shi, X.; Li, C.; Ayana, D.A.; Niu, J.Q.; Feng, J.Y.; Wang, J.; Jiang, Y.F. IL33 enhances humoral immunity against chronic HBV infection through activating CD4 (+) CXCR5 (+) TFH cells. J. Interferon Cytokine Res. 2015, 35, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [Green Version]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Sage, P.T.; Sharpe, A.H. T follicular regulatory cells. Immunol. Rev. 2016, 271, 246–259. [Google Scholar] [CrossRef]
- Vinuesa, C.G.; Linterman, M.A.; Goodnow, C.C.; Randall, K.L. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol. Rev. 2010, 237, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Goenka, R.; Barnett, L.G.; Silver, J.S.; O’Neill, P.J.; Hunter, C.A.; Cancro, M.P.; Laufer, T.M. Cutting edge: Dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J. Immunol. 2011, 187, 1091–1095. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Rigby, R.J.; Zotos, D.; Tsai, L.M.; Kawamoto, S.; Marshall, J.L.; Ramiscal, R.R.; Chan, T.D.; Gatto, D.; Brink, R.; et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med. 2011, 208, 1377–1388. [Google Scholar] [CrossRef] [Green Version]
- King, C.; Tangye, S.G.; Mackay, C.R. T Follicular Helper (TFH) Cells in Normal and Dysregulated Immune Responses. Annu. Rev. Immunol. 2008, 26, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Stohlman, S.A.; Atkinson, R.D.; Shlomchik, M.J.; Bergmann, C.C. Mechanisms of central nervous system viral persistence: The critical role of antibody and B cells. J. Immunol. 2002, 168, 1204–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Toriumi, H.; Wang, H.; Kuang, Y.; Guo, X.; Morimoto, K.; Fu, Z.F. Expression of MIP-1alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J. Virol. 2010, 84, 9642–9648. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, J.; Li, M.; Cui, M.; Fu, Z.F.; Zhao, L.; Zhou, M. A Recombinant Rabies Virus Expressing Fms-like Tyrosine Kinase 3 Ligand (Flt3L) Induces Enhanced Immunogenicity in Mice. Virol. Sin. 2019, 34, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, M.; Luo, Z.; Zhang, Y.; Cui, M.; Chen, H.; Fu, Z.F.; Zhao, L. Overexpression of interleukin-7 extends the humoral immune response induced by rabies vaccination. J. Virol. 2017, 91, e02324-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Zhou, M.; Wang, Z.; Yang, J.; Li, M.M.; Wang, K.L.; Cui, M.; Chen, H.C.; Fu, Z.F.; Zhao, L. Recombinant rabies virus expressing IL-21 enhances immunogenicity through activation of T follicular helper cells and germinal centre B cells. J. Gen. Virol. 2016, 97, 3154–3160. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Faber, M.; Faber, M.L.; Li, J.; Preuss, M.A.; Schnell, M.J.; Dietzschold, B. Dominance of a nonpathogenic glycoprotein gene over a pathogenic glycoprotein gene in rabies virus. J. Virol. 2007, 81, 7041–7047. [Google Scholar] [CrossRef] [Green Version]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Brilot, F.; Strowig, T.; Munz, C. NK cells interactions with dendritic cells shape innate and adaptive immunity. Front. Biosci. A J. Virtual Libr. 2008, 13, 6443–6454. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, L.; Sui, B.; Luo, Z.; Zhang, Y.; Wang, Y. Recombinant Rabies Virus Overexpressing OX40-Ligand Enhances Humoral Immune Responses by Increasing T Follicular Helper Cells and Germinal Center B Cells. Vaccines 2020, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, M.; Li, Y.; Luo, Z.; Chen, H.; Cui, M.; Fu, Z.F.; Zhao, L. Recombinant rabies virus with the glycoprotein fused with a DC-binding peptide is an efficacious rabies vaccine. Oncotarget 2018, 9, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Nutt, S.L.; Tarlinton, D.M. Germinal center B and follicular helper T cells: Siblings, cousins or just good friends? Nat. Immunol. 2011, 12, 472–477. [Google Scholar] [CrossRef]
- Koh, B.; Ulrich, B.J.; Nelson, A.S.; Panangipalli, G.; Kharwadkar, R.; Wu, W.; Xie, M.; Fu, Y.; Turner, M.J.; Paczesny, S.; et al. Bcl6 and Blimp1 reciprocally regulate ST2+ Treg cell development in the context of allergic airway inflammation. J. Allergy Clin. Immunol. 2020, 146, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iijima, K.; Dent, A.L.; Kita, H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J. Allergy Clin. Immunol. 2017, 139, 300–313. [Google Scholar] [CrossRef] [Green Version]
- Linterman, M.A.; Hill, D.L. Can follicular helper T cells be targeted to improve vaccine efficacy? F1000Research 2016, 1, 88. [Google Scholar] [CrossRef] [Green Version]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013, 38, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Deng, S.; Ye, H.; Yu, X.; Deng, Q.; Zhang, Y.; Jiang, L.; Li, J.; Yu, Y.; Han, W. The IL-33/ST2 pathway suppresses murine colon cancer growth and metastasis by upregulating CD40 L signaling. Biomed. Pharmacother. 2020, 127, 110232. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.A.I.; Okragly, A.J.; Hu, N.N.; Daniels, M.R.; Martin, A.P.; Koh, Y.T.; Kikly, K.; Benschop, R.J. Interleukin-33 Contributes Toward Loss of Tolerance by Promoting B-Cell-Activating Factor of the Tumor-Necrosis-Factor Family (BAFF)-Dependent Autoantibody Production. Front. Immunol. 2018, 9, 2871. [Google Scholar] [CrossRef]
- Haley, S.L.; Tzvetkov, E.P.; Meuwissen, S.; Plummer, J.R.; McGettigan, J.P. Targeting Vaccine-Induced Extrafollicular Pathway of B Cell Differentiation Improves Rabies Postexposure Prophylaxis. J. Virol. 2017, 91, e02435-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, W.A.; Okragly, A.J.; Patel, C.N.; Benschop, R.J. IL-33 released by alum is responsible for early cytokine production and has adjuvant properties. Sci. Rep. 2015, 5, 13146. [Google Scholar] [CrossRef] [Green Version]
- Cupedo, T. Human lymph node development: An inflammatory interaction. Immunol. Lett. 2011, 138, 4–6. [Google Scholar] [CrossRef]
- Flamar, A.-L.; Klose, C.S.; Moeller, J.B.; Mahlakõiv, T.; Bessman, N.J.; Zhang, W.; Moriyama, S.; Stokic-Trtica, V.; Rankin, L.C.; Putzel, G.G.; et al. Interleukin-33 Induces the Enzyme Tryptophan Hydroxylase 1 to Promote Inflammatory Group 2 Innate Lymphoid Cell-Mediated Immunity. Immunity 2020, 52, 606–619. [Google Scholar] [CrossRef]
- Nagarkar, D.R.; Ramirez-Carrozzi, V.; Choy, D.; Lee, K.; Soriano, R.; Jia, G.; Abbas, A.R.; Modrusan, Z.; Pappu, R.; Arron, J.R. IL-13 mediates IL-33-dependent mast cell and type 2 innate lymphoid cell effects on bronchial epithelial cells. J. Allergy Clin. Immunol. 2015, 136, 202–205. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.; Qiao, X.; Wang, X.; Liu, J.; Niu, X.; Zhong, M. Downregulated miR-524-5p Participates in the Tumor Microenvironment of Ameloblastoma by Targeting the Interleukin-33 (IL-33)/Suppression of Tumorigenicity 2 (ST2) Axis. Med. Sci. Monit. 2020, 26, e921863. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Gleeson, M.W.; Noelle, R.J.; Erickson, L.D. The rise and fall of long-lived humoral immunity: Terminal differentiation of plasma cells in health and disease. Immunol. Rev. 2003, 194, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutsky, K.; Curtis, D.; Bongiorno, E.K.; Barkhouse, D.A.; Kean, R.B.; Dietzschold, B.; Hooper, D.C.; Faber, M. Intramuscular Inoculation of Mice with the Live-Attenuated Recombinant Rabies Virus TriGAS Results in a Transient Infection of the Draining Lymph Nodes and a Robust, Long-Lasting Protective Immune Response against Rabies. J. Virol. 2013, 87, 1834–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Z.; Zhao, L.; Sun, M.; Gao, T.; Wang, Y.; Sui, B.; Li, Y. Overexpression of Interleukin-33 in Recombinant Rabies Virus Enhances Innate and Humoral Immune Responses through Activation of Dendritic Cell-Germinal Center Reactions. Vaccines 2022, 10, 34. https://doi.org/10.3390/vaccines10010034
Mi Z, Zhao L, Sun M, Gao T, Wang Y, Sui B, Li Y. Overexpression of Interleukin-33 in Recombinant Rabies Virus Enhances Innate and Humoral Immune Responses through Activation of Dendritic Cell-Germinal Center Reactions. Vaccines. 2022; 10(1):34. https://doi.org/10.3390/vaccines10010034
Chicago/Turabian StyleMi, Zhizhong, Ling Zhao, Ming Sun, Ting Gao, Yong Wang, Baokun Sui, and Yingying Li. 2022. "Overexpression of Interleukin-33 in Recombinant Rabies Virus Enhances Innate and Humoral Immune Responses through Activation of Dendritic Cell-Germinal Center Reactions" Vaccines 10, no. 1: 34. https://doi.org/10.3390/vaccines10010034
APA StyleMi, Z., Zhao, L., Sun, M., Gao, T., Wang, Y., Sui, B., & Li, Y. (2022). Overexpression of Interleukin-33 in Recombinant Rabies Virus Enhances Innate and Humoral Immune Responses through Activation of Dendritic Cell-Germinal Center Reactions. Vaccines, 10(1), 34. https://doi.org/10.3390/vaccines10010034




