COVID-19 Vaccines Cost-Effectiveness Analysis: A Scenario for Iran
Abstract
:1. Introduction
2. Material and Methods
2.1. The Cost-Effectiveness Model/Measurements
2.1.1. Demographic Data
2.1.2. Disease Burden Estimation
2.1.3. Cost Measurements
2.1.4. Effectiveness Measurements
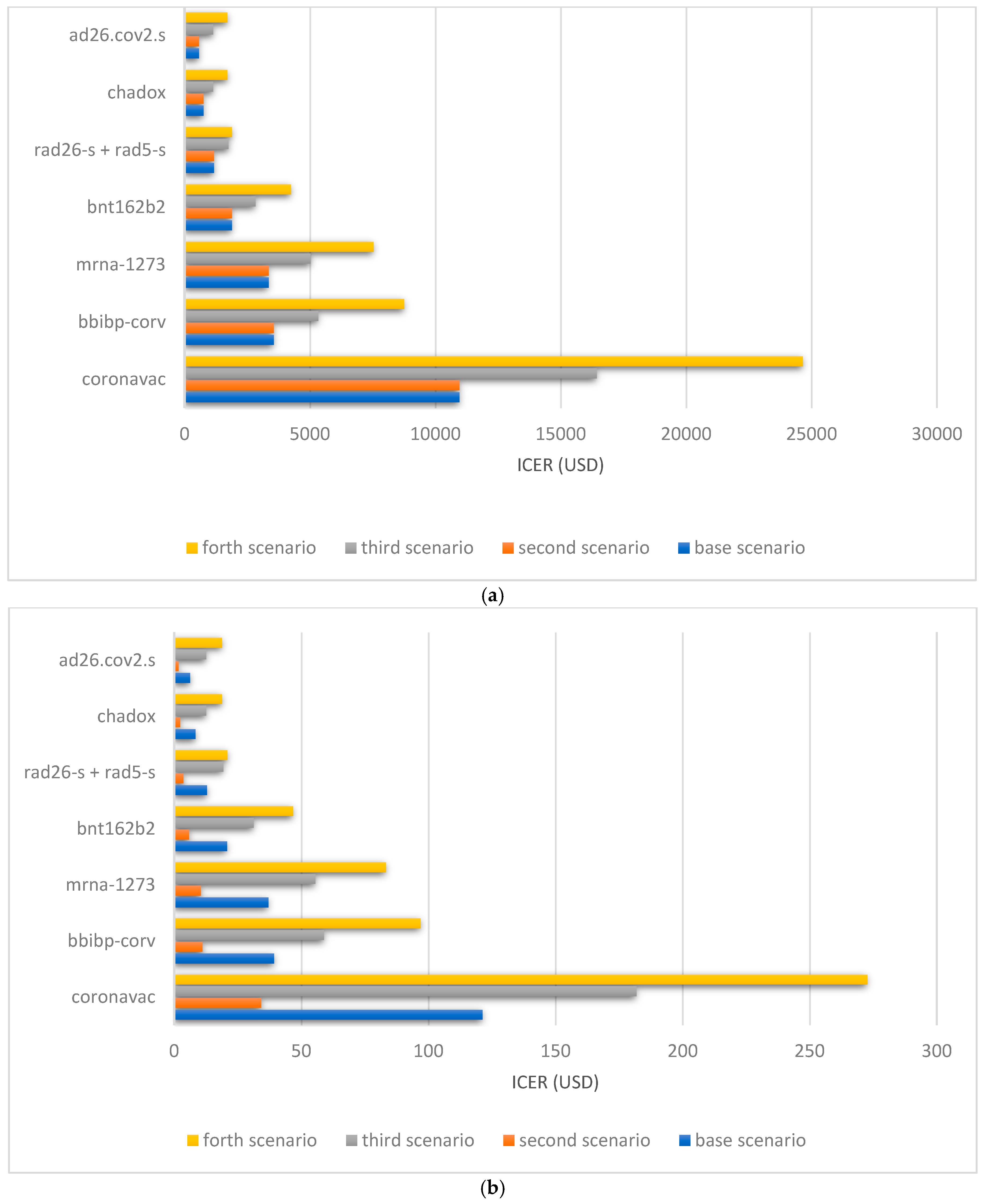
3. Results
3.1. Vaccines Profile
3.2. Cost-Effectiveness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 17 July 2021).
- WHO. WHO Coronavirus (COVID-19) Dashboard, Overview of all Countries. Available online: https://covid19.who.int/ (accessed on 1 July 2021).
- WHO. The current COVID-19 situation, Iran (Islamic Republic of). Available online: https://www.who.int/countries/irn/ (accessed on 18 July 2021).
- Güner, H.R.; Hasanoğlu, I.; Aktaş, F. COVID-19: Prevention and control measures in community. Turk. J. Med Sci. 2020, 50, 571–577. [Google Scholar] [CrossRef]
- Yu, X.; Yang, R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir. Viruses 2020, 14, 474–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, E.A.; Wu, J.W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 2020, 35, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1922. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Zhang, A.L.; Wang, Y.; Molina, M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 14857–14863. [Google Scholar] [CrossRef]
- Najafimehr, H.; Mohamed Ali, K.; Safari, S.; Yousefifard, M.; Hosseini, M. Estimation of basic reproduction number for COVID-19 and the reasons for its differences. Int. J. Clin. Pract. 2020, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checcucci, E.; Piramide, F.; Pecoraro, A.; Amparore, D.; Campi, R.; Fiori, C.; Elhage, O.; Kotecha, P.; Vyakarnam, A.; Serni, S. The vaccine journey for COVID-19: A comprehensive systematic review of current clinical trials in humans. Panminerva Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Covid-19 Candidate Vaccine Landscape and Tracker. 2021. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 24 July 2021).
- Covid-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 17 July 2021).
- Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine (accessed on 29 August 2021).
- Harder, T.; Koch, J.; Vygen-Bonnet, S.; Külper-Schiek, W.; Pilic, A.; Reda, S.; Scholz, S.; Wichmann, O. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: Interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Tracking Coronavirus Vaccination Around the World. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 17 July 2021).
- Moubarak, M.; Kasozi, K.I.; Hetta, H.F.; Shaheen, H.M.; Rauf, A.; Al-kuraishy, H.M.; Qusti, S.; Alshammari, E.M.; Ayikobua, E.T.; Ssempijja, F.; et al. The Rise of SARS-CoV-2 Variants and the Role of Convalescent Plasma Therapy for Management of Infections. Life 2021, 11, 734. [Google Scholar] [CrossRef]
- Konings, F.; Perkins, M.D.; Kuhn, J.H.; Pallen, M.J.; Alm, E.J.; Archer, B.N.; Barakat, A.; Bedford, T.; Bhiman, J.N.; Caly, L.; et al. SARS-CoV-2 Variants of Interest and Concern naming scheme conducive for global discourse. Nat. Microbiol. 2021, 6, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Pöhlmann, S.; Hoffmann, M. Mutation D614G increases SARS-CoV-2 transmission. Signal Transduct. Target. Ther. 2021, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Sander, A.-L.; Yadouleton, A.; Moreira-Soto, A.; Tchibozo, C.; Hounkanrin, G.; Badou, Y.; Fischer, C.; Krause, N.; Akogbeto, P.; de Oliveira Filho, E.F. An Observational Laboratory-Based Assessment of SARS-CoV-2 Molecular Diagnostics in Benin, Western Africa. MSphere 2021, 6, e00979-20. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.d.S.; Mishra, S.; Crispim, M.A.; Sales, F.C.; Hawryluk, I.; McCrone, J.T. Genomics and epidemiology of the P. 1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ 2021. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Fowlkes, A. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection among Frontline Workers Before and During B. 1.617. 2 (Delta) Variant Predominance—Eight US Locations, December 2020–August 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1167–1169. [Google Scholar] [CrossRef]
- Bollyky, T.J.; Gostin, L.O.; Hamburg, M.A. The Equitable Distribution of COVID-19 Therapeutics and Vaccines. JAMA 2020, 323, 2462–2463. [Google Scholar] [CrossRef]
- Statistical Center of Iran (s.c.o.), Population and Housing Census, Census. 2016. Available online: https://www.amar.org.ir/english/Population-and-Housing-Censuses/ (accessed on 20 June 2021).
- WHO. COVID-19 Clinical Management: Living Guidance, 25 January 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 17 July 2021).
- Mohanty, S.K.; Dubey, M.; Mishra, U.S.; Sahoo, U. Impact of COVID-19 Attributable Deaths on Longevity, Premature Mortality and DALY: Estimates of USA, Italy, Sweden and Germany. MedRxiv 2020. [Google Scholar] [CrossRef]
- Andrasfay, T.; Goldman, N. Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations. Proc. Natl. Acad. Sci. USA 2021, 118, e2014746118. [Google Scholar] [CrossRef]
- How Much is the Cost of Each Covid-19 Patient? Available online: https://www.irna.ir/news/83902086 (accessed on 19 July 2021).
- How Much Will a Covid-19 Vaccine Cost? The Financial Times. Available online: https://www.ft.com/content/80f20d71-d7eb-4386-b0f2-0b19e4aed94d (accessed on 29 August 2021).
- McCarthy, N. The Cost per Jab of Covid-19 Vaccine Candidates. Available online: https://www.statista.com/chart/23658/reported-cost-per-dose-of-covid-19-vaccines/ (accessed on 15 June 2021).
- Deborah Abrams Kaplan, P.W. The Price Tags on the COVID-19 Vaccines. Available online: https://www.managedhealthcareexecutive.com/view/the-price-tags-on-the-covid-19-vaccines (accessed on 29 August 2021).
- Baraniuk, C. What do we know about China’s covid-19 vaccines? BMJ 2021, 373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Sinovac Shot Shown 78% Effective in Brazil after Data Confusion. Bloomberg News. Available online: https://www.bloomberg.com/news/articles/2021-01-07/sinovac-covid-shot-78-effective-in-brazil-trial-folha-reports (accessed on 19 July 2021).
- Kozok, F. Turkey Finds Chinese Vaccine Efficacy Rate of 91.25% in Trial. Available online: https://www.bloomberg.com/news/articles/2020-12-24/turkey-finds-chinese-vaccine-efficacy-rate-of-91-25-in-trial (accessed on 19 July 2021).
- Aditya, A. Sinovac’s Vaccine Approved by Indonesia for Emergency Use. Available online: https://www.bloomberg.com/news/articles/2021-01-11/sinovac-s-covid-vaccine-approved-by-indonesia-for-emergency-use (accessed on 19 July 2021).
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; Villela, E.F.d.M.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- WHO. Evidence Assessment: Sinopharm/BBIBP COVID-19 vaccine. 2021. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf (accessed on 2 June 2021).
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Nogrady, B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nature 2021, 595, 339–340. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Chung, H.; He, S.; Nasreen, S.; Sundaram, M.E.; Buchan, S.A.; Wilson, S.E.; Chen, B.; Calzavara, A.; Fell, D.; Peter, C. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: Test negative design study. BMJ 2021, 374, n1943. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Moline, H.L.; Whitaker, M.; Deng, L.; Rhodes, J.C.; Milucky, J.; Pham, H.; Patel, K.; Anglin, O.; Reingold, A.; Chai, S.J.; et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years—COVID-NET, 13 States, February-April 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1088–1093. [Google Scholar] [CrossRef]
- Abdoli, A.; Aalizadeh, R.; Aminianfar, H.; Kianmehr, Z.; Azimi, E.; Emamipour, N.; Jamshidi, H.; Hosseinpour, M.; Taqavian, M.; Jalili, H. Safety and Potency of COVIran Barekat Inactivated Vaccine Candidate for SARS-CoV-2: A Preclinical Study. bioRxiv 2021. [Google Scholar] [CrossRef]
- Results of the Coviran Barekat Vaccine Trials [In Persian]. Available online: https://setad.ir/news/1665/ (accessed on 9 July 2021).
- Mozafari, A.; Miladinia, M.; Sabri, A.; Movaseghi, F.; Gholamzadeh Baeis, M. The challenge of deciding between home-discharge versus hospitalization in COVID-19 patients: The role of initial imaging and clinicolaboratory data. Clin. Epidemiol. Glob. Health 2021, 10, 100673. [Google Scholar] [CrossRef]
- AlQahtani, M.; Bhattacharyya, S.; Alawadi, A.; Al Mahmeed, H.; Al Sayed, J.; Justman, J.; El-Sadr, V.M.; Hidary, J. Morbidity and mortality from COVID-19 post-vaccination breakthrough infections in association with vaccines and the emergence of variants in Bahrain. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Ophinni, Y.; Hasibuan, A.S.; Widhani, A.; Maria, S.; Koesnoe, S.; Yunihastuti, E.; Karjadi, T.H.; Rengganis, I.; Djauzi, S. COVID-19 Vaccines: Current Status and Implication for Use in Indonesia. Acta Med. Indones. 2020, 52, 388. [Google Scholar] [PubMed]
- Kim, J.J. The Role of Cost-Effectiveness in U.S. Vaccination Policy. N. Engl. J. Med. 2011, 365, 1760–1761. [Google Scholar] [CrossRef] [PubMed]
- The World Bank, Data. GDP per Capita (Current US$)—Iran, Islamic Rep. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=IR (accessed on 28 August 2021).
- Hagens, A.; İnkaya, A.Ç.; Yildirak, K.; Sancar, M.; van der Schans, J.; Acar Sancar, A.; Ünal, S.; Postma, M.; Yeğenoğlu, S. COVID-19 Vaccination Scenarios: A Cost-Effectiveness Analysis for Turkey. Vaccines 2021, 9, 399. [Google Scholar] [CrossRef]
- Reddy, K.P.; Fitzmaurice, K.P.; Scott, J.A.; Harling, G.; Lessells, R.J.; Panella, C.; Shebl, F.M.; Freedberg, K.A.; Siedner, M.J. Clinical outcomes and cost-effectiveness of COVID-19 vaccination in South Africa. medRxiv 2021. [Preprint]. [Google Scholar] [CrossRef]
| Phase of Study | Developers | Vaccine Name | Vaccine Platform | Route of Administration | Number of Doses/Schedule | |
|---|---|---|---|---|---|---|
| 1 | 4 | Sinovac Research and Development Co., Ltd. | CoronaVac | Inactivated virus | IM | 2/day 0 + 14 |
| 2 | Sinopharm + China national Biotech group Co + Beijing Institute of Biological Products | BBIBP-CorV | Inactivated virus | IM | 2/day 0 + 21 | |
| 3 | AstraZeneca + University of Oxford | ChAdOx1-S-(AZD1222) | Viral vector (Non-replicating) | IM | 1–2/day 0 + 28 | |
| 4 | CanSino biological Inc./Beijing institute of biological products | Recombinant novel coronavirus vaccine (adenovirus type 5 vector) | Viral vector (non-replicating) | IM | 1/day 0 | |
| 5 | Janssen Pharmaceutical | Ad26.COV2.S | Viral vector (non-replicating) | IM | 1–2/day 0 or day 0 + 56 | |
| 6 | Moderna + National Institute of Allergy and Infectious Diseases | mRNA -1273 | RNA based vaccine | IM | 2/day 0 + 28 | |
| 7 | Moderna + National Institute of Allergy and Infectious Diseases | mRNA-1273.351 | RNA based vaccine | IM | 3/day 0 or day 0 + 28 or day 56 | |
| 8 | Pfizer/BioNTech + Fosun Pharma | BNT162b2 (3 LNP-mRNAs), also known as “Comirnaty” | RNA based vaccine | IM | 2/day 0 + 21 | |
| 9 | 3 | Sinopharm + China National biotech Group Co + Wuhan Institute of Biological Products | Inactivated SARS-CoV-2 vaccine (Vero cell) | Inactivated virus | IM | 2/day 0 + 21 |
| 10 | Gamaleya Research Institute; Health Ministry of the Russian Federation | Gam-COVID-Vac Adeno-based (rAd26-S + rAd5-S) | Viral vector (Non-replicating) | IM | 2/day 0 + 21 | |
| 11 | Novavax Gaithersburg, Maryland, USA | Sars-cov-2 rS/Matrix M1-adjuvant (full length recombinant SARS cov-2 glycoprotein nanoparticle vaccine adjuvant with Matrix M) NVX-CoV2373 | Protein subunit | IM | 2/day 0 + 21 | |
| 12 | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | Recombinant SARS-CoV-2 vaccine (CHO Cell) | Protein subunit | IM | 2–3/ Day 0 + 28 or Day 0 + 28 + 56 | |
| 13 | CureVac AG Tübingen, Germany | CVnCoV Vaccine | RNA based vaccine | IM | 2/ Day 0 + 28 | |
| 14 | Institute of Medical Biology + Chinese Academy of Medical Sciences | SARS-CoV-2 vaccine (Vero cells) | Inactivated virus | IM | 2/ Day 0 + 28 | |
| 15 | Research Institute for Biological Safety Problems, Rep. of Kazakhstan | QazCovid-in®-COVID-19 inactivated vaccine | Inactivated virus | IM | 2/ Day 0 + 21 | |
| 16 | Zydus Cadila Ahmedabad, Gujarat, India | nCov vaccine | DNA based vaccine | ID | 3/ Day 0 + 28 + 56 | |
| 17 | Bharat Biotech International Limited, Hyderabad, India | Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152): covaxin | Inactivated virus | IM | 2/ Day 0 + 14 | |
| 18 | Sanofi Pasteur + GSK Lyon, France-London, England | VAT00002: SARS-CoV-2 S protein with adjuvant | Protein subunit | IM | 2/ Day 0 + 21 | |
| 19 | Shenzhen Kangtai Biological Products Co., Ltd. Shenzhen, China | Inactivated sars-cov-2 vaccine (verocell) | Inactivated virus | IM | 2/day 0 + 28 | |
| 20 | Vaxine Pty Ltd./CinnaGen Co Adelaide, Australia-Tehran, Iran | COVAX-19 | Protein subunit | IM | 2/day 0 + 21 | |
| 21 | Instituto Finlay de Vacunas Havana, Cuba | FINLAY-FR-2 anti-SARS-CoV-2 Vaccine (RBD chemically conjugated to tetanus toxoid plus adjuvant) | Protein subunit | IM | 2/Day 0 + 28 | |
| 22 | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” Koltsovo, Novosibirsk Oblast, Russia | EpiVacCorona (based on peptide antigens for the prevention of COVID-19) | Protein subunit | IM | 2/Day 0 + 21 | |
| 23 | West China Hospital + Sichuan University | RBD (baculovirus production expressed in Sf9 cells) recombinant SARS-CoV-2 vaccine (Sf9 Cell) | Protein subunit | IM | 2/day 0 + 28 | |
| 24 | Academy of Military Science (AMS), Walvax biotechnology, and Suzhou Abogen Biosciences Shanghai, China | SARS-cov-2 mRNA vaccine (ARCoV) | RNA based vaccine | IM | 2/day 0 + 14 or day 0 + 28 | |
| 25 | Center for Genetic Engineering and Biotechnology (CIGB) Havana, Cuba | CIGB-66 (RBD+aluminium hydroxide) | Protein subunit | IM | 3/Day 0 + 14 + 28 or Day 0 +28 + 56 | |
| 26 | Valneva, National Institute for Health Research, United Kingdom | VLA2001 | Inactivated virus | IM | 2/day 0 + 21 | |
| 27 | Nanogen pharmaceutical biotechnology Ho Chi Minh, Vietnam | Recombinant sars-cov-2 spike protein, aluminum adjuvant (nanocovax) | Protein subunit | IM | 2/day 0 + 21 | |
| 28 | Erciyes University, Turkey | ERUCOV-VAC, inactivated virus | Inactivated virus | IM | 2/day 0 + 21 | |
| 29 | SK Bioscience Co. Gyeonggi-do, South Korea | GBP510 | Protein subunit | IM | 2/day 0 + 28 |
| Developers | Vaccine Name | Country | Type of Candidate Vaccine | Number of Doses/Route | Timing of Doses (Days) | Price per Dose |
|---|---|---|---|---|---|---|
| Sinovac Research and Development Co. | CoronaVac | China | inactivated | 2/IM | 0, 14 | 30 USD |
| Beijing Institute of Biological Products/Sinopharm | BBIBP-CorV | China | inactivated | 2/IM | 0, 21 | 36 USD |
| Moderna/NAIAD | mRNA-1273 | USA | LNP-encapsulated mRNA | 2/IM | 0, 28 | 37 USD |
| BioNTech/Fosun Pharma/Pfizer | BNT162b2 | USA, Germany | 3 LNP-mRNAs | 2/IM | 0, 21 | 19.5 USD |
| Gamaleya Research institute/ Sputnik | rAd26-S+rAd5-S | Russia | Adenovirus base | 2/IM | 0, 21 | 10 USD |
| University of Oxford/ AstraZeneca | ChAdOx1-S | UK | chimpanzee adenovirus-vectored vaccine | 2/IM | 0, 28 | 5.5 USD |
| Janssen Pharmaceutical | Ad26.COV2.S | USA, Netherland | Viral vector (non-replicating) | 1–2/IM | 0 or 0, 56 | 10 USD |
| Base Scenario | Scenario 2 | Scenario 3 | Scenario 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine Name | ICER Death | ICER DALY | Cost-Benefit | ICER Death | ICER DALY | Cost-Benefit | ICER Death | ICER DALY | Cost-Benefit | ICER Death | ICER DALY | Cost-Benefit | |
| CoronaVac | Efficacy: 0.78 | 3944.7 | 43.6 | 15.5 | 3944.7 | 12.3 | 15.5 | 5917.1 | 65.4 | 10.0 | 8875.7 | 98.1 | 5.9 |
| Effectiveness: 0.46 | 10,957.7 | 121.2 | 8.9 | 10,957.7 | 34.2 | 8.9 | 16,436.5 | 181.8 | 5.6 | 24,654.8 | 272.7 | 3.1 | |
| BBIBP-CorV | Efficacy: 0.78 | 4721.6 | 52.2 | 12.7 | 4721.6 | 14.7 | 12.7 | 7082.4 | 78.3 | 8.1 | 10,382.9 | 114.8 | 4.8 |
| Effectiveness: 0.9 | 3555.5 | 39.3 | 14.8 | 3555.5 | 11.1 | 14.8 | 5333.3 | 58.9 | 9.5 | 8761.4 | 96.9 | 5.3 | |
| mRNA-1273 | Efficacy: 0.94 | 3342.8 | 36.9 | 15.1 | 3342.8 | 10.4 | 15.1 | 5014.2 | 55.4 | 9.7 | 7521.3 | 83.2 | 5.7 |
| Effectiveness: 0.94 | 3349.9 | 37.0 | 15.1 | 3349.9 | 10.4 | 15.1 | 5024.8 | 55.5 | 9.7 | 7537.3 | 83.3 | 5.7 | |
| BNT162b2 | Efficacy: 0.95 | 1728.5 | 19.1 | 29.9 | 1728.5 | 5.4 | 29.9 | 2592.7 | 28.6 | 19.6 | 3889.1 | 43.0 | 12.0 |
| Effectiveness: 0.91 | 1883.8 | 20.8 | 28.6 | 1883.8 | 5.8 | 28.6 | 2825.7 | 31.2 | 18.7 | 4238.6 | 46.8 | 11.7 | |
| rAd26-S + rAd5-s | Efficacy: 0.91 | 953.4 | 10.5 | 57.1 | 953.4 | 2.9 | 57.1 | 1430.1 | 15.8 | 37.7 | 2145.2 | 23.7 | 23.4 |
| Effectiveness: 0.82 | 1166.8 | 12.9 | 51.5 | 1166.8 | 3.6 | 51.5 | 1750.3 | 19.3 | 34.0 | 1889.6 | 20.9 | 25.0 | |
| ChAdOx1 nCoV-19 | Efficacy: 0.82 | 618.5 | 6.8 | 98.6 | 618.5 | 1.9 | 98.6 | 927.8 | 10.2 | 65.4 | 1391.8 | 15.3 | 40.9 |
| Effectiveness: 0.74 | 756.7 | 8.3 | 89.1 | 756.7 | 2.3 | 89.1 | 1135.0 | 12.5 | 59.0 | 1702.6 | 18.8 | 36.9 | |
| Ad26.COV2.S | Efficacy: 0.66 | 904.5 | 10.0 | 83.4 | 904.5 | 2.8 | 83.4 | 1809.0 | 20.0 | 41.2 | 2713.5 | 30.0 | 25.6 |
| Effectiveness: 0.84 | 566.8 | 6.2 | 105.6 | 566.8 | 1.7 | 105.6 | 1133.7 | 12.5 | 52.3 | 1700.6 | 18.8 | 32.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaezi, A.; Meysamie, A. COVID-19 Vaccines Cost-Effectiveness Analysis: A Scenario for Iran. Vaccines 2022, 10, 37. https://doi.org/10.3390/vaccines10010037
Vaezi A, Meysamie A. COVID-19 Vaccines Cost-Effectiveness Analysis: A Scenario for Iran. Vaccines. 2022; 10(1):37. https://doi.org/10.3390/vaccines10010037
Chicago/Turabian StyleVaezi, Atefeh, and Alipasha Meysamie. 2022. "COVID-19 Vaccines Cost-Effectiveness Analysis: A Scenario for Iran" Vaccines 10, no. 1: 37. https://doi.org/10.3390/vaccines10010037






