Circulating Cytokines in Metastatic Breast Cancer Patients Select Different Prognostic Groups and Patients Who Might Benefit from Treatment beyond Progression
Abstract
:1. Introduction
2. Materials and Methods
- Group 1. Patients with both PFS and OS below the median;
- Group 2. Patients with PFS below the median and OS above the median;
- Group 3. Patients with PFS above the median and OS below the median;
- Group 4. Patients with both PFS and OS above the median.
2.1. Plasma Collection
2.2. Cytokines
2.3. Cytokine Measurement
2.4. Statistical Analyses
3. Results
3.1. Patient Population
3.2. Healthy Volunteers
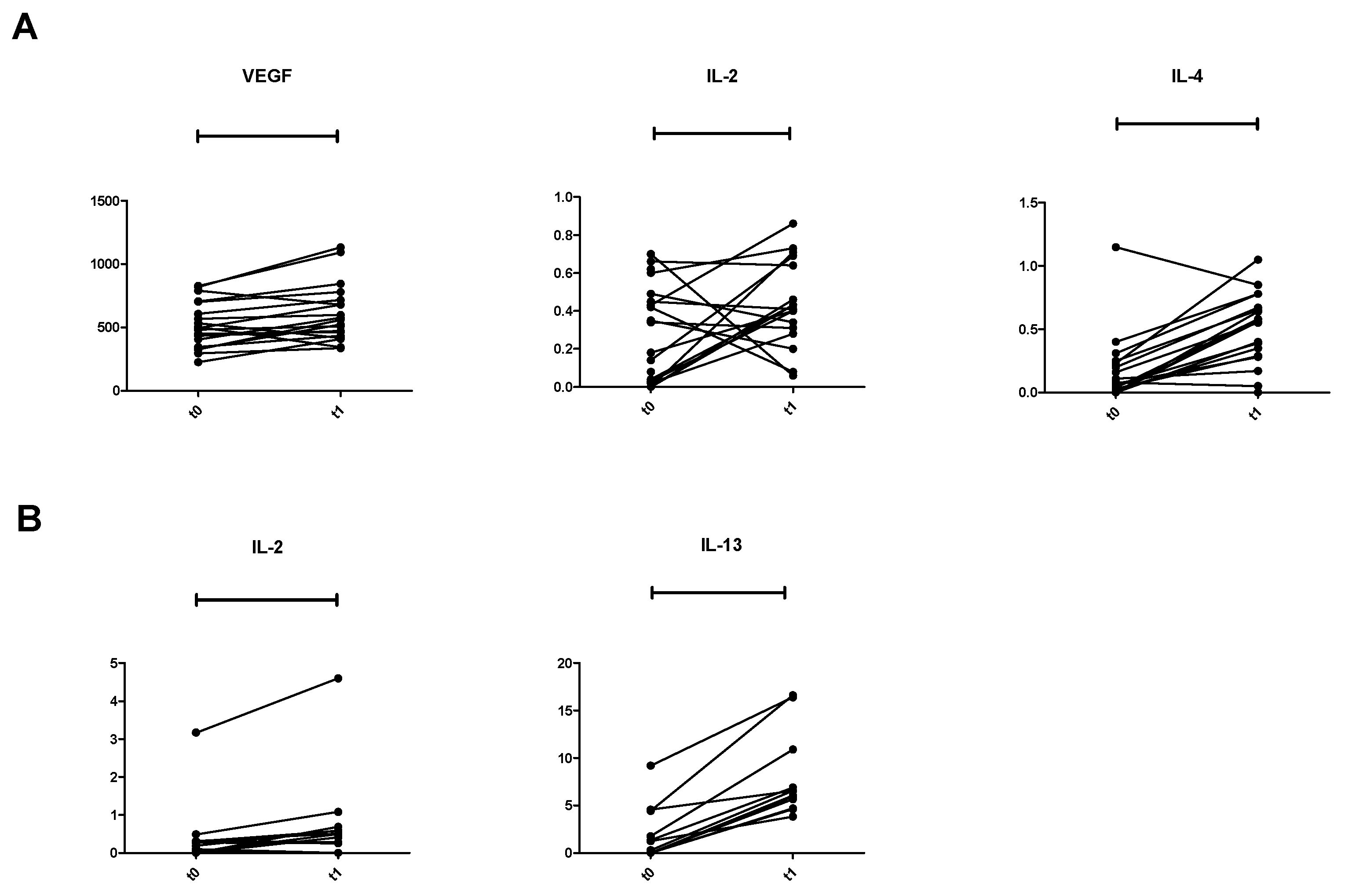
3.3. Treatment Effects
- Group 1 (patients with both PFS and OS below the median): 12 patients (29.2%), median PFS 2.8 months (range 2.5–3.6), and median OS 4.6 months (range 3.0–10.8).
- Group 2 (patients with PFS below the median and OS above the median): 9 patients (21.9%), median PFS 2.8 months (range 2.7–3.5), and median OS 15 months (range 11.2–23.8).
- Group 3 (patients with PFS above the median and OS below the median): 9 patients (21.9%), median PFS 5.3 months (range 3.7–7.6), and median OS 7.9 months (range 5.3–9.8).
- Group 4 (patients with both PFS and OS above the median): 11 patients (26.8%), median PFS 9.9 months (range 4.5–24.4), and median OS 19.6 months (range 11.7–33.7).
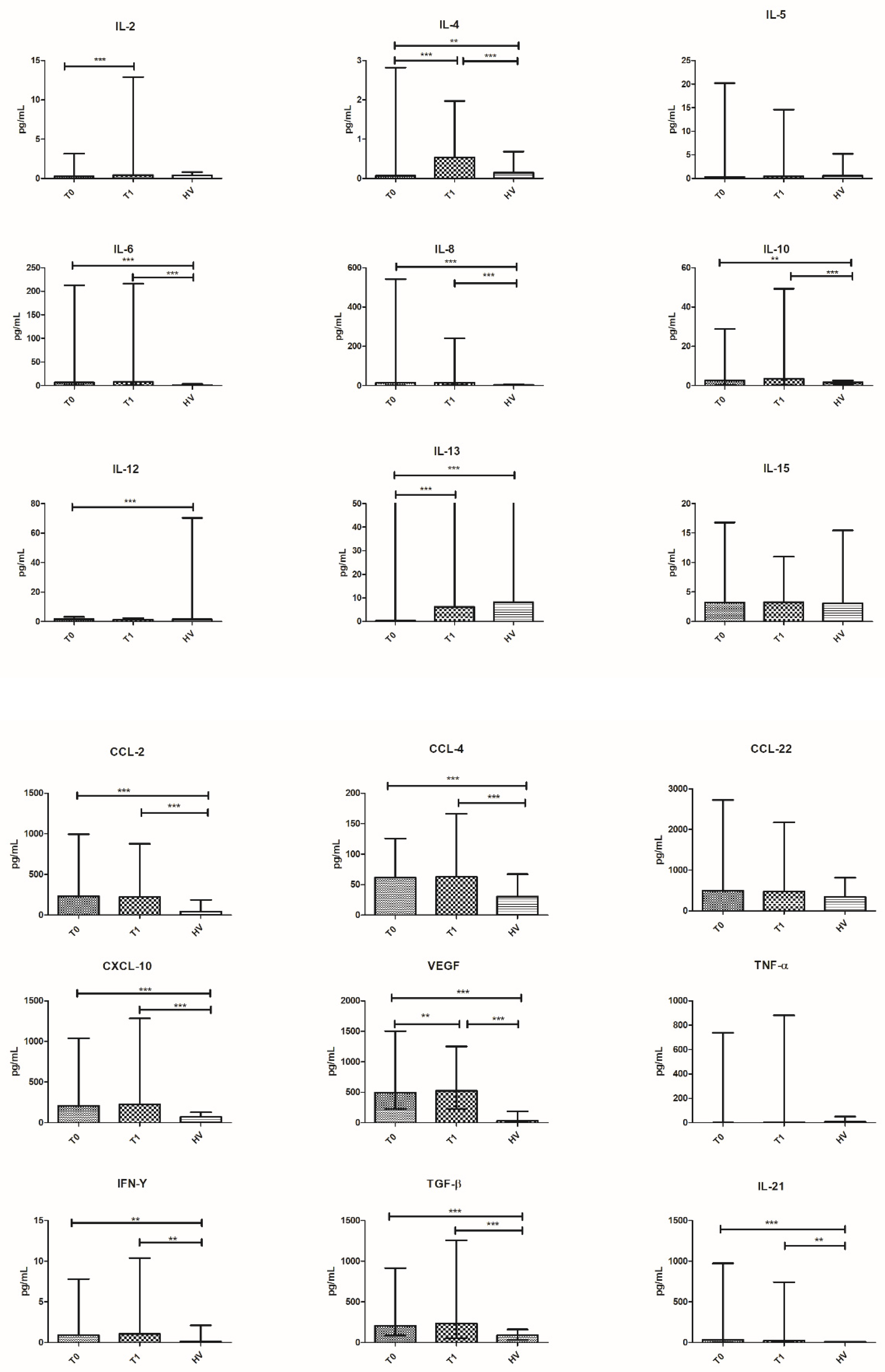
3.4. Cytokine Profile in 15 HV and in 41 Patients
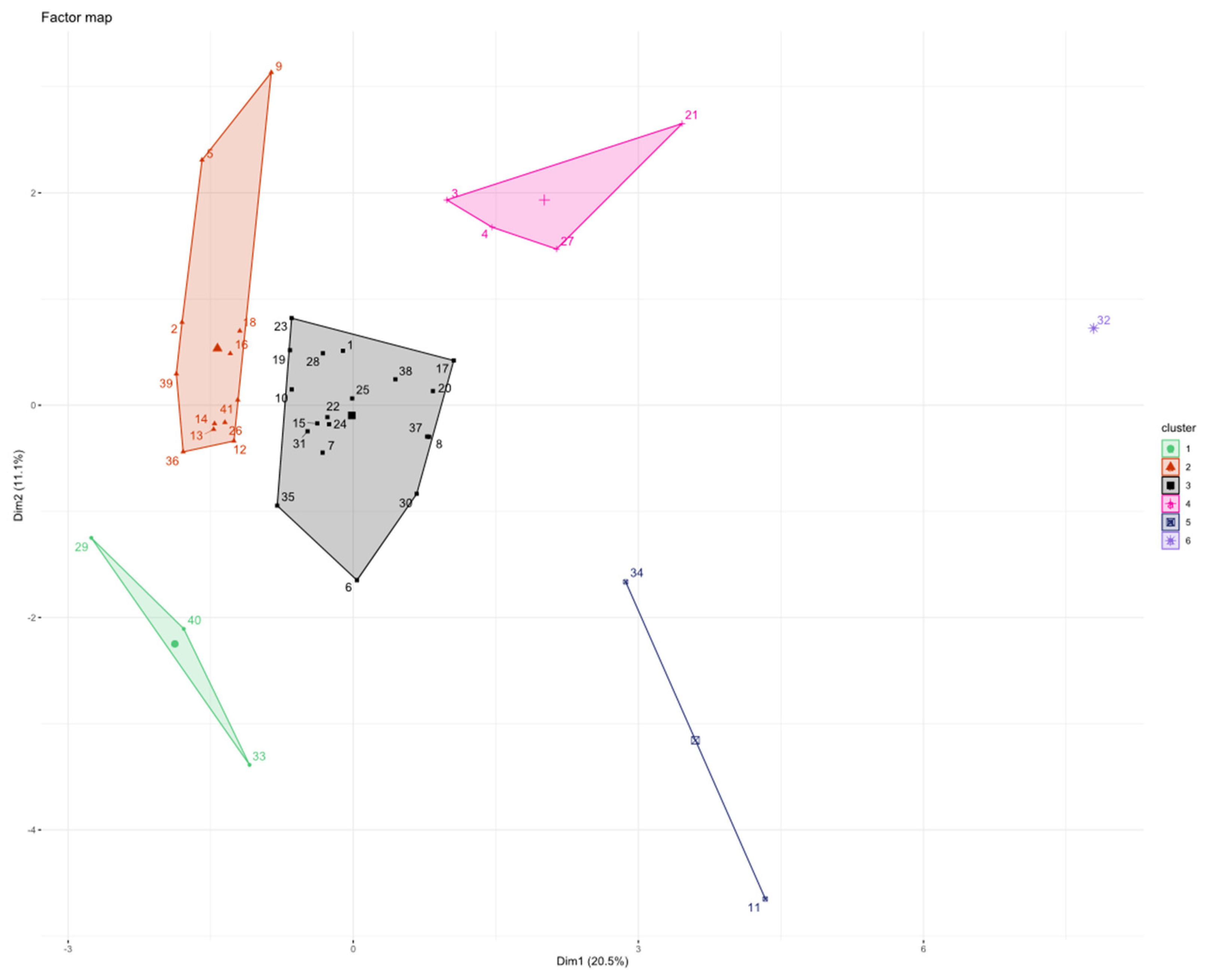
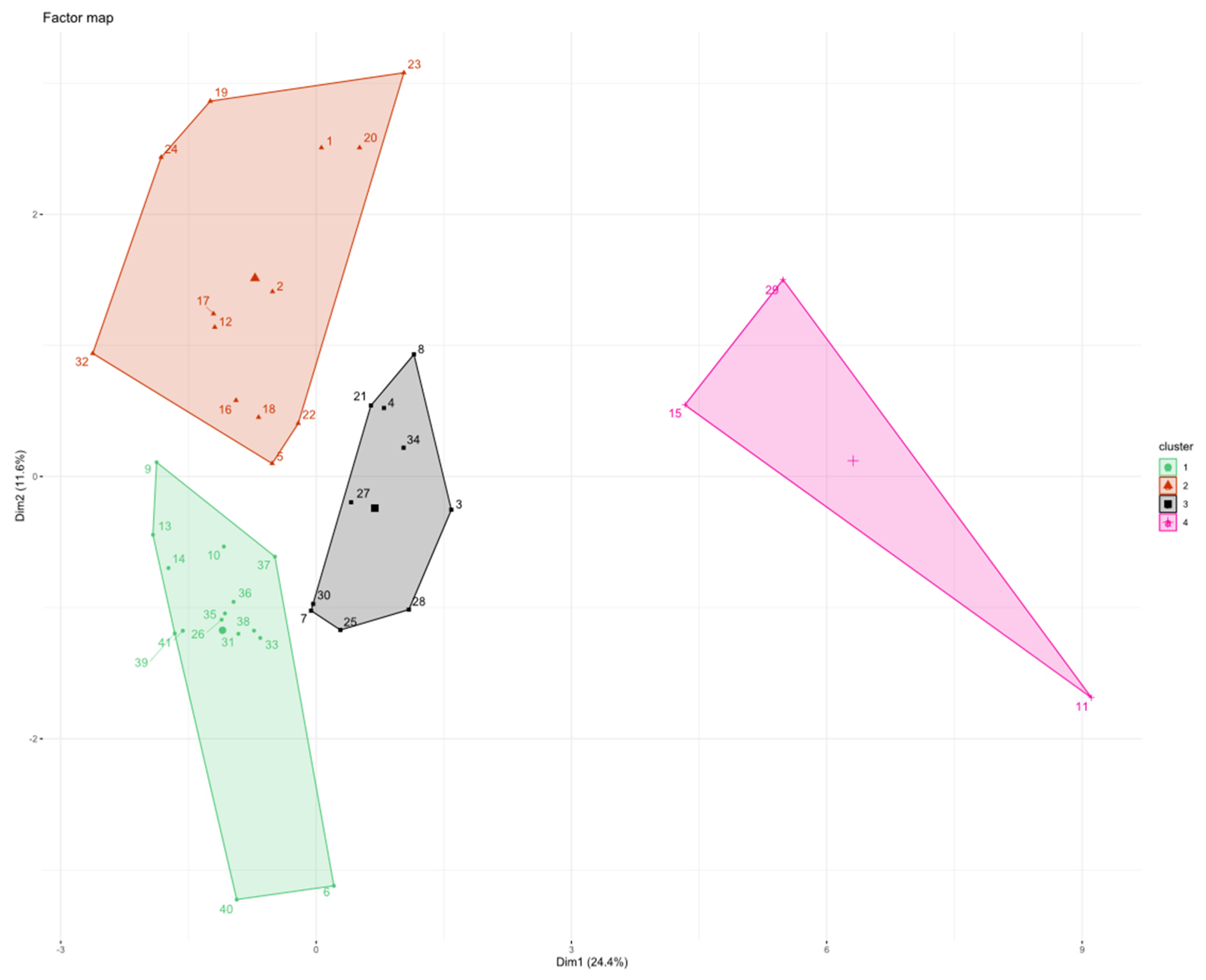
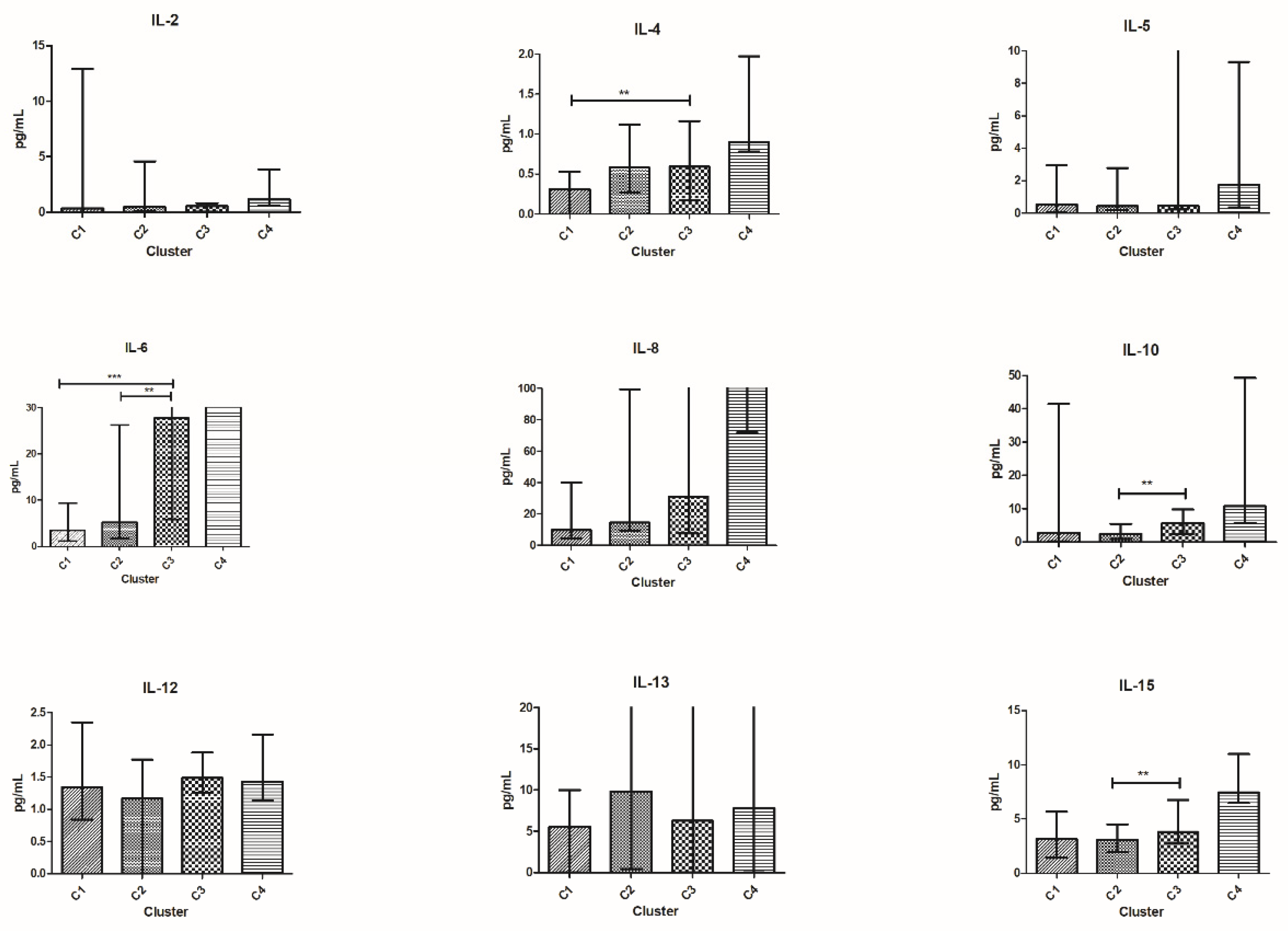
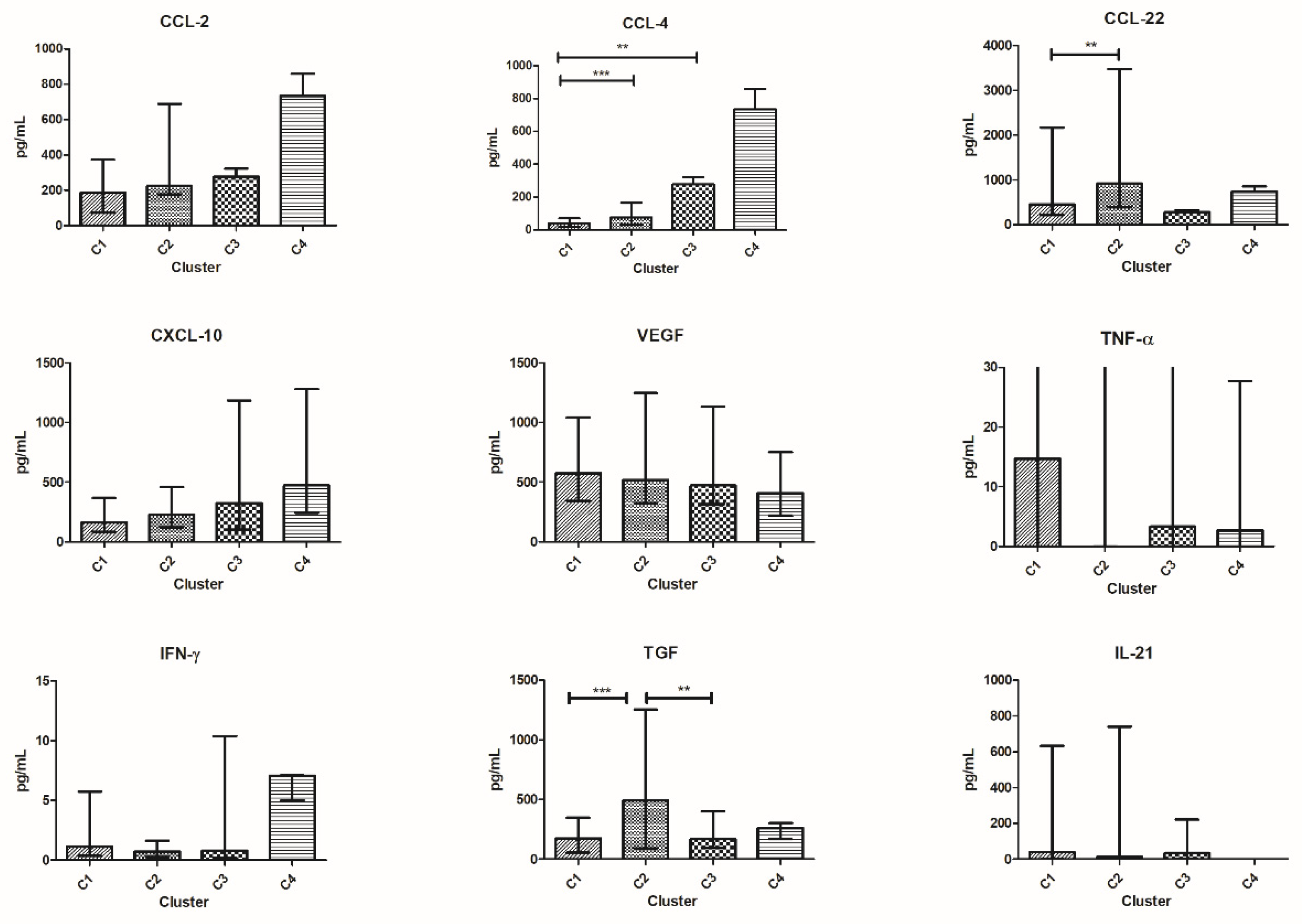
3.5. Patients’ Clusterization and Their Cytokine Profile at T0
- C1T0 = 3 patients (1 patient from group 3 and 2 patients from group 4);
- C2T0 = 12 patients (1 patient from group 1, 7 patients from group 2, and 4 patients from group 4);
- C3T0 = 19 patients (8 patients from group 1, 1 patient from group 2, 6 patients from group 3, and 4 patients from group 4);
- C4T0 = 4 patients (2 patients from group 1, 1 patient from group 2, and 1 patient from group 3);
- C5T0 = 2 patients (1 patient from group 1 and 1 patient from group 3);
- C6T0 = 1 patient from group 4.
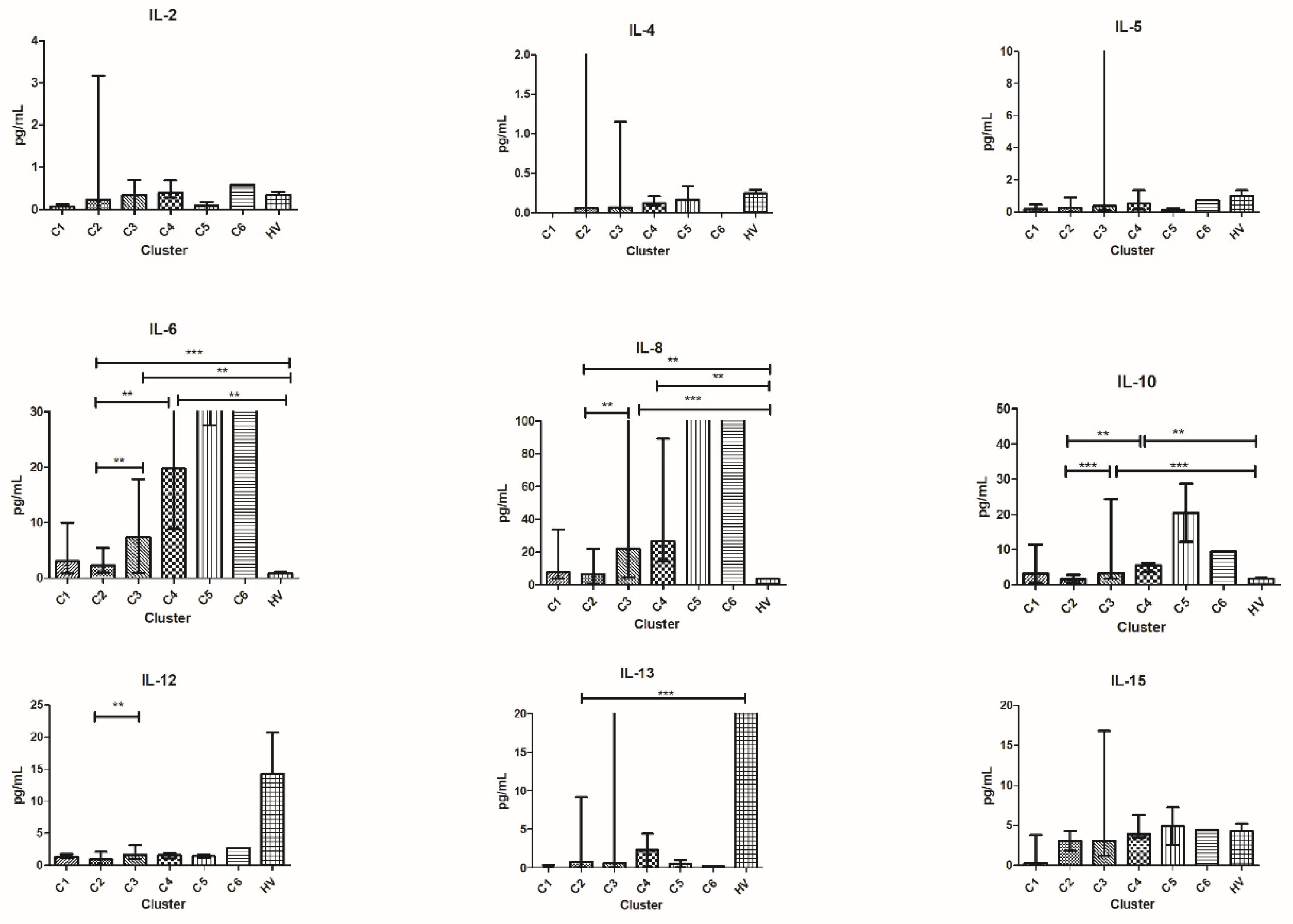
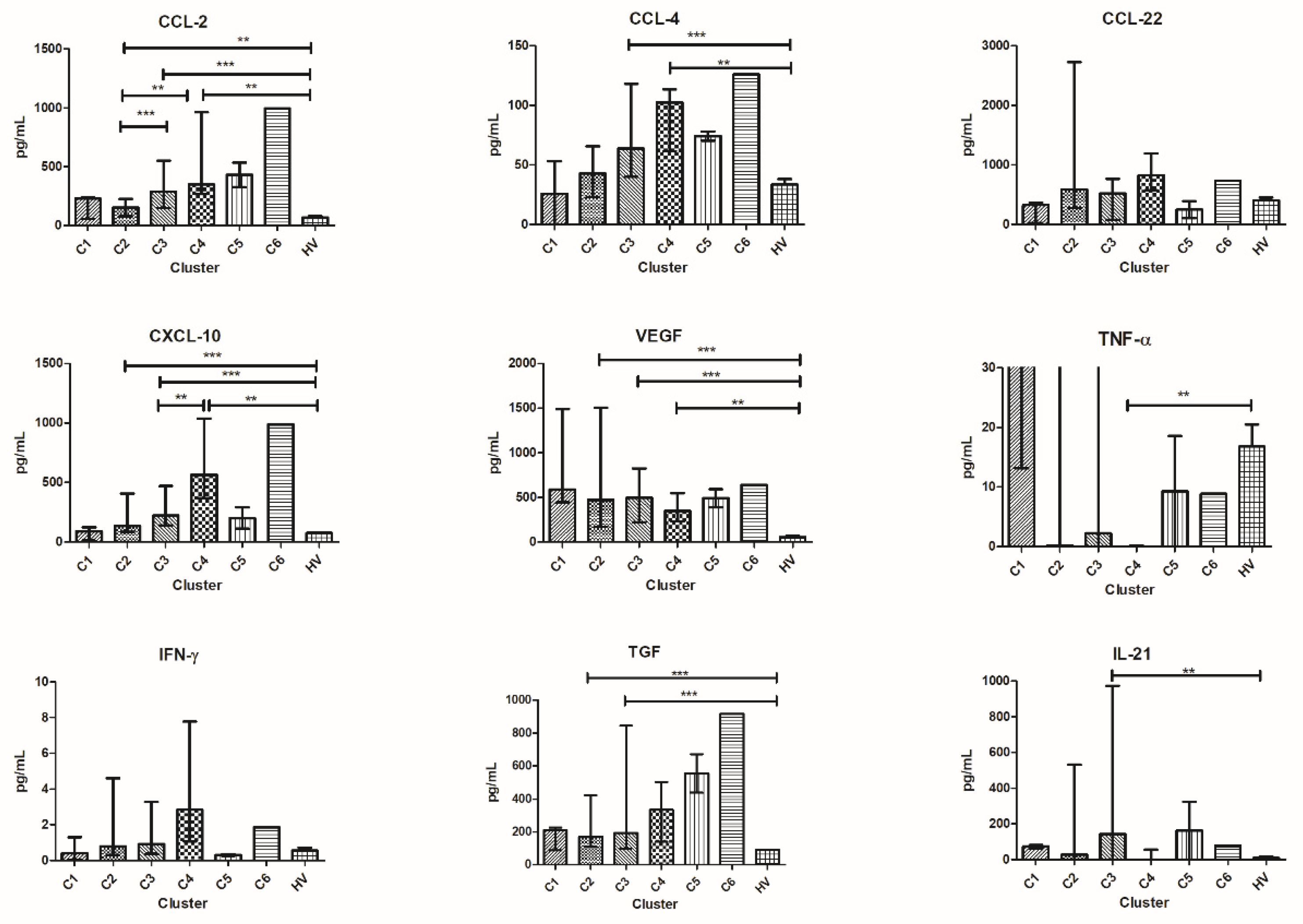
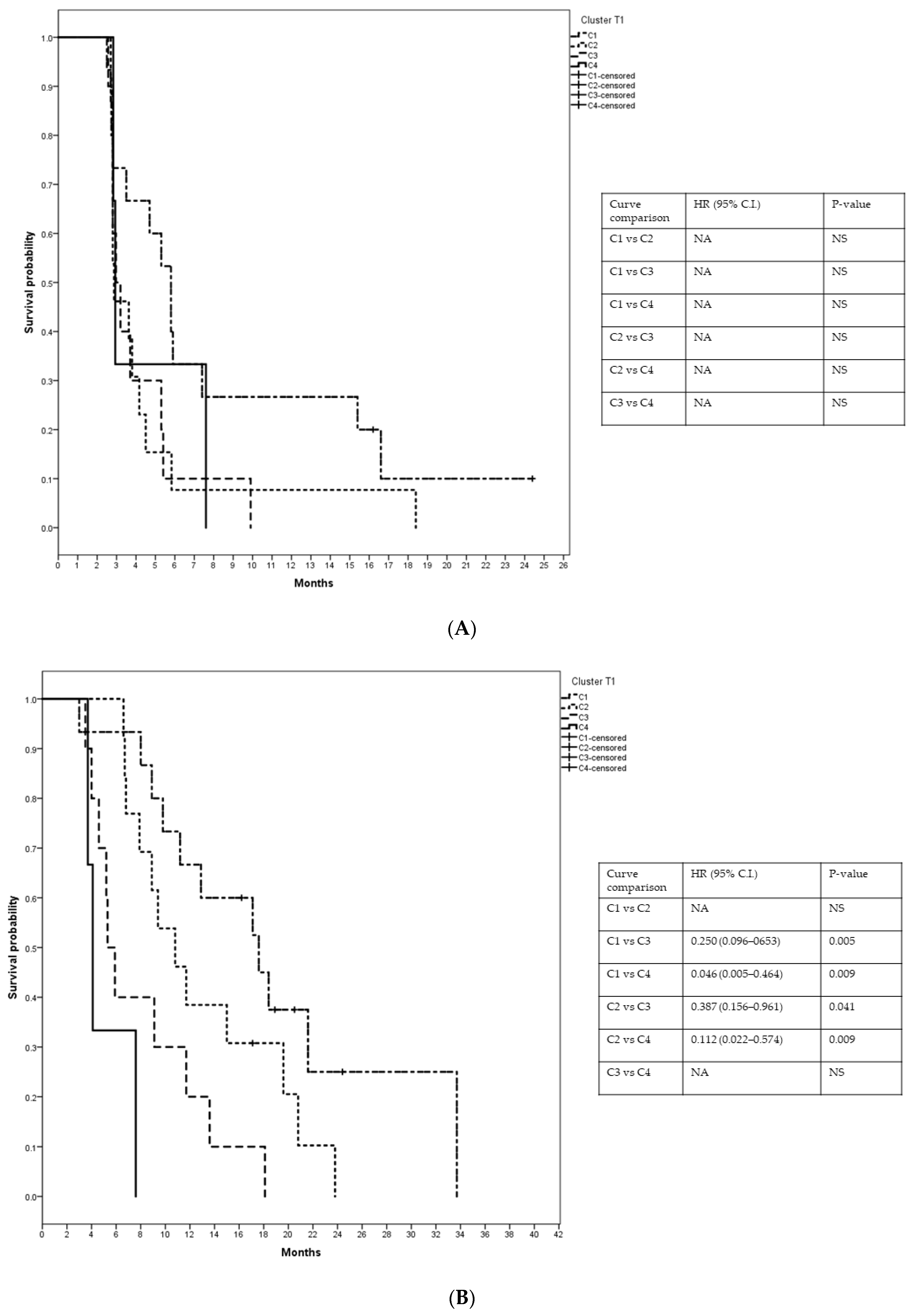
3.6. Patients’ Clusterization and Their Cytokine Profile at T1
- C1T1 = 15 patients (1 patient from group 1, 4 patients from group 2, 3 patients from group 3, and 7 patients from group 4);
- C2T1 = 13 patients (4 patients from group 1, 4 patients from group 2, 3 patients from group 3, and 2 patients from group 4);
- C3T1 = 10 patients (5 patients from group 1, 1 patient from group 2, 2 patients from group 3, and 2 patients from group 4);
- C4T1 = 3 patients (2 patients from group 1 and 1 patient from group 3).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remédios, C.; et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014, 20, 1301–1309. [Google Scholar] [CrossRef]
- Eribulin mesylate (Halaven) for breast cancer. Med. Lett. Drugs Ther. 2011, 53, 30–31.
- Pean, E.; Klaar, S.; Gil Berglund, E.; Salmonson, T.; Borregaard, J.; Hofland, K.F.; Ersbøll, J.; Abadie, E.; Giuliani, R.; Pignatti, F. The European Medicines Agency Review of Eribulin for the Treatment of Patients with Locally Advanced or Metastatic Breast Cancer: Summary of the Scientific Assessment of the Committee for Medicinal Products for Human Use. Clin. Cancer Res. 2012, 18, 4491–4497. [Google Scholar] [CrossRef]
- Garrone, O.; Miraglio, E.; Vandone, A.M.; Vanella, P.; Lingua, D.; Merlano, M.C. Eribulin in advanced breast cancer: Safety, efficacy and new perspectives. Future Oncol. 2017, 13, 2759–2769. [Google Scholar] [CrossRef]
- Ito, K.; Hamamichi, S.; Abe, T.; Akagi, T.; Shirota, H.; Kawano, S.; Asano, M.; Asano, O.; Yokoi, A.; Matsui, J.; et al. Antitumor effects of eribulin depend on modulation of the tumor microenvironment by vascular remodeling in mouse models. Cancer Sci. 2017, 108, 2273–2280. [Google Scholar] [CrossRef]
- Yoshida, T.; Ozawa, Y.; Kimura, T.; Sato, Y.; Kuznetsov, G.; Xu, S.; Uesugi, M.; Agoulnik, S.; Taylor, N.P.; Funahashi, Y.; et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial–mesenchymal transition (EMT) to mesenchymal–epithelial transition (MET) states. Br. J. Cancer 2014, 110, 1497–1505. [Google Scholar] [CrossRef]
- Ueda, S.; Saeki, T.; Takeuchi, H.; Shigekawa, T.; Yamane, T.; Kuji, I.; Osaki, A. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: A comparison to bevacizumab. Br. J. Cancer 2016, 114, 1212–1218. [Google Scholar] [CrossRef]
- Funahashi, Y.; Okamoto, K.; Adachi, Y.; Semba, T.; Uesugi, M.; Ozawa, Y.; Tohyama, O.; Uehara, T.; Kimura, T.; Watanabe, H.; et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014, 105, 1334–1342. [Google Scholar] [CrossRef]
- Abbona, A.; Falletta, A.; Paccagnella, M.; Astigiano, S.; Martini, S.; Denaro, N.; Ruatta, F.; Barbieri, O.; Merlano, M.C.; Garrone, O. Effect of eribulin on angiogenesis and endothelial adhesion molecules. BioRxiv 2021. [Google Scholar] [CrossRef]
- Garrone, O.; Michelotti, A.; Paccagnella, M.; Montemurro, F.; Vandone, A.M.; Abbona, A.; Geuna, E.; Vanella, P.; De Angelis, C.; Nigro, C.L.; et al. Exploratory analysis of circulating cytokines in patients with metastatic breast cancer treated with eribulin: The TRANSERI-GONO (Gruppo Oncologico del Nord Ovest) study. ESMO Open 2020, 5, e000876. [Google Scholar] [CrossRef]
- Schett, G.; Elewaut, D.; McInnes, I.; Dayer, J.-M.; Neurath, M.F. How Cytokines Networks Fuel Inflammation: Toward a cytokine-based disease taxonomy. Nat. Med. 2013, 19, 822–824. [Google Scholar] [CrossRef]
- Capone, F.; Guerriero, E.; Sorice, A.; Colonna, G.; Ciliberto, G.; Costantini, S. Serum Cytokinome Profile Evaluation: A Tool to Define New Diagnostic and Prognostic Markers of Cancer Using Multiplexed Bead-Based Immunoassays. Mediat. Inflamm. 2016, 2016, 3064643. [Google Scholar] [CrossRef]
- Glaab, E.; Baudot, A.; Krasnogor, N.; Valencia, A. Extending pathways and processes using molecular interaction networks to analyse cancer genome data. BMC Bioinform. 2010, 11, 597. [Google Scholar] [CrossRef]
- Jabeen, S.; Espinoza, J.A.; Torland, L.A.; Zucknick, M.; Kumar, S.; Haakensen, V.D.; Lüders, T.; Engebraaten, O.; Børresen-Dale, A.-L.; Kyte, J.A.; et al. Noninvasive profiling of serum cytokines in breast cancer patients and clinicopathological characteristics. OncoImmunology 2018, 8, e1537691. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Sakurai, M.; Yamamoto, Y.; Suzuki, E.; Tsuda, M.; Kataoka, T.R.; Hirata, M.; Nishie, M.; Nojiri, T.; Kumazoe, M.; et al. Alteration of specific cytokine expression patterns in patients with breast cancer. Sci. Rep. 2019, 9, 2924. [Google Scholar] [CrossRef]
- Hong, C.-C.; Yao, S.; McCann, S.E.; Dolnick, R.Y.; Wallace, P.; Gong, Z.; Quan, L.; Lee, K.P.; Evans, S.S.; Repasky, E.A.; et al. Pretreatment levels of circulating Th1 and Th2 cytokines, and their ratios, are associated with ER-negative and triple negative breast cancers. Breast Cancer Res. Treat. 2013, 139, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Junius, S.; Benoy, I.; Van Dam, P.; Vermeulen, P.; Van Marck, E.; Huget, P.; Dirix, L.Y. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int. J. Cancer 2002, 103, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Carranza-Rua, O.; Alfaro, C.; Oñate, C.; Martín-Algarra, S.; Perez, G.; Landazuri, S.F.; Gonzalez, Á.; Gross, S.; Rodriguez, I.; et al. Serum Interleukin-8 Reflects Tumor Burden and Treatment Response across Malignancies of Multiple Tissue Origins. Clin. Cancer Res. 2014, 20, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; A Weich, H.; Brokelmann, M.; Horiguchi, S.; Funata, N.; Ogawa, T.; Toi, M. Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br. J. Cancer 2005, 92, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Haynes, W. Bonferroni Correction. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; p. 154. [Google Scholar]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Zucknick, M.; Nome, M.; Dannenfelser, R.; Fleischer, T.; Kumar, S.; Lüders, T.; Gythfeldt, H.V.D.L.; Troyanskaya, O.; Kyte, J.A.; et al. Serum cytokine levels in breast cancer patients during neoadjuvant treatment with bevacizumab. OncoImmunology 2018, 7, e1457598. [Google Scholar] [CrossRef]
- I Semesiuk, N.; Zhylchuk, A.; Bezdenezhnykh, N.; Lykhova, A.; Vorontsova, A.L.; E Zhylchuk, V.; I Kudryavets, Y. Disseminated tumor cells and enhanced level of some cytokines in bone marrow and peripheral blood of breast cancer patients as predictive factors of tumor progression. Exp. Oncol. 2013, 35, 295–302. [Google Scholar]
- Aronica, M.A.; Goenka, S.; Boothby, M. IL-4-Dependent Induction of BCL-2 and BCL-XLIN Activated T Lymphocytes through a STAT6- and PI 3-Kinase-Independent Pathway. Cytokine 2000, 12, 578–587. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.-S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 2015, 75, 79–88. [Google Scholar] [CrossRef]
- König, A.; Vilsmaier, T.; Rack, B.; Friese, K.; Janni, W.; Jeschke, U.; Andergassen, U.; Trapp, E.; Jückstock, J.; Jäger, B.; et al. Determination of Interleukin-4, -5, -6, -8 and -13 in Serum of Patients with Breast Cancer Before Treatment and its Correlation to Circulating Tumor Cells. Anticancer. Res. 2016, 36, 3123–3130. [Google Scholar]
- Aversa, G.; Punnonen, J.; Cocks, B.G.; Malefyt, R.D.W.; Vega, F.; Zurawski, S.M.; Zurawski, G.; E De Vries, J. An interleukin 4 (IL-4) mutant protein inhibits both IL-4 or IL-13-induced human immunoglobulin G4 (IgG4) and IgE synthesis and B cell proliferation: Support for a common component shared by IL-4 and IL-13 receptors. J. Exp. Med. 1993, 178, 2213–2218. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Immune surveillance: A balance between protumor and antitumor immunity. Curr. Opin. Genet. Dev. 2008, 18, 11–18. [Google Scholar] [CrossRef]
- Zurawski, G.; de Vries, J.E. Interleukin 13 elicits a subset of the activities of its close relative interleukin 4. Stem Cells 1994, 12, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, G.; de Vries, J.E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 1994, 15, 19–26. [Google Scholar] [CrossRef]
- Setrerrahmane, S.; Xu, H. Tumor-related interleukins: Old validated targets for new anti-cancer drug development. Mol. Cancer 2017, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; Choueiri, T.K. IL-8 and cancer prognosis on immunotherapy. Nat. Med. 2020, 26, 650–651. [Google Scholar] [CrossRef]
- Chen, X.-L.; Chen, Z.-Q.; Zhu, S.-L.; Liu, T.-W.; Wen, Y.; Su, Y.-S.; Xi, X.-J.; Hu, Y.; Lian, L.; Liu, F.-B. Prognostic value of transforming growth factor-beta in patients with colorectal cancer who undergo surgery: A meta-analysis. BMC Cancer 2017, 17, 240. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.-D.; Zhan, Y.-T.; Zhu, Y.-H.; Li, Y.; Xie, D.; Guan, X.-Y. High levels of CCL2 or CCL4 in the tumor microenvironment predict unfavorable survival in lung adenocarcinoma. Thorac. Cancer 2018, 9, 775–784. [Google Scholar] [CrossRef]
- Li, J.; Shen, C.; Wang, X.; Lai, Y.; Zhou, K.; Li, P.; Liu, L.; Che, G. Prognostic value of TGF-β in lung cancer: Systematic review and meta-analysis. BMC Cancer 2019, 19, 61. [Google Scholar] [CrossRef]
- Wang, Q.; Schmoeckel, E.; Kost, B.P.; Kuhn, C.; Vattai, A.; Vilsmaier, T.; Mahner, S.; Mayr, D.; Jeschke, U.; Heidegger, H.H. Higher CCL22+ Cell Infiltration is Associated with Poor Prognosis in Cervical Cancer Patients. Cancers 2019, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Cytokines in the balance. Nat. Immunol. 2019, 20, 1557. [CrossRef] [PubMed]
- Haddad, R.; Concha-Benavente, F.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Kasper, S.; Vokes, E.E.; Worden, F.; et al. Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: A subgroup analysis of a randomized phase 3 clinical trial. Cancer 2019, 125, 3208–3218. [Google Scholar] [CrossRef]
- Vilsmaier, T.; SUCCESS Study Group; Rack, B.; Janni, W.; Jeschke, U.; Weissenbacher, T. Angiogenic cytokines and their influence on circulating tumour cells in sera of patients with the primary diagnosis of breast cancer before treatment. BMC Cancer 2016, 16, 547. [Google Scholar] [CrossRef]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number (%) |
|---|---|
| Age (median, range) | 62 (37–86) |
| ECOG PS (median, range) | 0 (0–2) |
| De novo disease | 14 (34.1) |
| ER status | |
| Positive | 34 (82.9) |
| Negative | 7 (17.1) |
| PgR status | |
| Positive | 28 (68.3) |
| Negative | 13 (31.7) |
| Triple negative | 6 (14.6) |
| HER2 status | |
| Positive | 3 (7.3) |
| Negative | 38 (92.7) |
| Neo/adjuvant CT (*) | 20 (74.1) |
| Adjuvant ET (*) | 22 (81.5) |
| Number of previous CT lines for advanced disease | |
| 1 | 10 (24.4) |
| 2 | 20 (48.8) |
| 3 | 5 (12.2) |
| ≥4 | 6 (14.6) |
| Median, range | 2 (1–6) |
| Number of previous ET lines for advanced disease | |
| 1 | 6 (14.6) |
| 2 | 15 (36.6) |
| ≥3 | 11 (26.8) |
| Median, range | 2 (0–4) |
| Number of organs involved | |
| 1 | 3 (7.3) |
| 2 | 14 (34.1) |
| ≥3 | 24 (58.5) |
| Median, range | 3 (1–6) |
| Most common metastatic sites | |
| Bone | 31 (75.6) |
| Liver | 29 (70.7) |
| Soft tissue | 27 (65.8) |
| Lung | 17 (41.5) |
| Pleura | 7 (17.1) |
| CNS | 5 (12.2) |
| Peritoneum | 4 (9.7) |
| Patient ID. | N. of Eribulin Cycles | ECOG PS at PD | Indolent Disease at PD | Symptoms at PD | CB | OS (mos) | N. of Previous ET + CT before Eribulin | Treatment at PD | Response to Treatment at PD |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | Stable | No | No | Yes | 23.4 | 2 + 2 | NPLD | SD |
| 18 | 4 | Stable | No | No | Yes | 21.3 | 2 + 2 | Nab-P | PD |
| 22 | 4 | Stable | No | No | Yes | 11.5 | 3 + 2 | mCAPE | PD |
| 19 | 4 | Stable | No | No | Yes | 12.5 | 2 + 2 | mVNR + CAPE | PD |
| 8 | 4 | Stable | No | No | Yes | 14.8 | 2 + 2 | Nab-P | SD |
| 24 | 4 | Stable | No | No | Yes | 18.1 | 1 + 1 | EVE-EXE | PD |
| 27 | 4 | Stable | No | No | Yes | 11.0 | 2 + 1 | Palbo + Letro | PD |
| 34 | 4 | Stable | No | No | Yes | 20.4 | 0 + 2 | CAPE | SD |
| 43 | 4 | Stable | No | No | Yes | 13.4 | 3 + 1 | PLD | SD |
| Patients (n = 41) | Patients (n = 41) | HV (n = 15) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cytokines at T0 | Median (Range) | p-Value vs. T1 | Cytokines at T1 | Median (Range) | p-Value vs. HV | Cytokines HV | Median (Range) | p-Value vs. T0 |
| IL-2 | 0.27 (0.00–3.17) | 0.0004 | IL-2 | 0.43 (0.00–12.90) | ns | IL-2 | 0.40 (0.00–0.80) | ns |
| IL-4 | 0.07 (0.00–2.82) | 0.0001 | IL-4 | 0.53 (0.00–1.97) | 0.0003 | IL-4 | 0.15 (0.08–0.68) | 0.0045 |
| IL-5 | 0.31 (0.00–20.2) | ns | IL-5 | 0.46 (0.06–14.60) | ns | IL-5 | 0.56 (0.04–5.24) | ns |
| IL-6 | 6.52 (0.82–212.4) | ns | IL-6 | 7.73 (1.15–216.1) | 0.0001 | IL-6 | 0.36 (0.00–3.70) | 0.0001 |
| IL-8 | 15.09 (0.57–542.4) | ns | IL-8 | 14.95 (3.17–240.6) | 0.0001 | IL-8 | 3.31 (2.02–5.76) | 0.0001 |
| IL-10 | 2.61 (0.37–28.7) | ns | IL-10 | 3.52 (0.16–49.26) | 0.0001 | IL-10 | 1.68 (0.90–2.56) | 0.0062 |
| IL-12 | 1.55 (0.0–3.17) | ns | IL-12 | 1.32 (0.00–2.35) | ns | IL-12 | 1.47 (0.47–70.36) | ns |
| Il-13 | 0.34 (0.00–1063) | 0.0001 | IL-13 | 6.17 (0.00–916) | ns | IL-13 | 8.14 (2.36–74.36) | 0.0001 |
| IL-15 | 3.23 (0.27–16.80) | ns | IL-15 | 3.27 (1.44–11.00) | ns | IL-15 | 3.10 (0.65–15.45) | ns |
| IL-21 | 32.53 (0.0–973.5) | ns | IL-21 | 22.04 (0.0–742.7) | 0.0018 | IL-21 | 0.00 (0.00–143.5) | 0.0008 |
| TGF-β | 204.8 (86.55–915.9) | ns | TGF-β | 233.9 (54.09–1255) | 0.0001 | TGF-β | 90.24 (31.33–160.2) | 0.0001 |
| TNF-α | 0.43 (0.0–737.7) | ns | TNF-α | 2.65 (0.00–879.8) | ns | TNF-α | 8.24 (2.50–50.00) | ns |
| VEGF | 495.2 (224.9–1503) | 0.0045 | VEGF | 523.7 (220.7–1248) | 0.0001 | VEGF | 30.70 (9.80–186.3) | 0.0001 |
| IFN-γ | 0.91 (0.03–7.79) | ns | IFN-γ | 1.04 (0.20–10.40) | 0.0039 | IFN-γ | 0.12 (0.00–2.10) | 0.0091 |
| CCL-2 | 231.4 (56.08–995.2) | ns | CCL-2 | 225.5 (74.09–878.1) | 0.0001 | CCL-2 | 44.72 (18.49–187.0) | 0.0001 |
| CCL-4 | 61.50 (0.00–125.8) | ns | CCL-4 | 62.73 (20.56–166.3) | 0.0002 | CCL-4 | 30.67 (4.27–67.20) | 0.0003 |
| CCL-22 | 497.4 (56.08–2727) | ns | CCL-22 | 476.2 (115.4–2173) | ns | CCL-22 | 347.2 (100.2–819.0) | ns |
| CXCL-10 | 210.1 (12.98–1039) | ns | CXCL-10 | 229.3 (82.88–1282) | 0.0001 | CXCL-10 | 71.70 (49.65–128.0) | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paccagnella, M.; Abbona, A.; Michelotti, A.; Geuna, E.; Ruatta, F.; Landucci, E.; Denaro, N.; Vanella, P.; Lo Nigro, C.; Galizia, D.; et al. Circulating Cytokines in Metastatic Breast Cancer Patients Select Different Prognostic Groups and Patients Who Might Benefit from Treatment beyond Progression. Vaccines 2022, 10, 78. https://doi.org/10.3390/vaccines10010078
Paccagnella M, Abbona A, Michelotti A, Geuna E, Ruatta F, Landucci E, Denaro N, Vanella P, Lo Nigro C, Galizia D, et al. Circulating Cytokines in Metastatic Breast Cancer Patients Select Different Prognostic Groups and Patients Who Might Benefit from Treatment beyond Progression. Vaccines. 2022; 10(1):78. https://doi.org/10.3390/vaccines10010078
Chicago/Turabian StylePaccagnella, Matteo, Andrea Abbona, Andrea Michelotti, Elena Geuna, Fiorella Ruatta, Elisabetta Landucci, Nerina Denaro, Paola Vanella, Cristiana Lo Nigro, Danilo Galizia, and et al. 2022. "Circulating Cytokines in Metastatic Breast Cancer Patients Select Different Prognostic Groups and Patients Who Might Benefit from Treatment beyond Progression" Vaccines 10, no. 1: 78. https://doi.org/10.3390/vaccines10010078
APA StylePaccagnella, M., Abbona, A., Michelotti, A., Geuna, E., Ruatta, F., Landucci, E., Denaro, N., Vanella, P., Lo Nigro, C., Galizia, D., Merlano, M., & Garrone, O. (2022). Circulating Cytokines in Metastatic Breast Cancer Patients Select Different Prognostic Groups and Patients Who Might Benefit from Treatment beyond Progression. Vaccines, 10(1), 78. https://doi.org/10.3390/vaccines10010078










