The Chimeric Binjari-Zika Vaccine Provides Long-Term Protection against ZIKA Virus Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. The Vaccine and Vaccination
2.3. Neutralizing Antibody Response Determination
2.4. ZIKV Challenge of IFNAR-/- Mice
2.5. Statistical Analysis
3. Results
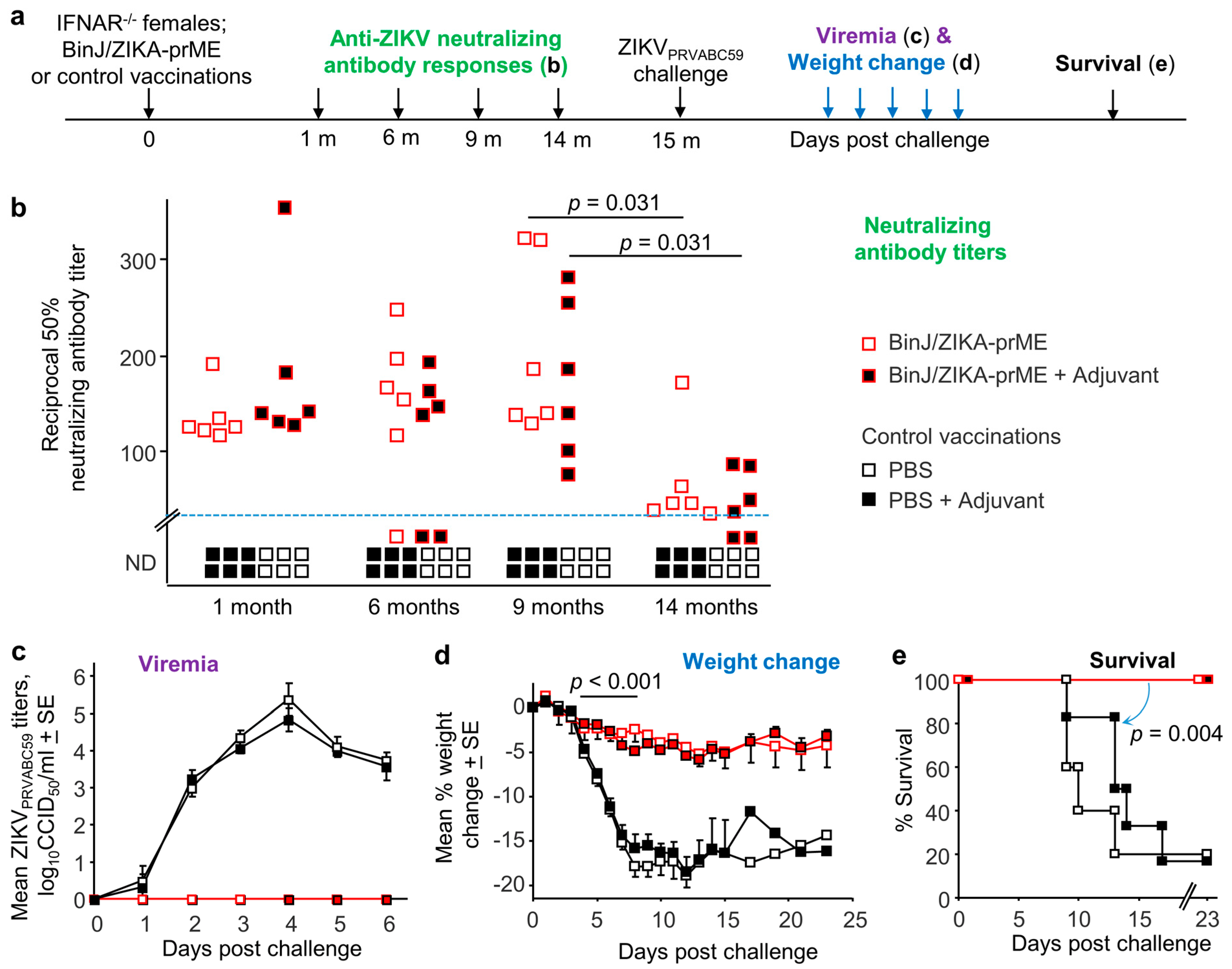
3.1. Maintenance of Neutralizing Antibody Responses after BinJ/ZIKA-prME Vaccination
3.2. BinJ/ZIKA-prME Vaccination Provides Long-Term Protection against Infection and Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, M.M.; Medronho, R.A.; Cunha, A. Zika virus in Brazil and worldwide: A narrative review. Paediatr. Int. Child Health 2021, 41, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Duttine, A.; Smythe, T.; Ribiero Calheiro de Sa, M.; Ferrite, S.; Zuurmond, M.; Moreira, M.E.; Collins, A.; Milner, K.; Kuper, H. Congenital Zika Syndrome-Assessing the Need for a Family Support Programme in Brazil. Int. J. Environ. Res. Public Health 2020, 17, 3559. [Google Scholar] [CrossRef] [PubMed]
- Adams Waldorf, K.M.; Olson, E.M.; Nelson, B.R.; Little, M.E.; Rajagopal, L. The Aftermath of Zika: Need for Long-Term Monitoring of Exposed Children. Trends Microbiol. 2018, 26, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the global burden of chikungunya and Zika viruses: A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Moreira, M.E.L.; Barros, L.B.P.; Costa, A.; Fernandes, S.; Kuper, H. Catastrophic expenditure on congenital Zika syndrome: Results of a cross-sectional study of caregivers of children in Rio de Janeiro, Brazil. Cad Saude Publica 2021, 37, e00007021. [Google Scholar] [CrossRef] [PubMed]
- Castanha, P.M.S.; Marques, E.T.A. A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development. Viruses 2020, 12, 1371. [Google Scholar] [CrossRef]
- Zhou, K.; Li, C.; Shi, W.; Hu, X.; Nandakumar, K.S.; Jiang, S.; Zhang, N. Current Progress in the Development of Zika Virus Vaccines. Vaccines 2021, 9, 1004. [Google Scholar] [CrossRef]
- Han, H.-H.; Diaz, C.; Acosta, C.J.; Liu, M.; Borkowski, A. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in healthy adults: An observer-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1282–1292. [Google Scholar] [CrossRef]
- Thomas, S.J.; Barrett, A. Zika vaccine pre-clinical and clinical data review with perspectives on the future development. Hum. Vaccin Immunother. 2020, 16, 2524–2536. [Google Scholar] [CrossRef]
- Harrison, J.J.; Hobson-Peters, J.; Colmant, A.M.G.; Koh, J.; Newton, N.D.; Warrilow, D.; Bielefeldt-Ohmann, H.; Piyasena, T.B.H.; O’Brien, C.A.; Vet, L.J.; et al. Antigenic Characterization of New Lineage II Insect-Specific Flaviviruses in Australian Mosquitoes and Identification of Host Restriction Factors. mSphere 2020, 5, e00095-20. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Hobson-Peters, J.; Bielefeldt-Ohmann, H.; Hall, R.A. Chimeric Vaccines Based on Novel Insect-Specific Flaviviruses. Vaccines 2021, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Peters, J.; Harrison, J.J.; Watterson, D.; Hazlewood, J.E.; Vet, L.J.; Newton, N.D.; Warrilow, D.; Colmant, A.M.G.; Taylor, C.; Huang, B.; et al. A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 2019, 11, eaax7888. [Google Scholar] [CrossRef] [PubMed]
- Vet, L.J.; Setoh, Y.X.; Amarilla, A.A.; Habarugira, G.; Suen, W.W.; Newton, N.D.; Harrison, J.J.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. Protective Efficacy of a Chimeric Insect-Specific Flavivirus Vaccine against West Nile Virus. Vaccines 2020, 8, 258. [Google Scholar] [CrossRef]
- Choo, J.J.Y.; Vet, L.J.; McMillan, C.L.D.; Harrison, J.J.; Scott, C.A.P.; Depelsenaire, A.C.I.; Fernando, G.J.P.; Watterson, D.; Hall, R.A.; Young, P.R.; et al. A chimeric dengue virus vaccine candidate delivered by high density microarray patches protects against infection in mice. NPJ Vaccines 2021, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Hazlewood, J.E.; Rawle, D.J.; Tang, B.; Yan, K.; Vet, L.J.; Nakayama, E.; Hobson-Peters, J.; Hall, R.A.; Suhrbier, A. A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines 2020, 8, 496. [Google Scholar] [CrossRef]
- Yan, K.; Vet, L.J.; Tang, B.; Hobson-Peters, J.; Rawle, D.J.; Le, T.T.; Larcher, T.; Hall, R.A.; Suhrbier, A. A Yellow Fever Virus 17D Infection and Disease Mouse Model Used to Evaluate a Chimeric Binjari-Yellow Fever Virus Vaccine. Vaccines 2020, 8, 368. [Google Scholar] [CrossRef]
- Setoh, Y.X.; Prow, N.A.; Peng, N.; Hugo, L.E.; Devine, G.; Hazlewood, J.E.; Suhrbier, A.; Khromykh, A.A. De Novo Generation and Characterization of New Zika Virus Isolate Using Sequence Data from a Microcephaly Case. mSphere 2017, 2, e00190-17. [Google Scholar] [CrossRef] [Green Version]
- Swann, J.B.; Hayakawa, Y.; Zerafa, N.; Sheehan, K.C.; Scott, B.; Schreiber, R.D.; Hertzog, P.; Smyth, M.J. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 2007, 178, 7540–7549. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Tajima, S.; Nakayama, E.; Kawai, Y.; Taniguchi, S.; Shibasaki, K.; Taira, M.; Maeki, T.; Lim, C.K.; Takasaki, T.; et al. Characterization of large and small-plaque variants in the Zika virus clinical isolate ZIKV/Hu/S36/Chiba/2016. Sci. Rep. 2017, 7, 16160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.J.; Le, T.T.T.; Dobbin, C.A.; Banovic, T.; Howard, C.B.; Flores, F.d.M.L.; Vanags, D.; Naylor, D.J.; Hill, G.R.; Suhrbier, A. Heat Shock Protein 10 Inhibits Lipopolysaccharide-induced Inflammatory Mediator Production. J. Biol. Chem. 2005, 280, 4037–4047. [Google Scholar] [CrossRef] [Green Version]
- La Linn, M.; Bellett, A.J.; Parsons, P.G.; Suhrbier, A. Complete removal of mycoplasma from viral preparations using solvent extraction. J. Virol. Methods 1995, 52, 51–54. [Google Scholar] [CrossRef]
- Nakayama, E.; Kato, F.; Tajima, S.; Ogawa, S.; Yan, K.; Takahashi, K.; Sato, Y.; Suzuki, T.; Kawai, Y.; Inagaki, T.; et al. Neuroinvasiveness of the MR766 strain of Zika virus in IFNAR-/- mice maps to prM residues conserved amongst African genotype viruses. PLoS Pathog. 2021, 17, e1009788. [Google Scholar] [CrossRef]
- Oya, A. Japanese encephalitis vaccine. Acta Paediatr. Jpn. 1988, 30, 175–184. [Google Scholar] [CrossRef]
- Hassert, M.; Wolf, K.J.; Schwetye, K.E.; DiPaolo, R.J.; Brien, J.D.; Pinto, A.K. CD4+T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018, 14, e1007237. [Google Scholar] [CrossRef]
- Ye, X.; Liu, X.; Shu, T.; Deng, W.; Liao, M.; Zheng, Y.; Zheng, X.; Zhang, X.; Li, T.; Fan, W.; et al. A Live-Attenuated Zika Virus Vaccine with High Production Capacity Confers Effective Protection in Neonatal Mice. J. Virol. 2021, 95, e0038321. [Google Scholar] [CrossRef]
- Adam, A.; Fontes-Garfias, C.R.; Sarathy, V.V.; Liu, Y.; Luo, H.; Davis, E.; Li, W.; Muruato, A.E.; Wang, B.; Ahatov, R.; et al. A genetically stable Zika virus vaccine candidate protects mice against virus infection and vertical transmission. NPJ Vaccines 2021, 6, 27. [Google Scholar] [CrossRef]
- Auguste, A.J.; Langsjoen, R.M.; Porier, D.L.; Erasmus, J.H.; Bergren, N.A.; Bolling, B.G.; Luo, H.; Singh, A.; Guzman, H.; Popov, V.L.; et al. Isolation of a novel insect-specific flavivirus with immunomodulatory effects in vertebrate systems. Virology 2021, 562, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Cagigi, A.; Lore, K. Immune Responses Induced by mRNA Vaccination in Mice, Monkeys and Humans. Vaccines 2021, 9, 61. [Google Scholar] [CrossRef]
- Weir, G.M.; Karkada, M.; Hoskin, D.; Stanford, M.M.; MacDonald, L.; Mansour, M.; Liwski, R.S. Combination of poly I:C and Pam3CSK4 enhances activation of B cells in vitro and boosts antibody responses to protein vaccines in vivo. PLoS ONE 2017, 12, e0180073. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.P.; Coirada, F.C.; de Souza Apostolico, J.; Tomita, N.; Fernandes, E.R.; Santos Souza, H.F.; Chura-Chambi, R.M.; Morganti, L.; Boscardin, S.B.; Rosa, D.S. Prime-boost with Chikungunya virus E2 envelope protein combined with Poly (I:C) induces specific humoral and cellular immune responses. Curr. Res. Immunol. 2021, 2, 23–31. [Google Scholar] [CrossRef]
- Hall, J.C.; Rosen, A. Type I interferons: Crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 2010, 6, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, C.L.; Abendroth, A.; McSharry, B.P.; Slobedman, B. Interferon-Independent Innate Responses to Cytomegalovirus. Front. Immunol. 2019, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Olagnier, D.; Scholte, F.E.; Chiang, C.; Albulescu, I.C.; Nichols, C.; He, Z.; Lin, R.; Snijder, E.J.; van Hemert, M.J.; Hiscott, J. Inhibition of dengue and chikungunya virus infections by RIG-I-mediated type I interferon-independent stimulation of the innate antiviral response. J. Virol. 2014, 88, 4180–4194. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.A.; Prow, N.A.; Schroder, W.A.; Ellis, J.J.; Cumming, H.E.; Gearing, L.J.; Poo, Y.S.; Taylor, A.; Hertzog, P.J.; Di Giallonardo, F.; et al. RNA-Seq analysis of chikungunya virus infection and identification of granzyme A as a major promoter of arthritic inflammation. PLoS Pathog. 2017, 13, e1006155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chackerian, B.; Peabody, D.S. Factors That Govern the Induction of Long-Lived Antibody Responses. Viruses 2020, 12, 74. [Google Scholar] [CrossRef] [Green Version]
- Krol, E.; Brzuska, G.; Szewczyk, B. Production and Biomedical Application of Flavivirus-like Particles. Trends Biotechnol. 2019, 37, 1202–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Yang, Y.; Muruato, A.E.; Zou, J.; Shan, C.; Nunes, B.T.; Medeiros, D.B.; Vasconcelos, P.F.; Weaver, S.C.; Rossi, S.L.; et al. Understanding Zika Virus Stability and Developing a Chimeric Vaccine through Functional Analysis. mBio 2017, 8, e02134-16. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.M.; Newton, N.D.; Modhiran, N.; Scott, C.A.P.; Venugopal, H.; Vet, L.J.; Young, P.R.; Hall, R.A.; Hobson-Peters, J.; Coulibaly, F.; et al. A unified route for flavivirus structures uncovers essential pocket factors conserved across pathogenic viruses. Nat. Commun. 2021, 12, 3266. [Google Scholar] [CrossRef]
- Clarke, E.C.; Bradfute, S.B. The use of mice lacking type I or both type I and type II interferon responses in research on hemorrhagic fever viruses. Part 1: Potential effects on adaptive immunity and response to vaccination. Antiviral. Res. 2020, 174, 104703. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazlewood, J.E.; Tang, B.; Yan, K.; Rawle, D.J.; Harrison, J.J.; Hall, R.A.; Hobson-Peters, J.; Suhrbier, A. The Chimeric Binjari-Zika Vaccine Provides Long-Term Protection against ZIKA Virus Challenge. Vaccines 2022, 10, 85. https://doi.org/10.3390/vaccines10010085
Hazlewood JE, Tang B, Yan K, Rawle DJ, Harrison JJ, Hall RA, Hobson-Peters J, Suhrbier A. The Chimeric Binjari-Zika Vaccine Provides Long-Term Protection against ZIKA Virus Challenge. Vaccines. 2022; 10(1):85. https://doi.org/10.3390/vaccines10010085
Chicago/Turabian StyleHazlewood, Jessamine E., Bing Tang, Kexin Yan, Daniel J. Rawle, Jessica J. Harrison, Roy A. Hall, Jody Hobson-Peters, and Andreas Suhrbier. 2022. "The Chimeric Binjari-Zika Vaccine Provides Long-Term Protection against ZIKA Virus Challenge" Vaccines 10, no. 1: 85. https://doi.org/10.3390/vaccines10010085





