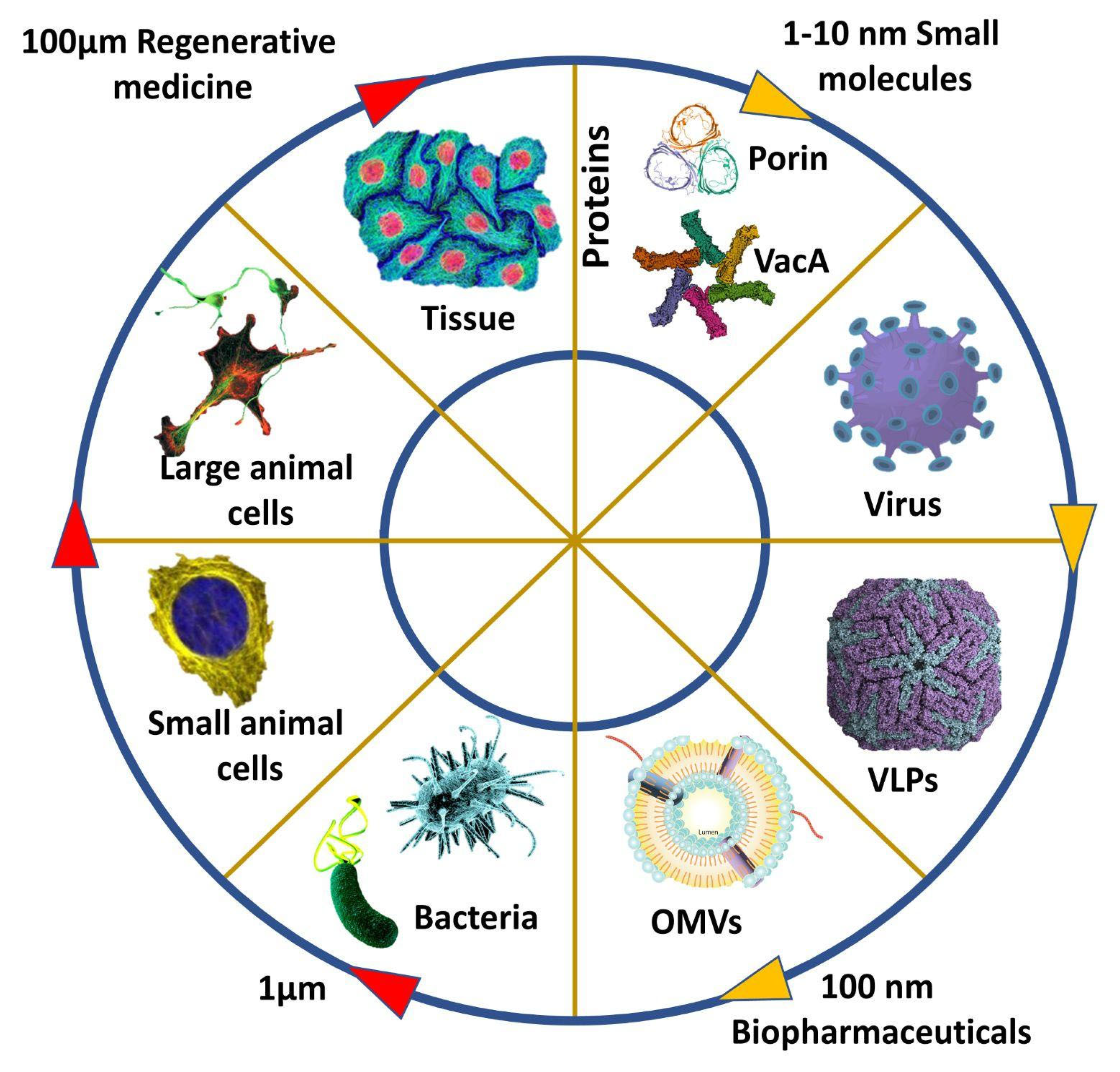
Outer Membrane Vesicles: An Emerging Vaccine Platform
Abstract
:1. Introduction
2. Formation of OMVs
3. Species Producing OMVs
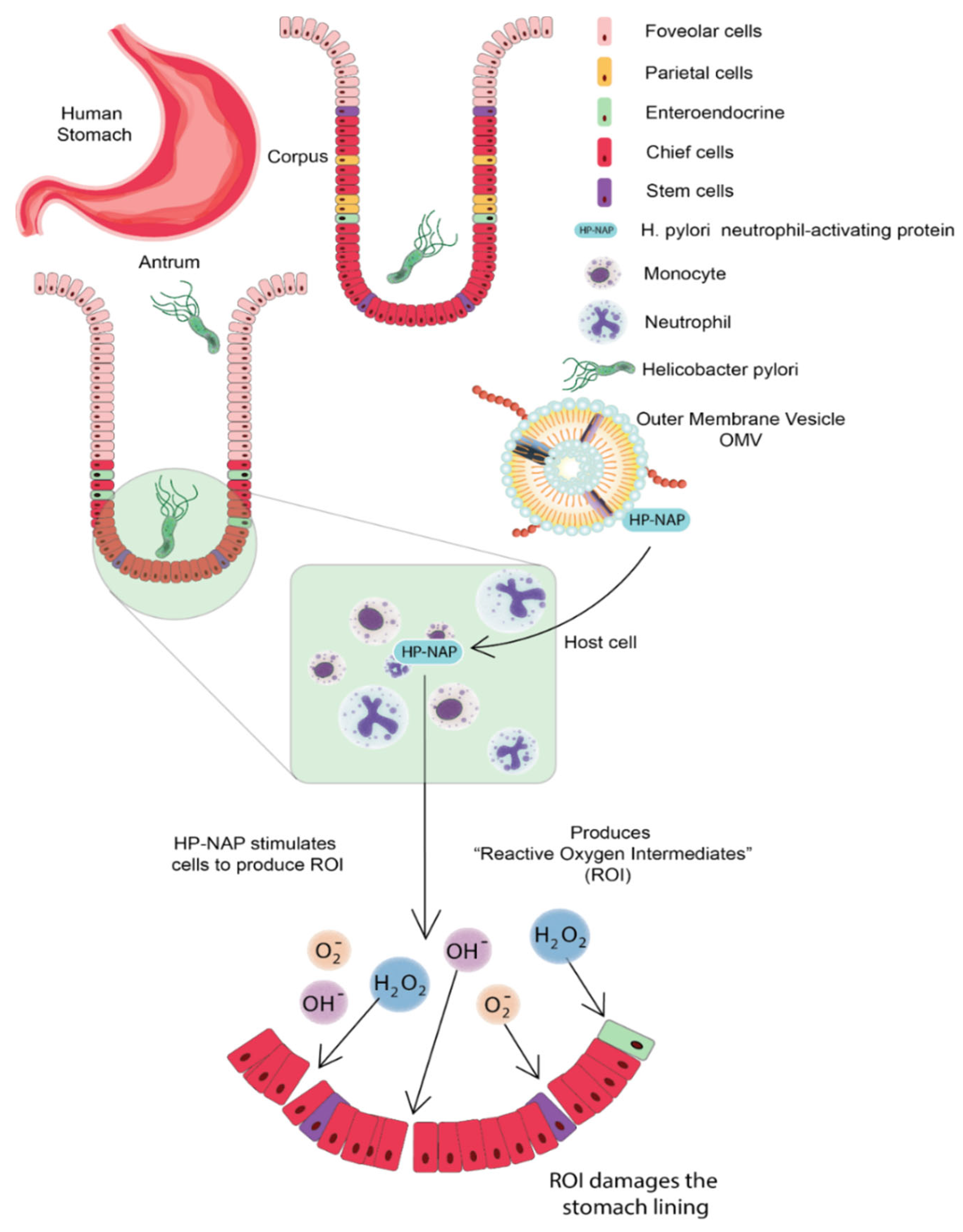
3.1. Helicobacter pylori
3.2. Neisseria meningitidis
3.3. Campylobacter jejuni
4. OMV-Based Vaccine Delivery
MERS-CoV and SARS-CoV-2
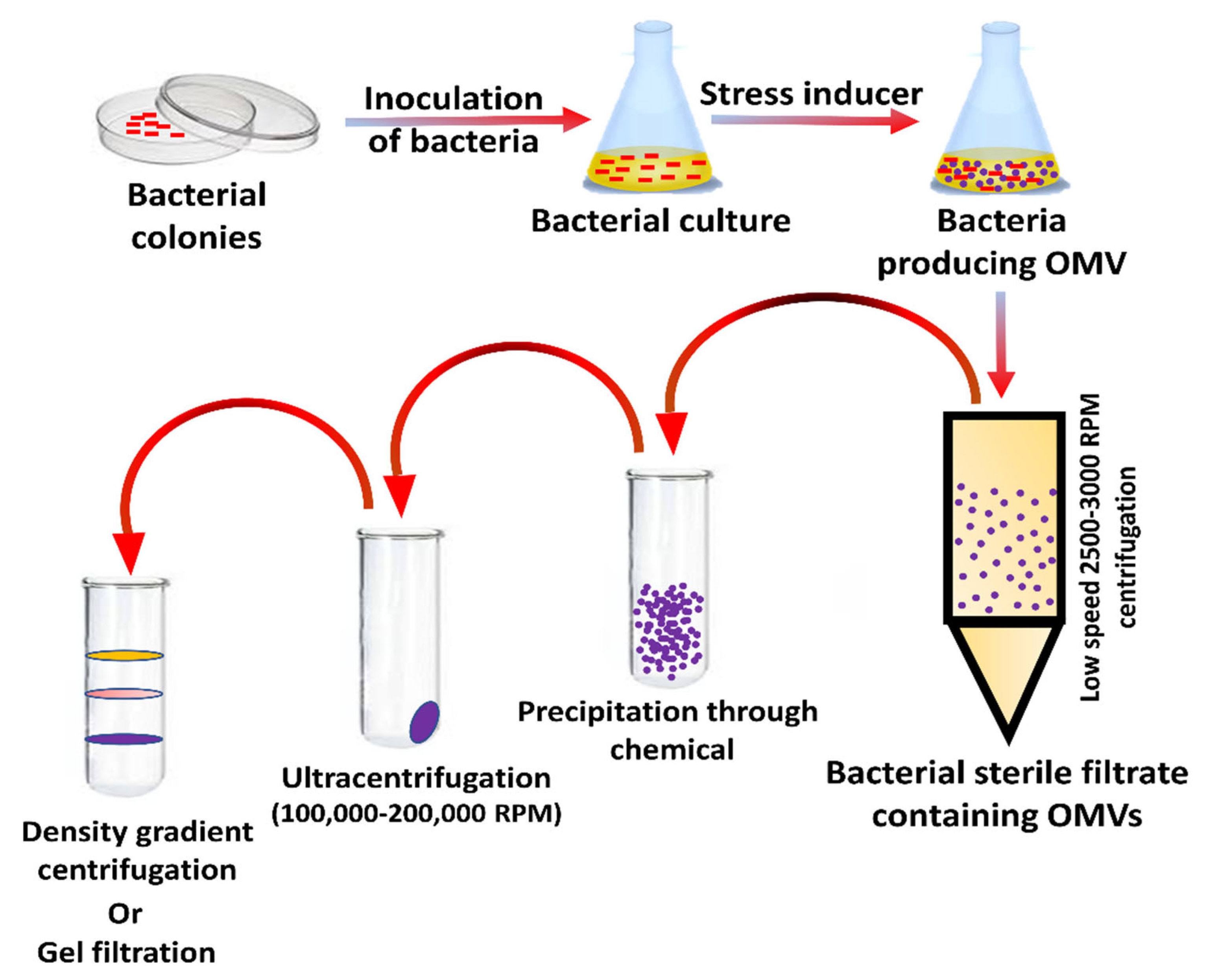
5. Preparation and Separation of OMVs
6. Uptake of OMVs by Host Cells
7. OMVs-Based Therapeutics
8. Native OMV Vaccines
9. Heterologous Vaccines
10. OMV-Based Novel Adjuvants
10.1. Mechanisms
10.2. Applications
10.3. Advantages
10.4. Limitations
11. Future Prospective and Scope
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fulsundar, S.; Harms, K.; Flaten, G.E.; Johnsen, P.J.; Chopade, B.A.; Nielsen, K.M. Gene Transfer Potential of Outer Membrane Vesicles of Acinetobacter Baylyi and Effects of Stress on Vesiculation. Appl. Environ. Microbiol. 2014, 80, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-Negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.M.; Jagannadham, M.V.Y. Biogenesis and Multifaceted Roles of Outer Membrane Vesicles from Gram-Negative Bacteria. Microbiology 2014, 160, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.N.; Das, J.Y. Electron Microscopic Observations on the Excretion of Cell-Wall Material by Vibrio Cholerae. Microbiology 1967, 49, 1–11. [Google Scholar] [CrossRef]
- Mayrand, D.; Grenier, D. Biological Activities of Outer Membrane Vesicles. Can. J. Microbiol. 1989, 35, 607–613. [Google Scholar] [CrossRef]
- Hussain, S.; Bernstein, H.D. The Bam Complex Catalyzes Efficient Insertion of Bacterial Outer Membrane Proteins into Membrane Vesicles of Variable Lipid Composition. J. Biol. Chem. 2018, 293, 2959–2973. [Google Scholar] [CrossRef]
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter Pylori Outer Membrane Vesicle Size Determines Their Mechanisms of Host Cell Entry and Protein Content. Front. Immunol. 2018, 9, 1466. [Google Scholar] [CrossRef]
- Bonnington, K.E.; Kuehn, M.J. Protein Selection and Export via Outer Membrane Vesicles. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 1612–1619. [Google Scholar] [CrossRef]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A Novel Mechanism for the Biogenesis of Outer Membrane Vesicles in Gram-Negative Bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [Green Version]
- Alfarouk, K.O.; Bashir, A.H.H.; Aljarbou, A.N.; Ramadan, A.M.; Muddathir, A.K.; AlHoufie, S.T.S.; Hifny, A.; Elhassan, G.O.; Ibrahim, M.E.; Alqahtani, S.S.; et al. The Possible Role of Helicobacter Pylori in Gastric Cancer and Its Management. Front. Oncol. 2019, 9, 75. [Google Scholar] [CrossRef]
- Parker, H.; Keenan, J.I. Composition and Function of Helicobacter Pylori Outer Membrane Vesicles. Microbes Infect. 2012, 14, 9–16. [Google Scholar] [CrossRef]
- Chmiela, M.; Walczak, N.; Rudnicka, K. Helicobacter Pylori Outer Membrane Vesicles Involvement in the Infection Development and Helicobacter Pylori-Related Diseases. J. Biomed. Sci. 2018, 25, 78. [Google Scholar] [CrossRef]
- Olofsson, A.; Vallström, A.; Petzold, K.; Tegtmeyer, N.; Schleucher, J.; Carlsson, S.; Haas, R.; Backert, S.; Wai, S.N.; Gröbner, G.; et al. Biochemical and Functional Characterization of Helicobacter Pylori Vesicles. Mol. Microbiol. 2010, 77, 1539–1555. [Google Scholar] [CrossRef]
- Mullaney, E.; Brown, P.A.; Smith, S.M.; Botting, C.H.; Yamaoka, Y.Y.; Terres, A.M.; Kelleher, D.P.; Windle, H.J. Proteomic and Functional Characterization of the Outer Membrane Vesicles from the Gastric Pathogen Helicobacter Pylori. Proteom. Clin. Appl. 2009, 3, 785–796. [Google Scholar] [CrossRef]
- Ismail, S.; Hampton, M.B.; Keenan, J.I. Helicobacter Pylori Outer Membrane Vesicles Modulate Proliferation and Interleukin-8 Production by Gastric Epithelial Cells. Infect. Immun. 2003, 71, 5670–5675. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The Immunology of Host Defence Peptides: Beyond Antimicrobial Activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Acevedo, R.; Fernandez, S.; Zayas, C.; Acosta, A.; Sarmiento, M.; Ferro, V.; Rosenqvist, E.; Campa, C.; Cardoso, D.; Garcia, L.; et al. Bacterial Outer Membrane Vesicles and Vaccine Applications. Front. Immunol. 2014, 5, 121. [Google Scholar] [CrossRef]
- Holst, J.; Martin, D.; Arnold, R.; Huergo, C.C.; Oster, P.; O’Hallahan, J.; Rosenqvist, E. Properties and Clinical Performance of Vaccines Containing Outer Membrane Vesicles from Neisseria Meningitidis. Vaccine 2009, 27, B3–B12. [Google Scholar] [CrossRef]
- Christodoulides, M.; Heckels, J. Novel Approaches to Neisseria Meningitidis Vaccine Design. Pathog. Dis. 2017, 75, ftx033. [Google Scholar] [CrossRef]
- Ge, Z.; Schauer, D.B.; Fox, J.G. In Vivo Virulence Properties of Bacterial Cytolethal-Distending Toxin. Cell. Microbiol. 2008, 10, 1599–1607. [Google Scholar] [CrossRef]
- Lara-Tejero, M.; Galán, J.E. CdtA, CdtB, and CdtC Form a Tripartite Complex That Is Required for Cytolethal Distending Toxin Activity. Infect. Immun. 2001, 69, 4358–4365. [Google Scholar] [CrossRef]
- Elmi, A.; Watson, E.; Sandu, P.; Gundogdu, O.; Mills, D.C.; Inglis, N.F.; Manson, E.; Imrie, L.; Bajaj-Elliott, M.; Wren, B.W.; et al. Campylobacter Jejuni Outer Membrane Vesicles Play an Important Role in Bacterial Interactions with Human Intestinal Epithelial Cells. Infect. Immun. 2012, 80, 4089–4098. [Google Scholar] [CrossRef]
- Jang, K.-S.; Sweredoski, M.J.; Graham, R.L.J.; Hess, S.; Clemons, W.M. Comprehensive Proteomic Profiling of Outer Membrane Vesicles from Campylobacter Jejuni. J. Proteom. 2014, 98, 90–98. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune Modulation by Bacterial Outer Membrane Vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Turkina, M.V.; Olofsson, A.; Magnusson, K.-E.; Arnqvist, A.; Vikström, E. Helicobacter Pylori Vesicles Carrying CagA Localize in the Vicinity of Cell–Cell Contacts and Induce Histone H1 Binding to ATP in Epithelial Cells. FEMS Microbiol. Lett. 2015, 362, fnv076. [Google Scholar] [CrossRef]
- Lapinet, J.A.; Scapini, P.; Calzetti, F.; Pérez, O.; Cassatella, M.A. Gene Expression and Production of Tumor Necrosis Factor Alpha, Interleukin-1β (IL-1β), IL-8, Macrophage Inflammatory Protein 1α (MIP-1α), MIP-1β, and Gamma Interferon-Inducible Protein 10 by Human Neutrophils Stimulated with Group B Meningococcal Outer Membrane Vesicles. Infect. Immun. 2000, 68, 6917–6923. [Google Scholar] [CrossRef]
- Jäger, J.; Marwitz, S.; Tiefenau, J.; Rasch, J.; Shevchuk, O.; Kugler, C.; Goldmann, T.; Steinert, M. Human Lung Tissue Explants Reveal Novel Interactions during Legionella Pneumophila Infections. Infect. Immun. 2014, 82, 275–285. [Google Scholar] [CrossRef]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane Vesicles Are Immunogenic Facsimiles of Salmonella Typhimurium That Potently Activate Dendritic Cells, Prime B and T Cell Responses, and Stimulate Protective Immunity in Vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef]
- van der Pol, L.; Stork, M.; van der Ley, P. Outer Membrane Vesicles as Platform Vaccine Technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef] [Green Version]
- Bouback, T.A.; Qadri, I.; Al-fassi, F.; Ali, M.A. Application of Secreted Bacterial Outer Membrane Vesicles (OMVs) to Develop A Candidate Vaccine for Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Middle-East J. Sci. Res. 2019, 27, 400–408. [Google Scholar]
- Shehata, M.M.; Mostafa, A.; Teubner, L.; Mahmoud, S.H.; Kandeil, A.; Elshesheny, R.; Boubak, T.A.; Frantz, R.; Pietra, L.L.; Pleschka, S.; et al. Bacterial Outer Membrane Vesicles (OMVs)-Based Dual Vaccine for Influenza A H1N1 Virus and MERS-CoV. Vaccines 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S. Another Decade, Another Coronavirus. New Engl. J. Med. 2020, 382, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; et al. Bioengineered Bacteria-Derived Outer Membrane Vesicles as a Versatile Antigen Display Platform for Tumor Vaccination via Plug-and-Display Technology. Nat. Commun. 2021, 12, 2041. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Sah, R.; Al-Tawfiq, J.A.; Al-Qaaneh, A.M.; Al-Jamea, L.H.; Woodman, A.; Al-Qahtani, M.; Haque, S.; Harapan, H.; et al. Recent Advances in Vaccine and Immunotherapy for COVID-19. Hum. Vaccines Immunother. 2020, 16, 3011–3022. [Google Scholar] [CrossRef]
- Gaspar, E.B.; Prudencio, C.R.; De Gaspari, E. Experimental Studies Using OMV in a New Platform of SARS-CoV-2 Vaccines. Hum. Vaccines Immunother. 2021, 17, 2965–2968. [Google Scholar] [CrossRef]
- Cai, W.; Kesavan, D.K.; Wan, J.; Abdelaziz, M.H.; Su, Z.; Xu, H. Bacterial Outer Membrane Vesicles, a Potential Vaccine Candidate in Interactions with Host Cells Based. Diagn. Pathol. 2018, 13, 95. [Google Scholar] [CrossRef]
- Klimentová, J.; Stulík, J. Methods of Isolation and Purification of Outer Membrane Vesicles from Gram-Negative Bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Rossi, O.; Pesce, I.; Giannelli, C.; Aprea, S.; Caboni, M.; Citiulo, F.; Valentini, S.; Ferlenghi, I.; MacLennan, C.A.; D’Oro, U.; et al. Modulation of Endotoxicity of Shigella Generalized Modules for Membrane Antigens (GMMA) by Genetic Lipid A Modifications: Relative Activation of TLR4 And TLR2 Pathways in Different Mutants *. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef]
- Tra, V.N.; Dube, D.H. Glycans in Pathogenic Bacteria—Potential for Targeted Covalent Therapeutics and Imaging Agents. Chem. Commun. 2014, 50, 4659–4673. [Google Scholar] [CrossRef]
- Rabets, A.; Bila, G.; Grytsko, R.; Samborskyy, M.; Rebets, Y.; Vari, S.G.; Pagneux, Q.; Barras, A.; Boukherroub, R.; Szunerits, S.; et al. The Potential of Developing Pan-Coronaviral Antibodies to Spike Peptides in Convalescent COVID-19 Patients. Arch. Immunol. Ther. Exp. 2021, 69, 5. [Google Scholar] [CrossRef]
- Qing, G.; Gong, N.; Chen, X.; Chen, J.; Zhang, H.; Wang, Y.; Wang, R.; Zhang, S.; Zhang, Z.; Zhao, X.; et al. Natural and Engineered Bacterial Outer Membrane Vesicles. Biophys. Rep. 2019, 5, 184–198. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and Phenotyping of Cellular Vesicles Using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Gerritzen, M.J.H.; Salverda, M.L.M.; Martens, D.E.; Wijffels, R.H.; Stork, M. Spontaneously Released Neisseria Meningitidis Outer Membrane Vesicles as Vaccine Platform: Production and Purification. Vaccine 2019, 37, 6978–6986. [Google Scholar] [CrossRef]
- Bilyy, R.O.; Shkandina, T.; Tomin, A.; Muñoz, L.E.; Franz, S.; Antonyuk, V.; Kit, Y.Y.; Zirngibl, M.; Fürnrohr, B.G.; Janko, C.; et al. Macrophages Discriminate Glycosylation Patterns of Apoptotic Cell-Derived Microparticles. J. Biol. Chem. 2012, 287, 496–503. [Google Scholar] [CrossRef]
- Windle, H.J. Isolation of Outer Membrane Vesicles from Helicobacter Pylori. In Helicobacter Pylori; Smith, S.M., Ed.; Springer: New York, NY, USA, 2021; pp. 123–130. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef]
- Fernández-Rojas, M.A.; Vaca, S.; Reyes-López, M.; de la Garza, M.; Aguilar-Romero, F.; Zenteno, E.; Soriano-Vargas, E.; Negrete-Abascal, E. Outer Membrane Vesicles of Pasteurella Multocida Contain Virulence Factors. MicrobiologyOpen 2014, 3, 711–717. [Google Scholar] [CrossRef]
- Davis, J.M.; Carvalho, H.M.; Rasmussen, S.B.; O’Brien, A.D. Cytotoxic Necrotizing Factor Type 1 Delivered by Outer Membrane Vesicles of Uropathogenic Escherichia Coli Attenuates Polymorphonuclear Leukocyte Antimicrobial Activity and Chemotaxis. Infect. Immun. 2006, 74, 4401–4408. [Google Scholar] [CrossRef]
- Macia, L.; Nanan, R.; Hosseini-Beheshti, E.; Grau, G.E. Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. Int. J. Mol. Sci. 2020, 21, 107. [Google Scholar] [CrossRef]
- Jung, A.L.; Hoffmann, K.; Herkt, C.E.; Schulz, C.; Bertrams, W.; Schmeck, B. Legionella Pneumophila Outer Membrane Vesicles: Isolation and Analysis of Their Pro-Inflammatory Potential on Macrophages. J. Vis. Exp. 2017, e55146. [Google Scholar] [CrossRef] [Green Version]
- Cecil, J.D.; O’Brien-Simpson, N.M.; Lenzo, J.C.; Holden, J.A.; Singleton, W.; Perez-Gonzalez, A.; Mansell, A.; Reynolds, E.C. Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo. Front. Immunol. 2017, 8, 1017. [Google Scholar] [CrossRef]
- Kim, O.Y.; Hong, B.S.; Park, K.-S.; Yoon, Y.J.; Choi, S.J.; Lee, W.H.; Roh, T.-Y.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Immunization with Escherichia Coli Outer Membrane Vesicles Protects Bacteria-Induced Lethality via Th1 and Th17 Cell Responses. J. Immunol. 2013, 190, 4092–4102. [Google Scholar] [CrossRef]
- Han, E.-C.; Choi, S.-Y.; Lee, Y.; Park, J.-W.; Hong, S.-H.; Lee, H.-J. Extracellular RNAs in Periodontopathogenic Outer Membrane Vesicles Promote TNF-α Production in Human Macrophages and Cross the Blood-Brain Barrier in Mice. FASEB J. 2019, 33, 13412–13422. [Google Scholar] [CrossRef]
- Mitra, S.; Sinha, R.; Nag, D.; Koley, H. Immunomodulatory Role of Outer Membrane Vesicles of Shigella in Mouse Model. Trials Vaccinol. 2015, 4, 56–60. [Google Scholar] [CrossRef]
- Chandler, C.E.; Ernst, R.K. Bacterial Lipids: Powerful Modifiers of the Innate Immune Response. F1000Research 2017, 6, 1334. [Google Scholar] [CrossRef]
- Ko, S.H.; Rho, D.J.; Jeon, J.I.; Kim, Y.-J.; Woo, H.A.; Kim, N.; Kim, J.M. Crude Preparations of Helicobacter Pylori Outer Membrane Vesicles Induce Upregulation of Heme Oxygenase-1 via Activating Akt-Nrf2 and MTOR–IκB Kinase–NF-ΚB Pathways in Dendritic Cells. Infect. Immun. 2016, 84, 2162–2174. [Google Scholar] [CrossRef]
- Schetters, S.T.T.; Jong, W.S.P.; Horrevorts, S.K.; Kruijssen, L.J.W.; Engels, S.; Stolk, D.; Daleke-Schermerhorn, M.H.; Garcia-Vallejo, J.; Houben, D.; Unger, W.W.J.; et al. Outer Membrane Vesicles Engineered to Express Membrane-Bound Antigen Program Dendritic Cells for Cross-Presentation to CD8+ T Cells. Acta Biomater. 2019, 91, 248–257. [Google Scholar] [CrossRef]
- Durant, L.; Stentz, R.; Noble, A.; Brooks, J.; Gicheva, N.; Reddi, D.; O’Connor, M.J.; Hoyles, L.; McCartney, A.L.; Man, R.; et al. Bacteroides Thetaiotaomicron-Derived Outer Membrane Vesicles Promote Regulatory Dendritic Cell Responses in Health but Not in Inflammatory Bowel Disease. Microbiome 2020, 8, 88. [Google Scholar] [CrossRef]
- Laughlin, R.C.; Alaniz, R.C. Outer Membrane Vesicles in Service as Protein Shuttles, Biotic Defenders, and Immunological Doppelgängers. Gut Microbes 2016, 7, 450–454. [Google Scholar] [CrossRef]
- Vaughan, A.T.; Gorringe, A.; Davenport, V.; Williams, N.A.; Heyderman, R.S. Absence of Mucosal Immunity in the Human Upper Respiratory Tract to the Commensal Bacteria Neisseria Lactamica but Not Pathogenic Neisseria Meningitidis during the Peak Age of Nasopharyngeal Carriage. J. Immunol. 2009, 182, 2231–2240. [Google Scholar] [CrossRef] [Green Version]
- Vidakovics, M.L.A.P.; Jendholm, J.; Mörgelin, M.; Månsson, A.; Larsson, C.; Cardell, L.-O.; Riesbeck, K. B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism. PLoS Pathog. 2010, 6, e1000724. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.-H.; Seo, S.H.; Kim, C.-U.; Jang, M.S.; Song, M.-S.; Lee, T.-Y.; Jeong, Y.-J.; Lee, M.-S.; Park, J.-H.; Lee, P.; et al. Bacterial Outer Membrane Vesicles Provide Broad-Spectrum Protection against Influenza Virus Infection via Recruitment and Activation of Macrophages. J. Innate. Immun. 2019, 11, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.J.; Kaparakis-Liaskos, M. The Therapeutic Benefit of Bacterial Membrane Vesicles. Int. J. Mol. Sci. 2017, 18, 1287. [Google Scholar] [CrossRef] [PubMed]
- Arigita, C.; Jiskoot, W.; Westdijk, J.; van Ingen, C.; Hennink, W.E.; Crommelin, D.J.A.; Kersten, G.F.A. Stability of Mono- and Trivalent Meningococcal Outer Membrane Vesicle Vaccines. Vaccine 2004, 22, 629–642. [Google Scholar] [CrossRef]
- Alves, N.J.; Turner, K.B.; Medintz, I.L.; Walper, S.A. Protecting Enzymatic Function through Directed Packaging into Bacterial Outer Membrane Vesicles. Sci. Rep. 2016, 6, 24866. [Google Scholar] [CrossRef]
- Bottero, D.; Gaillard, M.E.; Zurita, E.; Moreno, G.; Martinez, D.S.; Bartel, E.; Bravo, S.; Carriquiriborde, F.; Errea, A.; Castuma, C.; et al. Characterization of the Immune Response Induced by Pertussis OMVs-Based Vaccine. Vaccine 2016, 34, 3303–3309. [Google Scholar] [CrossRef]
- Finne, J.; Leinonen, M.; Mäkelä, P.H. ANTIGENIC SIMILARITIES BETWEEN BRAIN COMPONENTS AND BACTERIA CAUSING MENINGITIS: Implications for Vaccine Development and Pathogenesis. Lancet 1983, 322, 355–357. [Google Scholar] [CrossRef]
- Holst, J.; Oster, P.; Arnold, R.; Tatley, M.; Næss, L.; Aaberge, I.; Galloway, Y.; McNicholas, A.; O’Hallahan, J.; Rosenqvist, E.; et al. Vaccines against Meningococcal Serogroup B Disease Containing Outer Membrane Vesicles (OMV): Lessons from Past Programs and Implications for the Future. Hum. Vaccines Immunother. 2013, 9, 1241–1253. [Google Scholar] [CrossRef]
- Granoff, D.M. Review of Meningococcal Group B Vaccines. Clin. Infect. Dis. 2010, 50 (Suppl. S2), S54–S65. [Google Scholar] [CrossRef]
- Claassen, I.; Meylis, J.; van der Ley, P.; Peeters, C.; Brons, H.; Robert, J.; Borsboom, D.; van der Ark, A.; van Straaten, I.; Roholl, P.; et al. Production, Characterization and Control of a Neisseria Meningitidis Hexavalent Class 1 Outer Membrane Protein Containing Vesicle Vaccine. Vaccine 1996, 14, 1001–1008. [Google Scholar] [CrossRef]
- Tunheim, G.; Arnemo, M.; Næss, L.M.; Fjeldheim, Å.K.; Nome, L.; Bolstad, K.; Aase, A.; Mandiarote, A.; González, H.; González, D.; et al. Preclinical Immunogenicity and Functional Activity Studies of an A+W Meningococcal Outer Membrane Vesicle (OMV) Vaccine and Comparisons with Existing Meningococcal Conjugate- and Polysaccharide Vaccines. Vaccine 2013, 31, 6097–6106. [Google Scholar] [CrossRef]
- Zurita, M.E.; Wilk, M.M.; Carriquiriborde, F.; Bartel, E.; Moreno, G.; Misiak, A.; Mills, K.H.G.; Hozbor, D. A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection against Bordetella Pertussis, Including Pertactin Deficient Strains. Front. Cell. Infect. Microbiol. 2019, 9, 125. [Google Scholar] [CrossRef]
- Martins, P.; Machado, D.; Theizen, T.H.; Guarnieri, J.P.O.; Bernardes, B.G.; Gomide, G.P.; Corat, M.A.F.; Abbehausen, C.; Módena, J.L.P.; Melo, C.F.O.R.; et al. Outer Membrane Vesicles from Neisseria Meningitidis (Proteossome) Used for Nanostructured Zika Virus Vaccine Production. Sci. Rep. 2018, 8, 8290. [Google Scholar] [CrossRef]
- Muralinath, M.; Kuehn, M.J.; Roland, K.L.; Curtiss, R. Immunization with Salmonella Enterica Serovar Typhimurium-Derived Outer Membrane Vesicles Delivering the Pneumococcal Protein PspA Confers Protection against Challenge with Streptococcus Pneumoniae. Infect. Immun. 2011, 79, 887–894. [Google Scholar] [CrossRef]
- Schild, S.; Nelson, E.J.; Bishop, A.L.; Camilli, A. Characterization of Vibrio Cholerae Outer Membrane Vesicles as a Candidate Vaccine for Cholera. Infect. Immun. 2009, 77, 472–484. [Google Scholar] [CrossRef]
- Chen, L.; Valentine, J.L.; Huang, C.-J.; Endicott, C.E.; Moeller, T.D.; Rasmussen, J.A.; Fletcher, J.R.; Boll, J.M.; Rosenthal, J.A.; Dobruchowska, J.; et al. Outer Membrane Vesicles Displaying Engineered Glycotopes Elicit Protective Antibodies. Proc. Natl. Acad. Sci. USA 2016, 113, E3609–E3618. [Google Scholar] [CrossRef]
- Chen, D.J.; Osterrieder, N.; Metzger, S.M.; Buckles, E.; Doody, A.M.; DeLisa, M.P.; Putnam, D. Delivery of Foreign Antigens by Engineered Outer Membrane Vesicle Vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 3099–3104. [Google Scholar] [CrossRef]
- Shen, Y.; Torchia, M.L.G.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer Membrane Vesicles of a Human Commensal Mediate Immune Regulation and Disease Protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef]
- Siegemund, S.; Schütze, N.; Freudenberg, M.A.; Lutz, M.B.; Straubinger, R.K.; Alber, G. Production of IL-12, IL-23 and IL-27p28 by Bone Marrow-Derived Conventional Dendritic Cells Rather than Macrophages after LPS/TLR4-Dependent Induction by Salmonella Enteritidis. Immunobiol. 2008, 212, 739–750. [Google Scholar] [CrossRef]
- Tan, K.; Li, R.; Huang, X.; Liu, Q. Outer Membrane Vesicles: Current Status and Future Direction of These Novel Vaccine Adjuvants. Front. Microbiol. 2018, 9, 783. [Google Scholar] [CrossRef] [Green Version]
- Tavano, R.; Franzoso, S.; Cecchini, P.; Cartocci, E.; Oriente, F.; Capecchi, B.; Arico, B.; Papini, E. Self-Adjuvant and Immune-Stimulating Activity of the Anti-Meningococcus B Vaccine Candidate Neisseria Meningitidis Adhesin A as a Soluble Recombinant Antigen (NadAΔ351–405) or as Part of Bacterial Outer Membrane Vesicles. New Biotechnol. 2009, 25, S6. [Google Scholar] [CrossRef]
- Engineered Bacterial Outer Membrane Vesicles (OMVs) as Vaccines and Vaccine Adjuvants. Sci. -Bus. Exch. 2010, 3, 134. [CrossRef]
- Aghasadeghi, M.R.; Salmani, A.S.; Sadat, S.M.; Javadi, F.; Memarnejadian, A.; Vahabpour, R.; Zabihollahi, R.; Moshiri, A.; Siadat, S.D. Application of Outer Membrane Vesicle of Neisseria Meningitidis Serogroup B as a New Adjuvant to Induce Strongly Th1-Oriented Responses Against HIV-1. Curr. HIV Res. 2011, 9, 630–635. [Google Scholar] [CrossRef]
- Alatrakchi, N.; Di Martino, V.; Thibault, V.; Autran, B. Strong CD4 Th1 Responses to HIV and Hepatitis C Virus in HIV-Infected Long-Term Non-Progressors Co-Infected with Hepatitis C Virus. AIDS 2002, 16, 713–717. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, S.-H.; Kang, W.; Choi, Y.S.; Lee, S.-H.; Lee, S.-R.; You, S.; Lee, H.K.; Chang, K.-T.; Shin, E.-C. Adjuvant Effect of Bacterial Outer Membrane Vesicles with Penta-Acylated Lipopolysaccharide on Antigen-Specific T Cell Priming. Vaccine 2011, 29, 8293–8301. [Google Scholar] [CrossRef]
- Furuta, N.; Tsuda, K.; Omori, H.; Yoshimori, T.; Yoshimura, F.; Amano, A. Porphyromonas Gingivalis Outer Membrane Vesicles Enter Human Epithelial Cells via an Endocytic Pathway and Are Sorted to Lysosomal Compartments. Infect. Immun. 2009, 77, 4187–4196. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q. Engineered Bacterial Outer Membrane Vesicles as Multifunctional Delivery Platforms. Front. Mater. 2020, 7, 202. [Google Scholar] [CrossRef]
- Prior, J.T.; Davitt, C.; Kurtz, J.; Gellings, P.; McLachlan, J.B.; Morici, L.A. Bacterial-Derived Outer Membrane Vesicles Are Potent Adjuvants That Drive Humoral and Cellular Immune Responses. Pharmaceutics 2021, 13, 131. [Google Scholar] [CrossRef]
- Baumgarten, T.; Sperling, S.; Seifert, J.; von Bergen, M.; Steiniger, F.; Wick, L.Y.; Heipieper, H.J. Membrane Vesicle Formation as a Multiple-Stress Response Mechanism Enhances Pseudomonas Putida DOT-T1E Cell Surface Hydrophobicity and Biofilm Formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [Google Scholar] [CrossRef]
- Eberlein, C.; Baumgarten, T.; Starke, S.; Heipieper, H.J. Immediate Response Mechanisms of Gram-Negative Solvent-Tolerant Bacteria to Cope with Environmental Stress: Cis-Trans Isomerization of Unsaturated Fatty Acids and Outer Membrane Vesicle Secretion. Appl. Microbiol. Biotechnol. 2018, 102, 2583–2593. [Google Scholar] [CrossRef] [Green Version]
- de Jonge, E.F.; Balhuizen, M.D.; van Boxtel, R.; Wu, J.; Haagsman, H.P.; Tommassen, J. Heat Shock Enhances Outer-Membrane Vesicle Release in Bordetella spp. Curr. Res. Microb. Sci. 2021, 2, 100009. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashyap, D.; Panda, M.; Baral, B.; Varshney, N.; R, S.; Bhandari, V.; Parmar, H.S.; Prasad, A.; Jha, H.C. Outer Membrane Vesicles: An Emerging Vaccine Platform. Vaccines 2022, 10, 1578. https://doi.org/10.3390/vaccines10101578
Kashyap D, Panda M, Baral B, Varshney N, R S, Bhandari V, Parmar HS, Prasad A, Jha HC. Outer Membrane Vesicles: An Emerging Vaccine Platform. Vaccines. 2022; 10(10):1578. https://doi.org/10.3390/vaccines10101578
Chicago/Turabian StyleKashyap, Dharmendra, Mrutyunjaya Panda, Budhadev Baral, Nidhi Varshney, Sajitha R, Vasundhra Bhandari, Hamendra Singh Parmar, Amit Prasad, and Hem Chandra Jha. 2022. "Outer Membrane Vesicles: An Emerging Vaccine Platform" Vaccines 10, no. 10: 1578. https://doi.org/10.3390/vaccines10101578








