Attitudes toward COVID-19 Vaccination: A Survey of Chinese Patients with Rheumatic Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Survey Administration
2.2. Measures
2.3. Attitudes toward COVID-19 Vaccination
2.4. Current Status of Vaccination and Adverse Events
2.5. Knowledge Sources of the COVID-19 Vaccine
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Attitudes toward COVID-19 Vaccination
3.3. Knowledge of the COVID-19 Vaccination
3.4. Confidence in the Pandemic
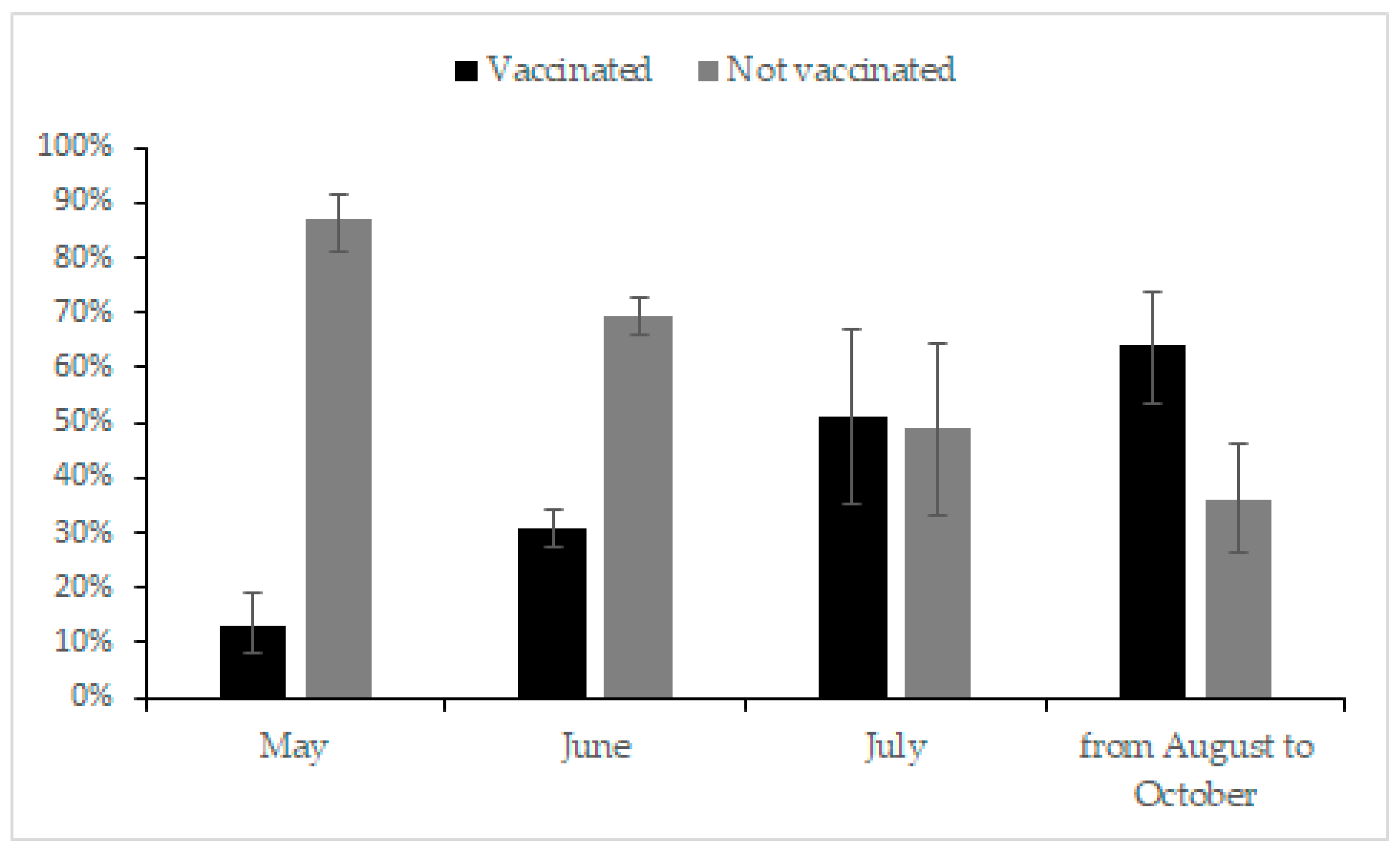
3.5. Current Status of Vaccination
3.6. Adverse Events after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard; World Health Organisation: Geneva, Switzerland, 2022. [Google Scholar]
- Xu, C.; Yi, Z.; Cai, R.; Chen, R.; Thong, B.Y.; Mu, R. Clinical outcomes of COVID-19 in patients with rheumatic diseases: A systematic review and meta-analysis of global data. Autoimmun. Rev. 2021, 20, 102778. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Wack, S.; Patton, T.; Ferris, L.K. COVID-19 vaccine safety and efficacy in patients with immune-mediated inflammatory disease: Review of available evidence. J. Am. Acad. Dermatol. 2021, 85, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Geng, Y.; Wang, Y.; Deng, X.; Li, G.; Zhao, J.; Ji, L.; Zhang, X.; Song, Z.; Zhang, H.; et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: A large real-world survey on inactivated COVID-19 vaccines. Ann. Rheum. Dis. 2022, 81, 443–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Guaracha-Basáñez, G.; Contreras-Yáñez, I.; Álvarez-Hernández, E.; Román-Montes, C.M.; Meza-López, Y.O.G.; Morales-Graciano, M.J.; Valverde-Hernández, S.S.; Peláez-Ballestas, I.; Pascual-Ramos, V. COVID-19 vaccine hesitancy among Mexican outpatients with rheumatic diseases. Hum. Vaccin. Immunother. 2021, 17, 5038–5047. [Google Scholar] [CrossRef]
- Felten, R.; Dubois, M.; Ugarte-Gil, M.F.; Chaudier, A.; Kawka, L.; Bergier, H.; Costecalde, C.; Pijnenburg, L.; Fort, J.; Chatelus, E.; et al. Vaccination against COVID-19: Expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021, 3, e243–e245. [Google Scholar] [CrossRef]
- Priori, R.; Pellegrino, G.; Colafrancesco, S.; Alessandri, C.; Ceccarelli, F.; di Franco, M.; Riccieri, V.; Scrivo, R.; Sili Scavalli, A.; Spinelli, F.R.; et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: A message for rheumatologists. Ann. Rheum. Dis. 2021, 80, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Agrawat, H.; Shukla, A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: An interview-based survey. Rheumatol. Int. 2021, 41, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Boekel, L.; Hooijberg, F.; van Kempen, Z.L.E.; Vogelzang, E.H.; Tas, S.W.; Killestein, J.; Nurmohamed, M.T.; Boers, M.; Kuijpers, T.W.; van Ham, S.M.; et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021, 3, e241–e243. [Google Scholar] [CrossRef]
- Yurttas, B.; Poyraz, B.C.; Sut, N.; Ozdede, A.; Oztas, M.; Uğurlu, S.; Tabak, F.; Hamuryudan, V.; Seyahi, E. Willingness to get the COVID-19 vaccine among patients with rheumatic diseases, healthcare workers and general population in Turkey: A web-based survey. Rheumatol. Int. 2021, 41, 1105–1114. [Google Scholar] [CrossRef]
- Ko, T.; Dendle, C.; Woolley, I.; Morand, E.; Antony, A. SARS-COV-2 vaccine acceptance in patients with rheumatic diseases: A cross-sectional study. Hum. Vaccin. Immunother. 2021, 17, 4048–4056. [Google Scholar] [CrossRef]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Kroger, A.; et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: Version 3. Arthritis Rheumatol. 2021, 73, e60–e75. [Google Scholar]
- Santosa, A.; Xu, C.; Arkachaisri, T.; Kong, K.O.; Lateef, A.; Lee, T.H.; Leong, K.H.; Low, A.H.L.; Sriranganathan, M.K.; Tan, T.C.; et al. Recommendations for COVID-19 vaccination in people with rheumatic disease: Developed by the Singapore Chapter of Rheumatologists. Int. J. Rheum. Dis. 2021, 24, 746–757. [Google Scholar] [CrossRef]
- Bijlsma, J.W. EULAR December 2020 View points on SARS-CoV-2 vaccination in patients with RMDs. Ann. Rheum. Dis. 2021, 80, 411–412. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Zhao, Q.; Alias, H.; Danaee, M.; Wong, L.P. Understanding COVID-19 vaccine demand and hesitancy: A nationwide online survey in China. PLoS Negl. Trop Dis. 2020, 14, e0008961. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Cambiaggi, M.; Vasco, A.; Maraniello, L.; Ansaldi, F.; Baldo, V.; Bonanni, P.; Calabrò, G.E.; Costantino, C.; de Waure, C.; et al. Attitudes and Beliefs on Influenza Vaccination during the COVID-19 Pandemic: Results from a Representative Italian Survey. Vaccines 2020, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- Pogue, K.; Jensen, J.L.; Stancil, C.K.; Ferguson, D.G.; Hughes, S.J.; Mello, E.J.; Burgess, R.; Berges, B.K.; Quaye, A.; Poole, B.D. Influences on Attitudes Regarding Potential COVID-19 Vaccination in the United States. Vaccines 2020, 8, 582. [Google Scholar] [CrossRef]
- Sallam, M.; Dababseh, D.; Eid, H.; Al-Mahzoum, K.; Al-Haidar, A.; Taim, D.; Yaseen, A.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. High Rates of COVID-19 Vaccine Hesitancy and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab Countries. Vaccines 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.D.; Pak, T.V.; Gribkova, E.I.; Galkina, G.A.; Loskutova, E.E.; Dorofeeva, V.V.; Dewey, R.S.; Nguyen, K.T.; Pham, D.T. Determinants of COVID-19 vaccine acceptance in a high infection-rate country: A cross-sectional study in Russia. Pharm. Pract. 2021, 19, 2276. [Google Scholar] [CrossRef] [PubMed]
- Epidemic Prevention and Control Trends. National Health Commission of the People’s Republic of China. 18 September 2021. Available online: http://www.nhc.gov.cn/xcs/yqfkdt/202109/bf2cf785ce0544ae818a76ffbb18ca79.shtml (accessed on 18 September 2021).
| Characteristic of the Participants | Data (N = 1022) |
|---|---|
| Gender | |
| Male | 203 (19.86%) |
| Female | 819 (80.14%) |
| Age (y) | |
| 18–24 | 68 (6.65%) |
| 25–35 | 306 (29.94%) |
| 36–55 | 514 (50.29%) |
| 56–70 | 111 (10.86%) |
| >70 | 9 (0.88%) |
| Invalid Data | 14 (1.37%) |
| Educational level | |
| Postgraduate degree or above | 53 (5.19%) |
| Bachelor’s degree | 263 (25.73%) |
| High school graduate or junior college | 394 (38.55%) |
| Less than high school | 316 (30.92%) |
| Monthly income (RMB) | |
| >10,000 | 71 (6.95%) |
| 5000–10,000 | 189 (18.49%) |
| 2500–5000 | 293 (28.67%) |
| <2500 | 418 (40.80%) |
| Did not report | 54 (5.09%) |
| Employment status | |
| Employed | 659 (64.48%) |
| Unemployed | 363 (35.52%) |
| Location | |
| City | 627 (61.35%) |
| Countryside | 395 (38.65%) |
| Disease status | |
| Inactive disease | 811 (79.35%) |
| Active disease | 212 (20.65%) |
| Disease diagnosis | |
| RA | 286 (27.98%) |
| SLE | 236 (23.09%) |
| AS | 171 (16.73%) |
| SSc | 131 (12.82%) |
| SS | 123 (12.04%) |
| Others | 229 (22.41%) |
| Factors | Parameter Estimates a | |||
|---|---|---|---|---|
| OR | 95% CI for OR | Sig. | ||
| Lower Bound | Upper Bound | |||
| Gender | ||||
| Male | 1.65 | 1.2 | 2.28 | 0.002 * |
| Location | ||||
| Countryside | 1.05 | 0.81 | 1.37 | 0.706 |
| Employment status | ||||
| Unemployed | 0.67 | 0.51 | 0.88 | 0.004 * |
| Disease status | ||||
| Active disease | 0.5 | 0.37 | 0.66 | 0.000 ** |
| Educational level (N = 1022) | 0.97 | 0.83 | 1.14 | 0.741 |
| Age (per 1 year) (N = 1010) | 1 | 0.9 | 1.11 | 0.939 |
| Monthly income (RMB) (N = 970) | 1.29 | 1.13 | 1.47 | 0.000 ** |
| Confidence (N = 1022) | ||||
| I will get seriously ill | 0.57 | 0.34 | 0.96 | 0.022 * |
| I will get a mild case | 1.29 | 0.79 | 2.08 | 0.302 |
| I will not be infected | ref | |||
| Have consulted a rheumatologist | 1.68 | 1.33 | 2.14 | 0.000 ** |
| Received knowledge and guidance from doctors | ||||
| 1.39 | 1.03 | 1.85 | 0.028 * | |
| Rheumatologist recommend vaccination | ||||
| 3.53 | 2.53 | 4.91 | 0.000 ** | |
| Reasons | 5-Point Likert Scale | ||||
|---|---|---|---|---|---|
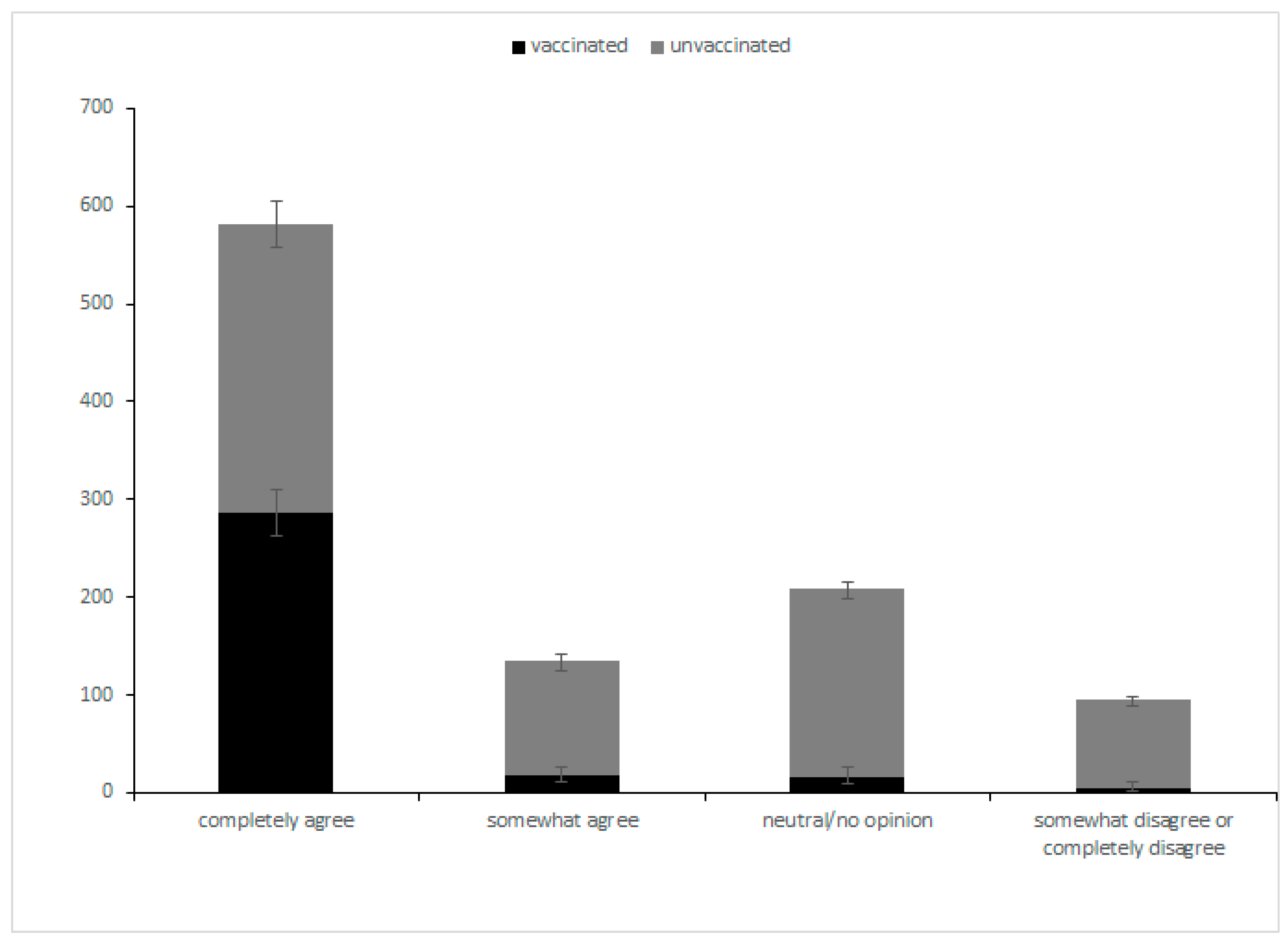
| Completely Disagree (%) | or Somewhat Disagree (%) | Neutral/No Opinion (%) | Somewhat Agree (%) | Completely Agree (%) | |
| Reasons for vaccinating (N = 324) | |||||
| Work requirements | 66 (20.4) | 9 (2.8) | 30 (9.3) | 19 (5.9) | 200 (61.7) |
| Community requirements | 70 (21.6) | 19 (5.9) | 28 (8.6) | 29 (9.0) | 178 (54.9) |
| Personal willingness | 11 (3.4) | 9 (2.8) | 24 (7.4) | 21 (6.5) | 259 (79.9) |
| Doctor’s recommendation | 55 (17.0) | 20 (6.2) | 35 (10.8) | 37 (11.4) | 177 (54.6) |
| Reasons for refusing (N = 305) | |||||
| Disease flare | 41 (13.8) | 15 (4.9) | 32 (10.5) | 27 (8.9) | 190 (62.0) |
| Adverse effect | 62 (20.7) | 19 (6.2) | 39 (12.8) | 27 (8.9) | 158 (51.5) |
| Causing COVID-19 infection | 128 (43.0) | 24 (7.9) | 45 (14.8) | 23 (7.5) | 85 (27.5) |
| Invalidity of vaccines | 139 (45.9) | 31 (10.2) | 46 (15.1) | 22 (7.2) | 67 (21.6) |
| Adverse Events | Date (N = 231) |
|---|---|
| Redness, swelling, and pain at the inoculation site | 92 (39.83%) |
| Weakness | 69 (29.87%) |
| Muscle soreness | 64 (27.71%) |
| Headache | 21 (9.09%) |
| Nausea | 13 (5.63%) |
| Fever | 12 (5.19%) |
| Flare | 5 (2.16%) |
| Rash and itch | 4 (1.73%) |
| Dizzy | 2 (0.87%) |
| Thrombus | 1 (0.43%) |
| Constipation | 1 (0.43%) |
| Cold | 1 (0.43%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.; Yao, Z.; Xu, D.; Xu, C.; Fang, W.; Guo, Z.; Wang, Y.; Huang, J.; Li, Q.; Zhang, H.; et al. Attitudes toward COVID-19 Vaccination: A Survey of Chinese Patients with Rheumatic Diseases. Vaccines 2022, 10, 1604. https://doi.org/10.3390/vaccines10101604
Yi Z, Yao Z, Xu D, Xu C, Fang W, Guo Z, Wang Y, Huang J, Li Q, Zhang H, et al. Attitudes toward COVID-19 Vaccination: A Survey of Chinese Patients with Rheumatic Diseases. Vaccines. 2022; 10(10):1604. https://doi.org/10.3390/vaccines10101604
Chicago/Turabian StyleYi, Zixi, Zhongqiang Yao, Dan Xu, Chuanhui Xu, Wenqiang Fang, Zhanfei Guo, Yong Wang, Jianlin Huang, Qin Li, Hong Zhang, and et al. 2022. "Attitudes toward COVID-19 Vaccination: A Survey of Chinese Patients with Rheumatic Diseases" Vaccines 10, no. 10: 1604. https://doi.org/10.3390/vaccines10101604
APA StyleYi, Z., Yao, Z., Xu, D., Xu, C., Fang, W., Guo, Z., Wang, Y., Huang, J., Li, Q., Zhang, H., Huang, A., Wu, L., Wu, Z., Guo, H., Zhang, F., Lu, J., Zhang, Z., Yu, Z., Da, Z., ... Mu, R. (2022). Attitudes toward COVID-19 Vaccination: A Survey of Chinese Patients with Rheumatic Diseases. Vaccines, 10(10), 1604. https://doi.org/10.3390/vaccines10101604







