A Possibility of Vasospastic Angina after mRNA COVID-19 Vaccination
Abstract
1. Introduction
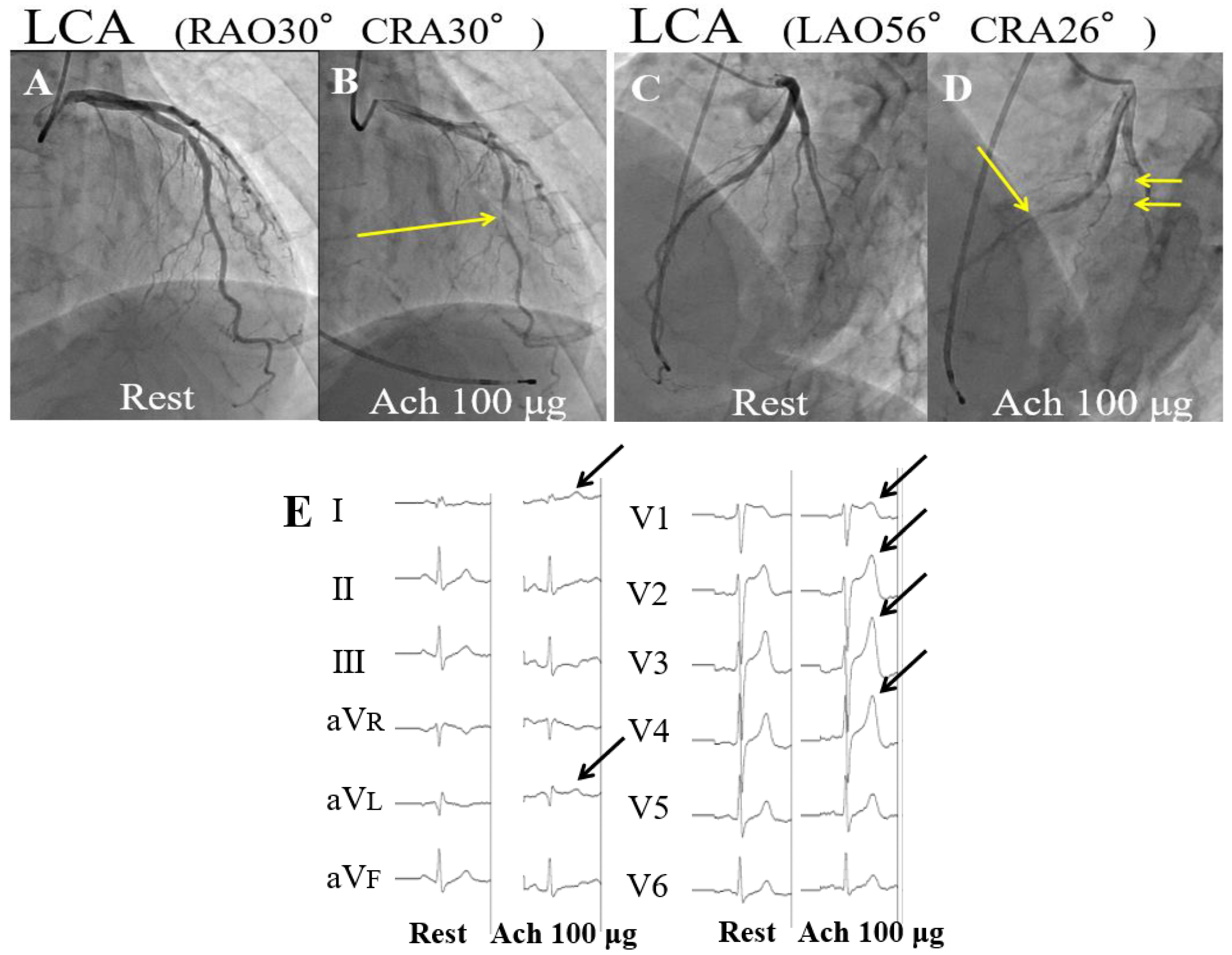
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VSA | vasospastic angina |
| COVID-19 | coronavirus disease 2019 |
| mRNA | messenger RNA |
| ACS | acute coronary syndrome |
| ACE2 | angiotensin-converting enzyme 2 |
| ECG | electrocardiogram |
| Ach | acetylcholine |
| LCA | left coronary artery |
| CCB | calcium channel blocker |
| AngII | angiotensin II |
| IL | interleukin |
| LNPs | lipid nanoparticles |
| ALDH2 | aldehyde dehydrogenase 2 |
| AFS | alcohol flushing syndrome |
| ARs | adrenoceptors |
References
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Aye, Y.N.; Mai, A.S.; Zhang, A.; Lim, O.Z.H.; Lin, N.; Ng, C.H.; Chan, M.Y.; Yip, J.; Loh, P.H.; Chew, N.W. Acute Myocardial Infarction and Myocarditis following COVID-19 Vaccination. QJM 2021, hcab252. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Penninger, J.M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ. J. 2013, 77, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Recinos, A., 3rd; LeJeune, W.S.; Sun, H.; Lee, C.Y.; Tieu, B.C.; Lu, M.; Hou, T.; Boldogh, I.; Tilton, R.G.; Brasier, A.R. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis 2007, 194, 125–133. [Google Scholar] [CrossRef]
- Murata, K.; Nakao, N.; Ishiuchi, N.; Fukui, T.; Katsuya, N.; Fukumoto, W.; Oka, H.; Yoshikawa, N.; Nagao, T.; Namera, A.; et al. Four cases of cytokine storm after COVID-19 vaccination: Case report. Front. Immunol. 2022, 13, 967226. [Google Scholar] [CrossRef]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomedicine 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Murase, Y.; Yamada, Y.; Hirashiki, A.; Ichihara, S.; Kanda, H.; Watarai, M.; Takatsu, F.; Murohara, T.; Yokota, M. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur. Heart J. 2004, 25, 970–977. [Google Scholar] [CrossRef]
- Kidde, J.; Gorabi, A.M.; Jamialahmadi, T.; Sahebkar, A. COVID-19 Is an Endothelial Disease: Implications of Nitric Oxide. Adv. Exp. Med. Biol. 2021, 1321, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, R.; Bristow, M.R.; Kantrowitz, N.; Baim, D.S.; Harrison, D.C. Histamine provocation of clinical coronary artery spasm: Implications concerning pathogenesis of variant angina pectoris. Am. Heart J. 1981, 102, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Kim, M.; Kwon, S.; Joo, J.; Kwak, S.H.; Kim, E.O.; Jung, J.; et al. Adverse Reactions of the Second Dose of the BNT162b2 mRNA COVID-19 Vaccine in Healthcare Workers in Korea. J. Korean Med. Sci. 2021, 36, e153. [Google Scholar] [CrossRef]
- Corsini, F.; Scaglione, A.; Iacomino, M.; Mascia, G.; Melorio, S.; Riccio, C.; Romano, S.; Vetrano, A.; Celardo, S.; Corsini, G.; et al. L’infarto miocardico acuto nell’ultrasettantacinquenne. Studio caso-controllo con una popolazione più giovane e rassegna della letteratura [Acute myocardial infarction in the elderly. A case-control study with a younger population and review of literature]. Monaldi Arch. Chest Dis. 2006, 66, 13–19. (In Italian) [Google Scholar] [CrossRef][Green Version]
- Marchionni, N.; Orso, F. Stable angina in the elderly: Diagnostic and therapeutic approach. J. Cardiovasc. Med. 2018, 19 (Suppl. 1), e84–e87. [Google Scholar] [CrossRef]
- Awaya, T.; Moroi, M.; Enomoto, Y.; Kunimasa, T.; Nakamura, M. What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions. Vaccines 2022, 28, 866. [Google Scholar] [CrossRef]
- Takahashi, J.; Suda, A.; Nishimiya, K.; Godo, S.; Yasuda, S.; Shimokawa, H. Pathophysiology and Diagnosis of Coronary Functional Abnormalities. Eur. Cardiol. 2021, 16, e30. [Google Scholar] [CrossRef]
- Mizuno, Y.; Hokimoto, S.; Harada, E.; Kinoshita, K.; Yoshimura, M.; Yasue, H. Variant Aldehyde Dehydrogenase 2 (ALDH2*2) in East Asians Interactively Exacerbates Tobacco Smoking Risk for Coronary Spasm—Possible Role of Reactive Aldehydes. Circ. J. 2016, 81, 96–102. [Google Scholar] [CrossRef]
- Barbato, E.; Wijns, W.; De Bruyne, B.; Mascia, G.; Piscione, F. Attuali concetti sulla fisiopatologia dei recettori beta-adrenergici nelle coronarie e sulle sue applicazioni cliniche [Current concepts of the physiopathology of coronary beta-adrenergic receptors, and their clinical applications]. Recenti Prog. Med. 2005, 96, 411–415. [Google Scholar] [PubMed]
- Kuda, Y.; Shibamoto, T.; Yang, W.; Zhang, T.; Tanida, M.; Kurata, Y. Blockade of β2-adrenoceptor, rather than β1-adrenoceptor, deteriorates cardiac anaphylaxis in isolated blood-perfused rat hearts. Cardiol. J. 2017, 24, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Ogino, J.; Oyama-Manabe, N.; Funayama, N. Vasospastic Angina: A Cause of Post-acute COVID-19 Syndrome. Intern. Med. 2022, 61, 2693–2695. [Google Scholar] [CrossRef] [PubMed]
- Vallejo Camazón, N.; Teis, A.; Martínez Membrive, M.J.; Llibre, C.; Bayés-Genís, A.; Mateu, L. Long COVID-19 and microvascular disease-related angina. Rev. Esp. Cardiol. Engl. Ed. 2022, 75, 444–446. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awaya, T.; Moroi, M.; Nakamura, F.; Toi, S.; Wakiya, M.; Enomoto, Y.; Kunimasa, T.; Nakamura, M. A Possibility of Vasospastic Angina after mRNA COVID-19 Vaccination. Vaccines 2022, 10, 1998. https://doi.org/10.3390/vaccines10121998
Awaya T, Moroi M, Nakamura F, Toi S, Wakiya M, Enomoto Y, Kunimasa T, Nakamura M. A Possibility of Vasospastic Angina after mRNA COVID-19 Vaccination. Vaccines. 2022; 10(12):1998. https://doi.org/10.3390/vaccines10121998
Chicago/Turabian StyleAwaya, Toru, Masao Moroi, Fuminori Nakamura, Satoru Toi, Momoko Wakiya, Yoshinari Enomoto, Taeko Kunimasa, and Masato Nakamura. 2022. "A Possibility of Vasospastic Angina after mRNA COVID-19 Vaccination" Vaccines 10, no. 12: 1998. https://doi.org/10.3390/vaccines10121998
APA StyleAwaya, T., Moroi, M., Nakamura, F., Toi, S., Wakiya, M., Enomoto, Y., Kunimasa, T., & Nakamura, M. (2022). A Possibility of Vasospastic Angina after mRNA COVID-19 Vaccination. Vaccines, 10(12), 1998. https://doi.org/10.3390/vaccines10121998




