Influenza Vaccination Reduces the Risk of Liver Cancer in Patients with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study
Abstract
1. Introduction
2. Methods
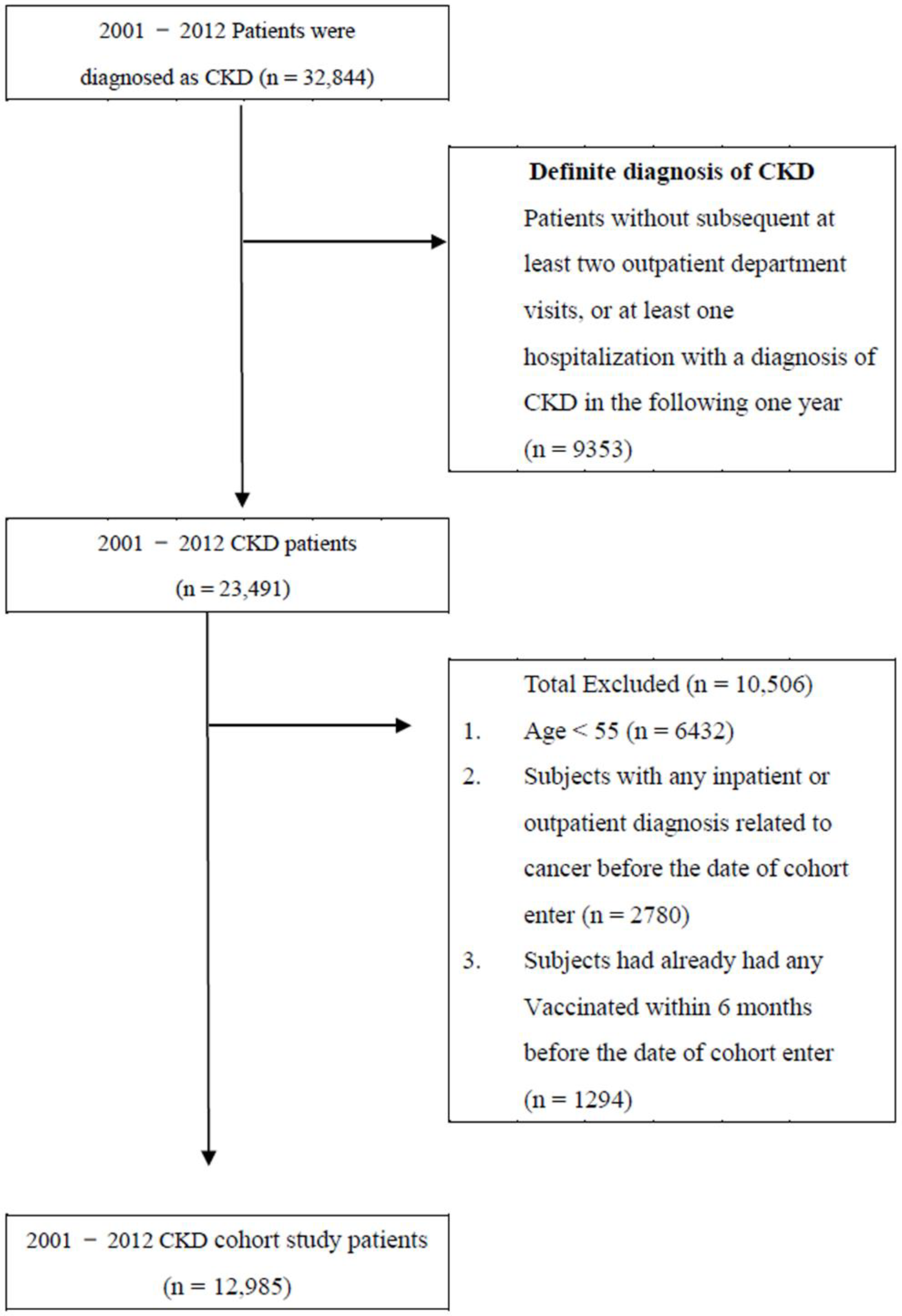
2.1. Patient Selection Process and the Primary Endpoint
2.2. Potential Confounders
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Main Findings
4.2. Mechanism of Liver Cancer Development in Patients with CKD
4.3. Mechanism Underlying the Association between Incidence of Liver Cancer and Influenza Vaccination
4.4. Effect of Influenza Vaccination in Patients with Different Characteristics
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2011, 2, 243–247. [Google Scholar] [CrossRef]
- Kazancioğlu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef]
- Na, S.Y.; Sung, J.Y.; Chang, J.H.; Kim, S.; Lee, H.H.; Park, Y.H.; Chung, W.; Oh, K.H.; Jung, J.Y. Chronic kidney disease in cancer patients: An independent predictor of cancer-specific mortality. Am. J. Nephrol. 2011, 33, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Cerutti, R.; Alfieri, C.M.; Ridruejo, E. An Update on Hepatocellular Carcinoma in Chronic Kidney Disease. Cancers 2021, 13, 3617. [Google Scholar] [CrossRef]
- Lafaro, K.J.; Demirjian, A.N.; Pawlik, T.M. Epidemiology of hepatocellular carcinoma. Surg. Oncol. Clin. N. Am. 2015, 24, 1–17. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Lo, W.-C.; Yang, Y.-W.; You, S.-L.; Chen, C.-J.; Lai, M.-S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. 2016, 115, 1076–1088. [Google Scholar] [CrossRef]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Yeh, H.; Chiang, C.C.; Yen, T.H. Hepatocellular carcinoma in patients with renal dysfunction: Pathophysiology, prognosis, and treatment challenges. World J. Gastroenterol. 2021, 27, 4104–4142. [Google Scholar] [CrossRef]
- Fang, Y.A.; Chen, C.I.; Liu, J.C.; Sung, L.C. Influenza Vaccination Reduces Hospitalization for Heart Failure in Elderly Patients with Chronic Kidney Disease: A Population-Based Cohort Study. Acta Cardiol. Sin. 2016, 32, 290–298. [Google Scholar] [CrossRef]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza vaccination in the elderly. Hum. Vaccines Immunother. 2018, 14, 540–549. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 27 June 2022).
- Mallia, P.; Johnston, S.L. Influenza infection and COPD. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 55–64. [Google Scholar] [CrossRef]
- Read, S.A.; Douglas, M.W. Virus induced inflammation and cancer development. Cancer Lett. 2014, 345, 174–181. [Google Scholar] [CrossRef]
- Rondy, M.; El Omeiri, N.; Thompson, M.G.; Levêque, A.; Moren, A.; Sullivan, S.G. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. J. Infect. 2017, 75, 381–394. [Google Scholar] [CrossRef]
- Chen, C.C.; Wu, C.H.; Lin, C.H.; Chiu, C.C.; Yang, T.Y.; Lei, M.H.; Yeh, H.T.; Jian, W.; Fang, Y.A.; Hao, W.R.; et al. Influenza Vaccination and Risk of Lung Cancer in Patients with Chronic Kidney Disease: A Nationwide, Population-Based Cohort Study. Cancers 2022, 14, 2926. [Google Scholar] [CrossRef]
- 2021 Government-Funded Influenza Vaccination Program. 17 September 2021. Available online: http://at.cdc.tw/GV3J16 (accessed on 23 January 2022).
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Zhang, X.; Wong, G.L.; Yip, T.C.; Tse, Y.K.; Liang, L.Y.; Hui, V.W.; Lin, H.; Li, G.L.; Lai, J.C.; Chan, H.L.; et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology 2022, 76, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Stengel, B. Chronic kidney disease and cancer: A troubling connection. J. Nephrol. 2010, 23, 253–262. [Google Scholar] [PubMed]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Uremic Toxins Induce Kidney Fibrosis by Activating Intrarenal Renin–Angiotensin–Aldosterone System Associated Epithelial-to-Mesenchymal Transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, Y.; Zhu, Z.; Su, X.; Ni, J.; Du, R.; Zhang, R.; Jin, W. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front. Med. 2016, 10, 320–329. [Google Scholar] [CrossRef]
- Nitta, T.; Kim, J.-S.; Mohuczy, D.; Behrns, K.E. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology 2008, 48, 909–919. [Google Scholar] [CrossRef]
- Wells, R.G. The epithelial to mesenchymal transition in liver fibrosis: Here today, gone tomorrow? Hepatology 2010, 51, 737–740. [Google Scholar] [CrossRef]
- Descamps-Latscha, B.; Herbelin, A.; Nguyen, A.T.; Roux-Lombard, P.; Zingraff, J.; Moynot, A.; Verger, C.; Dahmane, D.; de Groote, D.; Jungers, P.; et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J. Immunol. 1995, 154, 882–892. [Google Scholar]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015, 7, ps271. [Google Scholar] [CrossRef]
- Yamada, S.; Takashina, Y.; Watanabe, M.; Nagamine, R.; Saito, Y.; Kamada, N.; Saito, H. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget 2018, 9, 9925–9939. [Google Scholar] [CrossRef]
- Chai, E.Z.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem. J. 2015, 468, 1–15. [Google Scholar] [CrossRef]
- Jost, S.; Quillay, H.; Reardon, J.; Peterson, E.; Simmons, R.P.; Parry, B.A.; Bryant, N.N.P.; Binder, W.D.; Altfeld, M. Changes in Cytokine Levels and NK Cell Activation Associated with Influenza. PLoS ONE 2011, 6, e25060. [Google Scholar] [CrossRef]
- Krueger, P.D.; Lassen, M.G.; Qiao, H.; Hahn, Y.S. Regulation of NK cell repertoire and function in the liver. Crit. Rev. Immunol. 2011, 31, 43–52. [Google Scholar] [CrossRef]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.L.; Salomé-Desnoulez, S.; Deryuter, L.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947.e2936. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J Toxicol Environ. Health B Crit. Rev. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Watanabe, T. Renal complications of seasonal and pandemic influenza A virus infections. Eur. J. Pediatr. 2013, 172, 15–22. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Prieto, J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J. Hepatol. 2008, 48, 380–381. [Google Scholar] [CrossRef] [PubMed]
| Whole Cohort (n = 12,985) | Unvaccinated (n = 7490) | Vaccinated (n = 5495) | p a | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, years (Mean ± SD) | 70.98 (9.40) | 70.09 (10.26) | 72.18 (7.90) | <0.001 | |||
| 55–64 | 3989 | 30.72 | 2877 | 38.41 | 1112 | 20.24 | <0.001 |
| 65–74 | 4541 | 34.97 | 2139 | 28.56 | 2402 | 43.71 | |
| ≥75 | 4455 | 34.31 | 2474 | 33.03 | 1981 | 36.05 | |
| Gender | |||||||
| Female | 5712 | 43.99 | 3333 | 44.50 | 2379 | 43.29 | 0.172 |
| Male | 7273 | 56.01 | 4157 | 55.50 | 3116 | 56.71 | |
| CCI Index + | |||||||
| 0 | 1491 | 11.48 | 876 | 11.70 | 615 | 11.19 | 0.013 |
| 1 | 2043 | 15.73 | 1166 | 15.57 | 877 | 15.96 | |
| 2 | 2876 | 22.15 | 1589 | 21.21 | 1287 | 23.42 | |
| ≥3 | 6575 | 50.64 | 3859 | 51.52 | 2716 | 49.43 | |
| Diabetes | |||||||
| No | 6310 | 48.59 | 3355 | 44.79 | 2955 | 53.78 | <0.001 |
| Yes | 6675 | 51.41 | 4135 | 55.21 | 2540 | 46.22 | |
| Hypertension | |||||||
| No | 2555 | 19.68 | 1387 | 18.52 | 1168 | 21.26 | <0.001 |
| Yes | 10,430 | 80.32 | 6103 | 81.48 | 4327 | 78.74 | |
| Dyslipidemia | |||||||
| No | 6337 | 48.80 | 3386 | 45.21 | 2951 | 53.70 | <0.001 |
| Yes | 6648 | 51.20 | 4104 | 54.79 | 2544 | 46.30 | |
| Statin | |||||||
| <28 days | 7972 | 61.39 | 4786 | 63.90 | 3186 | 57.98 | <0.001 |
| 28–365 days | 2683 | 20.66 | 1576 | 21.04 | 1107 | 20.15 | |
| >365 days | 2330 | 17.94 | 1128 | 15.06 | 1202 | 21.87 | |
| Metformin | |||||||
| <28 days | 10,266 | 79.06 | 6045 | 80.71 | 4221 | 76.82 | <0.001 |
| 28–365 days | 1331 | 10.25 | 804 | 10.73 | 527 | 9.59 | |
| >365 days | 1388 | 10.69 | 641 | 8.56 | 747 | 13.59 | |
| RAASI | |||||||
| <28 days | 4114 | 31.68 | 2792 | 37.28 | 1322 | 24.06 | <0.001 |
| 28–365 days | 3751 | 28.89 | 2344 | 31.30 | 1407 | 25.61 | |
| >365 days | 5120 | 39.43 | 2354 | 31.43 | 2766 | 50.34 | |
| Aspirin | |||||||
| <28 days | 6715 | 51.71 | 4478 | 59.79 | 2237 | 40.71 | <0.001 |
| 28–365 days | 3149 | 24.25 | 1702 | 22.72 | 1447 | 26.33 | |
| >365 days | 3121 | 24.04 | 1310 | 17.49 | 1811 | 32.96 | |
| Level of Urbanization | |||||||
| Urban | 8785 | 67.65 | 5350 | 71.43 | 3435 | 62.51 | <0.001 |
| Suburban | 2806 | 21.61 | 1488 | 19.87 | 1318 | 23.99 | |
| Rural | 1394 | 10.74 | 652 | 8.70 | 742 | 13.50 | |
| Monthly income (TWD) | |||||||
| 0 | 1596 | 12.29 | 901 | 12.03 | 695 | 12.65 | <0.001 |
| 1–21,000 | 4486 | 34.55 | 2397 | 32.00 | 2089 | 38.02 | |
| 21,000–33,300 | 3788 | 29.17 | 1996 | 26.65 | 1792 | 32.61 | |
| ≥33,301 | 3115 | 23.99 | 2196 | 29.32 | 919 | 16.72 | |
| All Group (n = 12,985) | Unvaccinated (Total Follow-Up 21,919.2 Person-Years) | Vaccinated (Total Follow-Up 33,990.2 Person-Years) | Adjusted HR † (95% C.I.) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients with Cancer | Incidence Rate (per 105 Person-Years) (95% C.I.) | No. of Patients with Cancer | Incidence Rate (Per 105 Person-Years) (95% C.I.) | ||||||
| Whole cohort | |||||||||
| All season | 162 | 739.1 | (625.3, | 852.9) | 123 | 361.9 | (297.9, | 425.8) | 0.45 (0.35, 0.58) *** |
| Age, 55–64 a | |||||||||
| All season | 82 | 760.1 | (595.6, | 924.7) | 22 | 244.5 | (142.3, | 346.7) | 0.28 (0.17, 0.46) *** |
| Age, 65–74 b | |||||||||
| All season | 43 | 701.0 | (491.5, | 910.5) | 63 | 398.2 | (299.9, | 496.5) | 0.53 (0.35, 0.79) ** |
| Age, ≥75 c | |||||||||
| All season | 37 | 740.4 | (501.8, | 978.9) | 38 | 414.4 | (282.6, | 546.1) | 0.57 (0.35, 0.91) * |
| Female d | |||||||||
| All season | 60 | 622.6 | (465.1, | 780.2) | 39 | 260.3 | (178.6, | 342.1) | 0.39 (0.26, 0.60) *** |
| Male e | |||||||||
| All season | 102 | 830.4 | (669.3, | 991.6) | 84 | 441.9 | (347.4, | 536.4) | 0.49 (0.36, 0.67) *** |
| Unvaccinated | Vaccinated | p for Trend | |||
|---|---|---|---|---|---|
| 1 | 2–3 | ≥4 | |||
| Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | Adjusted HR (95%C.I.) | ||
| Main model † | 1.00 | 0.86 (0.63, 1.17) | 0.45 (0.31, 0.63) *** | 0.21 (0.14, 0.33) *** | <0.001 |
| Additional covariates ‡ | |||||
| Main model + Statin | 1.00 | 0.89 (0.65, 1.21) | 0.47 (0.33, 0.67) *** | 0.23 (0.15, 0.35) *** | <0.001 |
| Main model + Metformin | 1.00 | 0.86 (0.63, 1.18) | 0.45 (0.32, 0.64) *** | 0.22 (0.14, 0.33) *** | <0.001 |
| Main model + RAA | 1.00 | 0.91 (0.67, 1.23) | 0.47 (0.33, 0.67) *** | 0.23 (0.15, 0.36) *** | <0.001 |
| Main model + Aspirin | 1.00 | 0.91 (0.67, 1.24) | 0.49 (0.34, 0.69) *** | 0.24 (0.16, 0.36) *** | <0.001 |
| Subgroup effects | |||||
| Age, years | |||||
| 55–64 | 1.00 | 0.50 (0.27, 0.92) * | 0.33 (0.16, 0.69) ** | 0.07 (0.02, 0.27) *** | <0.001 |
| 65–74 | 1.00 | 1.22 (0.75, 1.99) | 0.58 (0.34, 0.97) * | 0.23 (0.13, 0.42) *** | <0.001 |
| ≥75 | 1.00 | 0.98 (0.55, 1.75) | 0.42 (0.21, 0.84) * | 0.38 (0.18, 0.80) * | 0.002 |
| Sex | |||||
| Female | 1.00 | 0.90 (0.55, 1.47) | 0.36 (0.19, 0.67) *** | 0.11 (0.04, 0.27) *** | <0.001 |
| Male | 1.00 | 0.85 (0.57, 1.26) | 0.50 (0.33, 0.77) ** | 0.28 (0.17, 0.45) *** | <0.001 |
| CCI Index + | |||||
| 0 | 1.00 | 1.00 (0.27, 3.65) | 0.75 (0.23, 2.44) | 0.46 (0.14, 1.54) | 0.204 |
| 1 | 1.00 | 0.57 (0.23, 1.40) | 0.65 (0.28, 1.47) | 0.23 (0.08, 0.62) ** | 0.004 |
| 2 | 1.00 | 0.75 (0.38, 1.47) | 0.57 (0.29, 1.11) | 0.20 (0.09, 0.45) *** | <0.001 |
| ≥3 | 1.00 | 0.89 (0.60, 1.32) | 0.28 (0.16, 0.49) *** | 0.17 (0.09, 0.32) *** | <0.001 |
| Diabetes | |||||
| No | 1.00 | 0.88 (0.56, 1.38) | 0.54 (0.33, 0.87) * | 0.23 (0.13, 0.39) *** | <0.001 |
| Yes | 1.00 | 0.81 (0.53, 1.23) | 0.34 (0.20, 0.57) *** | 0.20 (0.10, 0.38) *** | <0.001 |
| Dyslipidemia | |||||
| No | 1.00 | 0.80 (0.53, 1.19) | 0.37 (0.23, 0.59) *** | 0.21 (0.13, 0.35) *** | <0.001 |
| Yes | 1.00 | 0.97 (0.60, 1.57) | 0.59 (0.35, 1.01) | 0.22 (0.10, 0.45) *** | <0.001 |
| Hypertension | |||||
| No | 1.00 | 0.80 (0.42, 1.54) | 0.52 (0.26, 1.03) | 0.20 (0.09, 0.45) *** | <0.001 |
| Yes | 1.00 | 0.87 (0.61, 1.24) | 0.42 (0.27, 0.63) *** | 0.21 (0.13, 0.35) *** | <0.001 |
| Statin | |||||
| <28 days | 1.00 | 0.90 (0.63, 1.28) | 0.43 (0.28, 0.65) *** | 0.24 (0.15, 0.39) *** | <0.001 |
| 28–365 days | 1.00 | 0.80 (0.29, 2.18) | 0.77 (0.30, 1.92) | 0.24 (0.07, 0.87) * | 0.040 |
| >365 days | 1.00 | 0.97 (0.40, 2.36) | 0.47 (0.17, 1.32) | 0.17 (0.05, 0.60) ** | 0.003 |
| Metformin | |||||
| <28 days | 1.00 | 0.82 (0.57, 1.16) | 0.42 (0.28, 0.63) *** | 0.19 (0.11, 0.31) *** | <0.001 |
| 28–365 days | 1.00 | 1.15 (0.44, 2.99) | 0.86 (0.31, 2.38) | 0.59 (0.20, 1.80) | 0.346 |
| >365 days | 1.00 | 1.05 (0.42, 2.59) | 0.42 (0.15, 1.19) | 0.24 (0.09, 0.69) ** | 0.003 |
| RAASI | |||||
| <28 days | 1.00 | 0.86 (0.52, 1.41) | 0.36 (0.18, 0.70) ** | 0.09 (0.03, 0.28) *** | <0.001 |
| 28–365 days | 1.00 | 1.19 (0.67, 2.11) | 0.42 (0.20, 0.88) * | 0.36 (0.17, 0.77) ** | 0.002 |
| >365 days | 1.00 | 0.83 (0.47, 1.44) | 0.63 (0.37, 1.07) | 0.28 (0.15, 0.50) *** | <0.001 |
| Aspirin | |||||
| <28 days | 1.00 | 0.83 (0.54, 1.26) | 0.53 (0.33, 0.85) ** | 0.14 (0.06, 0.31) *** | <0.001 |
| 28–365 days | 1.00 | 1.10 (0.59, 2.05) | 0.45 (0.21, 0.96) * | 0.08 (0.02, 0.36) *** | <0.001 |
| >365 days | 1.00 | 1.15 (0.56, 2.36) | 0.54 (0.24, 1.20) | 0.57 (0.30, 1.10) | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.-R.; Yang, T.-Y.; Chen, C.-C.; Lin, K.-J.; Chiu, C.-C.; Fang, Y.-A.; Jian, W.; Lei, M.-H.; Yeh, H.-T.; Hsu, M.-H.; et al. Influenza Vaccination Reduces the Risk of Liver Cancer in Patients with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study. Vaccines 2022, 10, 2008. https://doi.org/10.3390/vaccines10122008
Hao W-R, Yang T-Y, Chen C-C, Lin K-J, Chiu C-C, Fang Y-A, Jian W, Lei M-H, Yeh H-T, Hsu M-H, et al. Influenza Vaccination Reduces the Risk of Liver Cancer in Patients with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study. Vaccines. 2022; 10(12):2008. https://doi.org/10.3390/vaccines10122008
Chicago/Turabian StyleHao, Wen-Rui, Tsung-Yeh Yang, Chun-Chao Chen, Kuan-Jie Lin, Chun-Chih Chiu, Yu-Ann Fang, William Jian, Meng-Huan Lei, Hsien-Tang Yeh, Min-Huei Hsu, and et al. 2022. "Influenza Vaccination Reduces the Risk of Liver Cancer in Patients with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study" Vaccines 10, no. 12: 2008. https://doi.org/10.3390/vaccines10122008
APA StyleHao, W.-R., Yang, T.-Y., Chen, C.-C., Lin, K.-J., Chiu, C.-C., Fang, Y.-A., Jian, W., Lei, M.-H., Yeh, H.-T., Hsu, M.-H., Chen, N.-H., Jong, H.-C., Zheng, J.-Q., & Liu, J.-C. (2022). Influenza Vaccination Reduces the Risk of Liver Cancer in Patients with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study. Vaccines, 10(12), 2008. https://doi.org/10.3390/vaccines10122008









