Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Heterogeneity Assessment
2.5. Quality Assessment
2.6. Outcome Measures
2.7. Statistical Analyses
3. Results
3.1. Study Selection and Characteristics
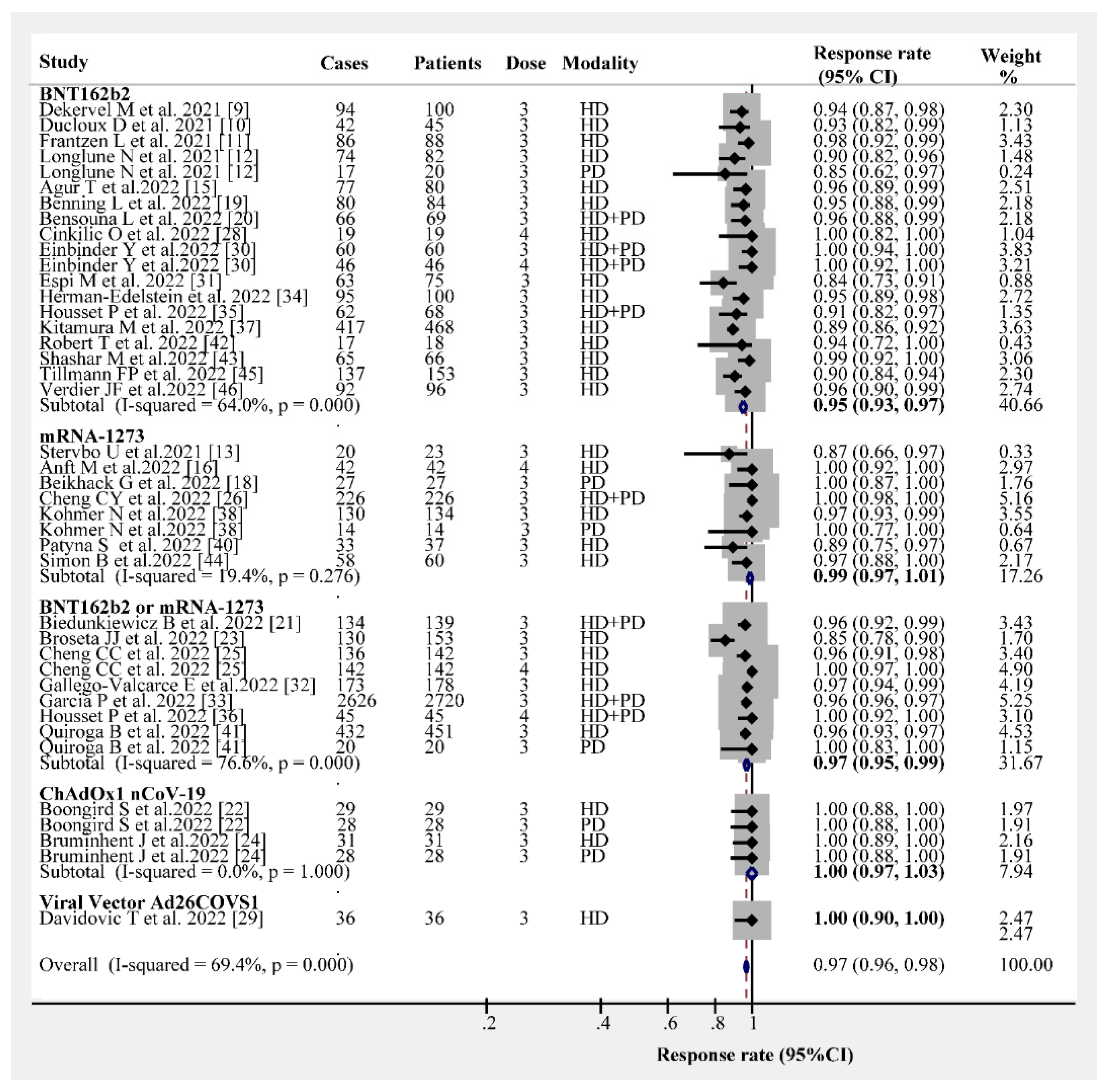
3.2. Post-Vaccination Humoral Immunogenicity Rates
3.3. Cellular Immunogenicity Rates
3.4. RRs for a Breakthrough SARS-CoV-2 Infection, All-Cause Death, and Admission
3.5. Adverse Events of the SARS-CoV-2 Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin Immunother. 2022, 18, 2002083. [Google Scholar] [PubMed]
- Geetha, D.; Kronbichler, A.; Rutter, M.; Bajpai, D.; Menez, S.; Weissenbacher, A.; Anand, S.; Lin, E.; Carlson, N.; Sozio, S.; et al. Impact of the COVID-19 pandemic on the kidney community: Lessons learned and future directions. Nat. Rev. Nephrol. 2022, 18, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.; Fischer, S.; Müller, L.; Lübke, N.; Hillebrandt, J.; Andrée, M.; Schmitz, M.; Schmidt, C.; Küçükköylü, S.; Koster, L.; et al. Impaired Immune Response to SARS-CoV-2 Vaccination in Dialysis Patients and in Kidney Transplant Recipients. Kidney360 2021, 2, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Babel, N.; Hugo, C.; Westhoff, T.H. Vaccination in patients with kidney failure: Lessons from COVID-19. Nat. Rev. Nephrol. 2022, 18, 708–723. [Google Scholar] [CrossRef]
- Piotrowska, M.; Zieliński, M.; Tylicki, L.; Biedunkiewicz, B.; Kubanek, A.; Ślizień, Z.; Polewska, K.; Tylicki, P.; Muchlado, M.; Sakowska, J.; et al. Local and Systemic Immunity Are Impaired in End-Stage-Renal-Disease Patients Treated with Hemodialysis, Peritoneal Dialysis and Kidney Transplant Recipients Immunized with BNT162b2 Pfizer-BioNTech SARS-CoV-2 Vaccine. Front. Immunol. 2022, 13, 832924. [Google Scholar] [CrossRef]
- Chen, J.J.; Lee, T.H.; Tian, Y.C.; Lee, C.C.; Fan, P.C.; Chang, C.H. Immunogenicity Rates after SARS-CoV-2 Vaccination in People with End-stage Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2131749. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- El Karoui, K.; De Vriese, A.S. COVID-19 in dialysis: Clinical impact, immune response, prevention, and treatment. Kidney Int. 2022, 101, 883–894. [Google Scholar] [CrossRef]
- Dekervel, M.; Henry, N.; Torreggiani, M.; Pouteau, L.M.; Imiela, J.P.; Mellaza, C.; Garnier, A.S.; Dujardin, A.; Asfar, M.; Ducancelle, A.; et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021, 14, 2349–2355. [Google Scholar] [CrossRef]
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021, 100, 702–704. [Google Scholar] [CrossRef]
- Frantzen, L.; Thibeaut, S.; Moussi-Frances, J.; Indreies, M.; Kiener, C.; Saingra, Y.; Santini, J.; Stroumza, P.; El-Haik, Y.; Cavaillé, G. COVID-19 vaccination in haemodialysis patients: Good things come in threes…. Nephrol. Dial. Transplant. 2021, 36, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Longlune, N.; Nogier, M.B.; Miedougé, M.; Gabilan, C.; Cartou, C.; Seigneuric, B.; Del Bello, A.; Marion, O.; Faguer, S.; Izopet, J.; et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol. Dial. Transplant. 2021, 36, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Stervbo, U.; Blazquez-Navarro, A.; Blanco, E.V.; Safi, L.; Meister, T.L.; Paniskaki, K.; Stockhausen, M.; Marheinecke, C.; Zimmer, G.; Wellenkötter, J.; et al. Improved cellular and humoral immunity upon a second BNT162b2 and mRNA-1273 boost in prime-boost vaccination no/low responders with end-stage renal disease. Kidney Int. 2021, 100, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, F.P.; Figiel, L.; Ricken, J.; Still, H.; Korte, C.; Plassmann, G.; von Landenberg, P. Evolution of SARS-CoV-2-Neutralizing Antibodies after Two Standard Dose Vaccinations, Risk Factors for Non-Response and Effect of a Third Dose Booster Vaccination in Non-Responders on Hemodialysis: A Prospective Multi-Centre Cohort Study. J. Clin. Med. 2021, 10, 5113. [Google Scholar] [CrossRef] [PubMed]
- Agur, T.; Zingerman, B.; Ben-Dor, N.; Alkeesh, W.; Steinmetz, T.; Rachamimov, R.; Korzets, A.; Rozen-Zvi, B.; Herman-Edelstein, M. Humoral Response to the Third Dose of BNT162b2 COVID-19 Vaccine among Hemodialysis Patients. Nephron 2022, 1–8. [Google Scholar] [CrossRef]
- Anft, M.; Blazquez-Navarro, A.; Frahnert, M.; Fricke, L.; Meister, T.L.; Roch, T.; Stervbo, U.; Pfaender, S.; Westhoff, T.H.; Babel, N. Inferior cellular and humoral immunity against Omicron and Delta variants of concern compared with SARS-CoV-2 wild type in hemodialysis patients immunized with 4 SARS-CoV-2 vaccine doses. Kidney Int. 2022, 102, 207–208. [Google Scholar] [CrossRef]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chen, J.; et al. Outcome and effect of vaccination in SARS-CoV-2 Omicron infection in hemodialysis patients: A cohort study. Nephrol. Dial. Transplant. 2022, 37, 1944–1950. [Google Scholar] [CrossRef]
- Beilhack, G.; Monteforte, R.; Frommlet, F.; Reindl-Schwaighofer, R.; Strassl, R.; Vychytil, A. Humoral Response to mRNA-1273 SARS-CoV-2 Vaccine in Peritoneal Dialysis Patients: Is Boostering after Six Months Adequate? Front. Med. 2022, 9, 905798. [Google Scholar] [CrossRef]
- Benning, L.; Klein, K.; Morath, C.; Bartenschlager, M.; Kim, H.; Buylaert, M.; Reineke, M.; Töllner, M.; Nusshag, C.; Kälble, F.; et al. Neutralizing Antibody Activity against the B.1.617.2 (delta) Variant before and after a Third BNT162b2 Vaccine Dose in Hemodialysis Patients. Front. Immunol. 2022, 13, 840136. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.L.; Housset, P. SARS-CoV-2 Antibody Response after a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e1. [Google Scholar] [CrossRef]
- Biedunkiewicz, B.; Tylicki, L.; Ślizień, W.; Lichodziejewska-Niemierko, M.; Dąbrowska, M.; Kubanek, A.; Rodak, S.; Polewska, K.; Tylicki, P.; Renke, M.; et al. Waning Humoral Response after COVID-19 mRNA Vaccination in Maintenance Dialysis Patients and Recovery after a Complementary Third Dose. Vaccines 2022, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Boongird, S.; Setthaudom, C.; Kitpermkiat, R.; Prasongtanakij, S.; Srisala, S.; Chuengsaman, P.; Nongnuch, A.; Assanatham, M.; Kiertiburanakul, S.; Malathum, K.; et al. Durability of Humoral and Cellular Immunity after an Extended Primary Series with Heterologous Inactivated SARS-CoV-2 Prime-Boost and ChAdOx1 nCoV-19 in Dialysis Patients (ICON3). Vaccines 2022, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Cuadrado, E.; Rodríguez, N.; Bedini, J.L.; Maduell, F. Humoral Response after Three Doses of mRNA-1273 or BNT162b2 SARS-CoV-2 Vaccines in Hemodialysis Patients. Vaccines 2022, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Bruminhent, J.; Setthaudom, C.; Kitpermkiat, R.; Kiertiburanakul, S.; Malathum, K.; Assanatham, M.; Nongnuch, A.; Phuphuakrat, A.; Chaumdee, P.; Janphram, C.; et al. Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients. Sci. Rep. 2022, 12, 3587. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Platen, L.; Christa, C.; Tellenbach, M.; Kappler, V.; Bester, R.; Liao, B.H.; Holzmann-Littig, C.; Werz, M.; Schönhals, E.; et al. Improved SARS-CoV-2 Neutralization of Delta and Omicron BA.1 Variants of Concern after Fourth Vaccination in Hemodialysis Patients. Vaccines 2022, 10, 1328. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Hsiao, S.H.; Fang, T.C.; Lin, Y.C.; Wang, J.C.; Hung, C.S.; Chen, T.H.; Sue, Y.M. SARS-CoV2 antibody response after a third dose of heterologous ChAdOx1 nCoV-19 and Moderna vaccine in chronic dialysis patients. J. Infect. 2022, 84, e98–e100. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Wu, H.H.L.; Cox, E.; Moore, J.; Clough, T.; Lamerton, E.; Donne, R.; O’Riordan, E.; Poulikakos, D. Humoral Response in Hemodialysis Patients Following COVID-19 Vaccination and Breakthrough Infections during Delta and Omicron Variant Predominance. Vaccines 2022, 10, 498. [Google Scholar] [CrossRef]
- Cinkilic, O.; Anft, M.; Blazquez-Navarro, A.; Meister, T.L.; Roch, T.; Stervbo, U.; Pfaender, S.; Westhoff, T.H.; Babel, N. Inferior humoral and sustained cellular immunity against wild-type and omicron variant of concern in hemodialysis patients immunized with 3 SARS-CoV-2 vaccine doses compared with 4 doses. Kidney Int. 2022, 101, 1287–1289. [Google Scholar] [CrossRef]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mähr, H.; Lhotta, K.; Zitt, E. Humoral and Cellular Immune Response after a 3-Dose Heterologous SARS-CoV-2 Vaccination Using the mRNA-BNT162b2 and Viral Vector Ad26COVS1 Vaccine in Hemodialysis Patients. Front. Immunol. 2022, 13, 907615. [Google Scholar] [CrossRef]
- Einbinder, Y.; Perl, J.; Nacasch, N.; Bnaya, A.; Shavit, L.; Erez, D.; Shashar, M.; Halperin, T.; Grupper, A.; Benchetrit, S.; et al. Humoral Response and SARS-CoV-2 Infection Risk following the Third and Fourth Doses of the BNT162b2 Vaccine in Dialysis Patients. Am. J. Nephrol. 2022, 53, 586–590. [Google Scholar] [CrossRef]
- Espi, M.; Charmetant, X.; Barba, T.; Mathieu, C.; Pelletier, C.; Koppe, L.; Chalencon, E.; Kalbacher, E.; Mathias, V.; Ovize, A.; et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022, 101, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Valcarce, E.; Shabaka, A.; Leon-Poo, M.; Gruss, E.; Acedo-Sanz, J.M.; Cordón, A.; Cases-Corona, C.; Fernandez-Juarez, G. Humoral Response Following Triple Dose of mRNA Vaccines against SARS-CoV-2 in Hemodialysis Patients: Results after 1 Year of Follow-up. Front. Med. 2022, 9, 927546. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Han, J.; Montez-Rath, M.E.; Sun, S.; Shang, T.; Parsonnet, J.; Chertow, G.M.; Anand, S.; Schiller, B.; Abra, G. SARS-CoV-2 Booster Vaccine Response among Patients Receiving Dialysis. Clin. J. Am. Soc. Nephrol. 2022, 17, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Ben-Dor, N.; Agur, T.; Guetta, T.; Raiter, A.; Meisel, E.; Alkeesh, W.; Ori, Y.; Rozen-Zvi, B.; Zingerman, B. BNT162b2 Booster Vaccination Induced Immunity against SARS-CoV-2 Variants among Hemodialysis Patients. Vaccines 2022, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Housset, P.; Kubab, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Caudwell, V.; Faucon, A.L. Waning but persistent humoral response 6 months after the third dose of the mRNA BNT162b2 vaccine in hemodialysis and peritoneal dialysis patients. J. Nephrol. 2022, 35, 783–785. [Google Scholar] [CrossRef]
- Housset, P.; Kubab, S.; Hanafi, L.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Caudwell, V.; Faucon, A.L. Humoral response after a fourth “booster” dose of a Coronavirus disease 2019 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int. 2022, 101, 1289–1290. [Google Scholar] [CrossRef]
- Kitamura, M.; Takazono, T.; Yamaguchi, K.; Tomura, H.; Yamamoto, K.; Harada, T.; Funakoshi, S.; Mukae, H.; Nishino, T. Favorable Humoral Response to Third Dose of BNT162b2 in Patients Undergoing Hemodialysis. J. Clin. Med. 2022, 11, 2090. [Google Scholar] [CrossRef]
- Kohmer, N.; Rabenau, H.F.; Ciesek, S.; Krämer, B.K.; Göttmann, U.; Keller, C.; Rose, D.; Blume, C.; Thomas, M.; Lammert, A.; et al. Heterologous immunization with BNT162b2 followed by mRNA-1273 in dialysis patients: Seroconversion and presence of neutralizing antibodies. Nephrol. Dial. Transplant. 2022, 37, 1132–1139. [Google Scholar] [CrossRef]
- Mosconi, G.; Fantini, M.; Righini, M.; Flachi, M.; Semprini, S.; Hu, L.; Chiappo, F.; Veterani, B.; Ambri, K.; Ferrini, F.; et al. Efficacy of SARS-CoV-2 Vaccination in Dialysis Patients: Epidemiological Analysis and Evaluation of the Clinical Progress. J. Clin. Med. 2022, 11, 4723. [Google Scholar] [CrossRef]
- Patyna, S.; Eckes, T.; Koch, B.F.; Sudowe, S.; Oftring, A.; Kohmer, N.; Rabenau, H.F.; Ciesek, S.; Avaniadi, D.; Steiner, R.; et al. Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response. Vaccines 2022, 10, 585. [Google Scholar] [CrossRef]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Orero, E.; Tejedor, S.; Mantecón, C.J.J.; Gómez Pérez, V.O.; Marín Franco, A.J.; Alfaro Sánchez, C.; Puerta Carretero, M.; et al. Humoral Response to Third Dose of SARS-CoV-2 Vaccines in the CKD Spectrum. Clin. J. Am. Soc. Nephrol. 2022, 17, 872–876. [Google Scholar] [CrossRef]
- Robert, T.; Lano, G.; Giot, M.; Fourié, T.; de Lamballeri, X.; Jehel, O.; Bouchouareb, D.; Brunet, P.; Ninove, L.; Burtey, S. Humoral response after SARS-CoV-2 vaccination in patients undergoing maintenance haemodialysis: Loss of immunity, third dose and non-responders. Nephrol. Dial. Transplant. 2022, 37, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Shashar, M.; Nacasch, N.; Grupper, A.; Benchetrit, S.; Halperin, T.; Erez, D.; Rozenberg, I.; Shitrit, P.; Sela, Y.; Wand, O.; et al. Humoral Response to Pfizer BNT162b2 Vaccine Booster in Maintenance Hemodialysis Patients. Am. J. Nephrol. 2022, 53, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Rubey, H.; Gromann, M.; Knopf-Völkerer, A.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. SARS-CoV-2 Antibody and T Cell Response after a Third Vaccine Dose in Hemodialysis Patients Compared with Healthy Controls. Vaccines 2022, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, F.P.; Figiel, L.; Ricken, J.; Still, H.; Korte, C.; Plaßmann, G.; Harth, A.; Jörres, A.; von Landenberg, P. Effect of Third and Fourth mRNA-Based Booster Vaccinations on SARS-CoV-2 Neutralizing Antibody Titer Formation, Risk Factors for Non-Response, and Outcome after SARS-CoV-2 Omicron Breakthrough Infections in Patients on Chronic Hemodialysis: A Prospective Multicenter Cohort Study. J. Clin. Med. 2022, 11, 3187. [Google Scholar]
- Verdier, J.F.; Boyer, S.; Chalmin, F.; Jeribi, A.; Egasse, C.; Maggi, M.F.; Auvray, P.; Yalaoui, T. Response to three doses of the Pfizer/BioNTech BNT162b2 COVID-19 vaccine: A retrospective study of a cohort of haemodialysis patients in France. BMC Nephrol. 2022, 23, 189. [Google Scholar] [CrossRef]
- Park, S.; Gatchalian, K.K.; Oh, H. Association of Homologous and Heterologous Vaccine Boosters with SARS-CoV-2 Infection in BBIBP-CorV Vaccinated Healthcare Personnel. Cureus 2022, 14, e27323. [Google Scholar] [CrossRef]
- Matula, Z.; Gönczi, M.; Bekő, G.; Kádár, B.; Ajzner, É.; Uher, F.; Vályi-Nagy, I. Antibody and T Cell Responses against SARS-CoV-2 Elicited by the Third Dose of BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) Vaccines Using a Homologous or Heterologous Booster Vaccination Strategy. Vaccines 2022, 10, 539. [Google Scholar] [CrossRef]
- Haase, M.; Lesny, P.; Anderson, M.; Cloherty, G.; Stec, M.; Haase-Fielitz, A.; Haarhaus, M.; Santos-Araújo, C.; Veiga, P.M.; Macario, F. Humoral immunogenicity and tolerability of heterologous ChAd/BNT compared with homologous BNT/BNT and ChAd/ChAd SARS-CoV-2 vaccination in hemodialysis patients: A multicenter prospective observational study. J. Nephrol. 2022, 35, 1467–1478. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal Centers. Annu. Rev. Immunol. 2022, 40, 413–442. [Google Scholar] [CrossRef]
- Muecksch, F.; Wang, Z.; Cho, A.; Gaebler, C.; Ben Tanfous, T.; DaSilva, J.; Bednarski, E.; Ramos, V.; Zong, S.; Johnson, B.; et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature 2022, 607, 128–134. [Google Scholar] [CrossRef]
- Naito, T.; Yan, Y.; Tabe, Y.; Seyama, K.; Deshpande, G.A. Real-world evidence for the effectiveness and breakthrough of BNT162b2 mRNA COVID-19 vaccine at a medical center in Japan. Hum. Vaccin Immunother. 2022, 18, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Behera, P.; Singh, A.K.; Subba, S.H.; Mc, A.; Sahu, D.P.; Chandanshive, P.D.; Pradhan, S.K.; Parida, S.P.; Mishra, A.; Patro, B.K.; et al. Effectiveness of COVID-19 vaccine (Covaxin) against breakthrough SARS-CoV-2 infection in India. Hum. Vaccin Immunother. 2022, 18, 2034456. [Google Scholar] [CrossRef] [PubMed]
- Monge, S.; Rojas-Benedicto, A.; Olmedo, C.; Mazagatos, C.; José Sierra, M.; Limia, A.; Martín-Merino, E.; Larrauri, A.; Hernán, M.A.; IBERCovid. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: A nationwide cohort study. Lancet Infect. Dis. 2022, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Sibbel, S.; McKeon, K.; Luo, J.; Wendt, K.; Walker, A.G.; Kelley, T.; Lazar, R.; Zywno, M.L.; Connaire, J.J.; Tentori, F.; et al. Real-World Effectiveness and Immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Patients on Hemodialysis. J. Am. Soc. Nephrol. 2022, 33, 49–57. [Google Scholar] [CrossRef]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chen, J.; et al. Severity of COVID-19 after Vaccination among Hemodialysis Patients: An Observational Cohort Study. Clin. J. Am. Soc. Nephrol. 2022, 17, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Morgan, C.; Kerschmann, R.; Beyer, P.; Dittrich, M.; et al. SARS-CoV-2 Vaccine Antibody Response and Breakthrough Infection in Patients Receiving Dialysis. Ann. Intern. Med. 2022, 175, 371–378. [Google Scholar] [CrossRef]
- Seraceni, S.; Zocca, E.; Cervone, T.E.; Tomassetti, F.; Polidori, I.; Valisi, M.; Broccolo, F.; Calugi, G.; Bernardini, S.; Pieri, M. T-Cell Assay after COVID-19 Vaccination Could Be a Useful Tool? A Pilot Study on Interferon-Gamma Release Assay in Healthcare Workers. Diseases 2022, 10, 49. [Google Scholar] [CrossRef]
- Yang, L.M.; Costales, C.; Ramanathan, M.; Bulterys, P.L.; Murugesan, K.; Schroers-Martin, J.; Alizadeh, A.A.; Boyd, S.D.; Brown, J.M.; Nadeau, K.C.; et al. Cellular and humoral immune response to SARS-CoV-2 vaccination and booster dose in immunosuppressed patients: An observational cohort study. J. Clin. Virol. 2022, 153, 105217. [Google Scholar] [CrossRef]
- Zaleska, J.; Kwasnik, P.; Paziewska, M.; Purkot, J.; Szabelak, A.; Jurek, M.; Masny, N.; Dziatkiewicz, I.; Pronobis-Szczylik, B.; Piebiak, A.; et al. Response to anti-SARS-CoV-2 mRNA vaccines in multiple myeloma and chronic lymphocytic leukemia patients. Int. J. Cancer 2022. [Google Scholar] [CrossRef]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response after COVID-19 Booster Vaccination and Association with COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef] [PubMed]




| Study | N | Age (Years) | Sex (Male%) | KPT Modality (N) | Vaccine Type | Homologous or Heterologous Vaccinations | Dose | Prior SARS-CoV-2 Infection | Antibody Outcome | Follow Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Dekervel M et al., 2021 [9] | 100 | 55–85 | 57.0 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Ducloux D et al., 2021 [10] | 45 | 69 ± 10 | 59.0 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Frantzen L et al., 2021 [11] | 88 | 76 (65–83) | 73.0 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Longlune N et al., 2021 [12] | 102 | 64 ± 14 | 75.5 | HD 82; PD 20 | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Stervbo U et al., 2021 [13] | 23 | 64 (52–73) | 68.0 | HD | mRNA-1273 | Heterologous | 3 | No | Anti-RBD IgG | 4 weeks |
| Tillmann FP et al., 2021 [14] | 17 | 64 ± 8 | 64.0 | HD | mRNA-1273 | Heterologous | 3 | No | Anti-RBD IgG | 4–5 weeks |
| Agur T et al., 2022 [15] | 80 | 65.2 ± 12.8 | 63.4 | HD | BNT162b2 | Homologous | 3 | No | Anti-RBD IgG | 3 weeks |
| Anft M et al., 2022 [16] | 42 | 81(66–84) | 57.1 | HD | mRNA-1273 | Homologous | 4 | No | Neutralizing Antibodies; IFNγ | 8–9 weeks |
| Ashby D et al., 2022 [17] | 1126 | 61 (51–80) | 59.0 | HD | BNT162b2 or mRNA-1273 | Homologous or heterologous | 3 | Mixed | NR | NR |
| Beikhack G et al., 2022 [18] | 27 | 54.3 (33–76) | 63.0 | PD | mRNA-1273 | Homologous | 3 | No | Anti-RBD IgG | 4 weeks |
| Benning L et al., 2022 [19] | 84 | 72 (62–79) | 69.0 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Bensouna L et al., 2022 [20] | 69 | 68 (53–76) | 65.0 | HD 38; PD 31 | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 3 weeks |
| Biedunkiewicz B et al., 2022 [21] | 139 | 69 (57–75) | 63.3 | HD 129; PD 10 | BNT162b2 or mRNA-1273 | Homologous | 3 | No | Anti-S IgG | 2 weeks |
| Boongird S et al., 2022 [22] | 57 | 18–59 | 68.4 | HD 29; PD 28 | ChAdOx1 nCoV-19 | Heterologous | 3 | No | Anti-RBD IgG | 2 weeks |
| Broseta JJ et al., 2022 [23] | 153 | 72.12 ± 14.44 | 54.2 | HD | BNT162b2 or mRNA-1273 | Homologous | 3 | No | Anti-RBD IgG | 2 weeks |
| Bruminhent J et al., 2022 [24] | 59 | 51 (42–54) | 58.0 | HD 31; PD 28 | ChAdOx1 nCoV-19 | Heterologous | 3 | No | Anti-RBD IgG | 2 weeks |
| Cheng CC et al., 2022 [25] | 142 | 72.6 (61.5–80.6) | 61.2 | HD | BNT162b2 or mRNA-1273 | Heterologous | 3; 4 | Mixed | Neutralizing antibodies | 4 weeks |
| Cheng CY et al., 2022 [26] | 19 | 60.84 ± 13.36 | 57.9 | HD 13; PD 6 | mRNA-1273 | Heterologous | 3 | No | Anti-RBD IgG | 2–3 weeks |
| Chinnadurai et al., 2022 [27] | 12 | 61 (49–72) | 64.0 | HD | BNT162b2 | Homologous or heterologous | 3 | No | Anti-S IgG | 4 weeks |
| Cinkilic O et al., 2022 [28] | 19 | NR | NR | HD | BNT162b2 | Homologous | 4 | No | Anti-S IgG | 4–6 weeks |
| Davidovic T et al., 2022 [29] | 36 | 66.9 ± 15.9 | 66.7 | HD | Viral Vector Ad26COVS1 | Heterologous | 3 | No | Anti-RBD IgG; IFNγ | 4 weeks |
| Einbinder Y et al., 2022 [30] | 106 | 70.5 ± 14.2 | 67.0 | HD 75; PD 31 | BNT162b2 | Homologous | 3; 4 | No | Anti-S IgG | 2–3 weeks |
| Espi M et al., 2022 [31] | 75 | 69.6 ± 15.0 | 42.1 | HD | BNT162b2 | Homologous | 3 | Mixed | Anti-RBD IgG; IFNγ | 2 weeks |
| Gallego-Valcarce E et al., 2022 [32] | 178 | 68.7 ± 14.5 | 63.5 | HD | BNT162b2 or mRNA-1273 | Homologous | 3 | No | Anti-RBD IgG | 4 weeks |
| Garcia P et al., 2022 [33] | 2720 | NR | NR | HD; PD | BNT162b2 or mRNA-1273 | Homologous | 3 | Mixed | Anti-RBD IgG | 2 weeks |
| Herman-Edelstein et al., 2022 [34] | 100 | 72 ± 12 | 70.0 | HD | BNT162b2 | Homologous | 3 | Mixed | Anti-RBD IgG; IFNγ | 2–3 weeks |
| Housset P et al., 2022 [35] | 68 | 66 (53.8–76.3) | 65.0 | HD 34; PD 34 | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Housset P et al., 2022 [36] | 45 | 72 (56–79) | 57.8 | HD 17; PD 18 | BNT162b2 or mRNA-1273 | Homologous | 4 | No | Anti-S IgG | 4 weeks |
| Kitamura M et al., 2022 [37] | 468 | 69 ± 11 | 65.0 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 3 weeks |
| Kohmer N et al., 2022 [38] | 148 | 69.6 ± 14.2 | 56.7 | HD 134; PD 14 | mRNA-1273 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Mosconi G et al., 2022 [39] | 109 | 69.2 ± 13.5 | 70.1 | HD 105 PD 4 | BNT162b2 or mRNA-1273 | Homologous | 3 | NR | NR | NR |
| Patyna S et al., 2022 [40] | 37 | 62 (52–72.5) | 69.0 | HD | mRNA-1273 | Heterologous | 3 | No | Anti-RBD IgG; IFNγ; IL2 | 2 weeks |
| Quiroga B et al., 2022 [41] | 451 | NR | NR | HD | BNT162b2 or mRNA-1273 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Robert T et al., 2022 [42] | 18 | 68.9 ± 13.7 | 60.0 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Shashar M et al., 2022 [43] | 66 | 75 ± 10.2 | 59.1 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 2–3 weeks |
| Simon B et al.2022 [44] | 60 | 66 (34–83) | 71.7 | HD | mRNA-1273 | Heterologous | 3 | No | Anti-RBD IgG; IFNγ; | 6 weeks |
| Tillmann FP et al., 2022 [45] | 153 | 67.4 ± 15.8 | 60.8 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
| Verdier JF et al., 2022 [46] | 96 | 71.1 ± 13.1 | 72.5 | HD | BNT162b2 | Homologous | 3 | No | Anti-S IgG | 4 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, H.; Bao, W.; Fu, S.; Jin, H. Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2070. https://doi.org/10.3390/vaccines10122070
Yang X, Zhang H, Bao W, Fu S, Jin H. Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis. Vaccines. 2022; 10(12):2070. https://doi.org/10.3390/vaccines10122070
Chicago/Turabian StyleYang, Xiuhong, Hua Zhang, Wenjing Bao, Shunkun Fu, and Huimin Jin. 2022. "Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis" Vaccines 10, no. 12: 2070. https://doi.org/10.3390/vaccines10122070
APA StyleYang, X., Zhang, H., Bao, W., Fu, S., & Jin, H. (2022). Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis. Vaccines, 10(12), 2070. https://doi.org/10.3390/vaccines10122070







