An Impact of COVID-19 on Cancer Care: An Update
Abstract
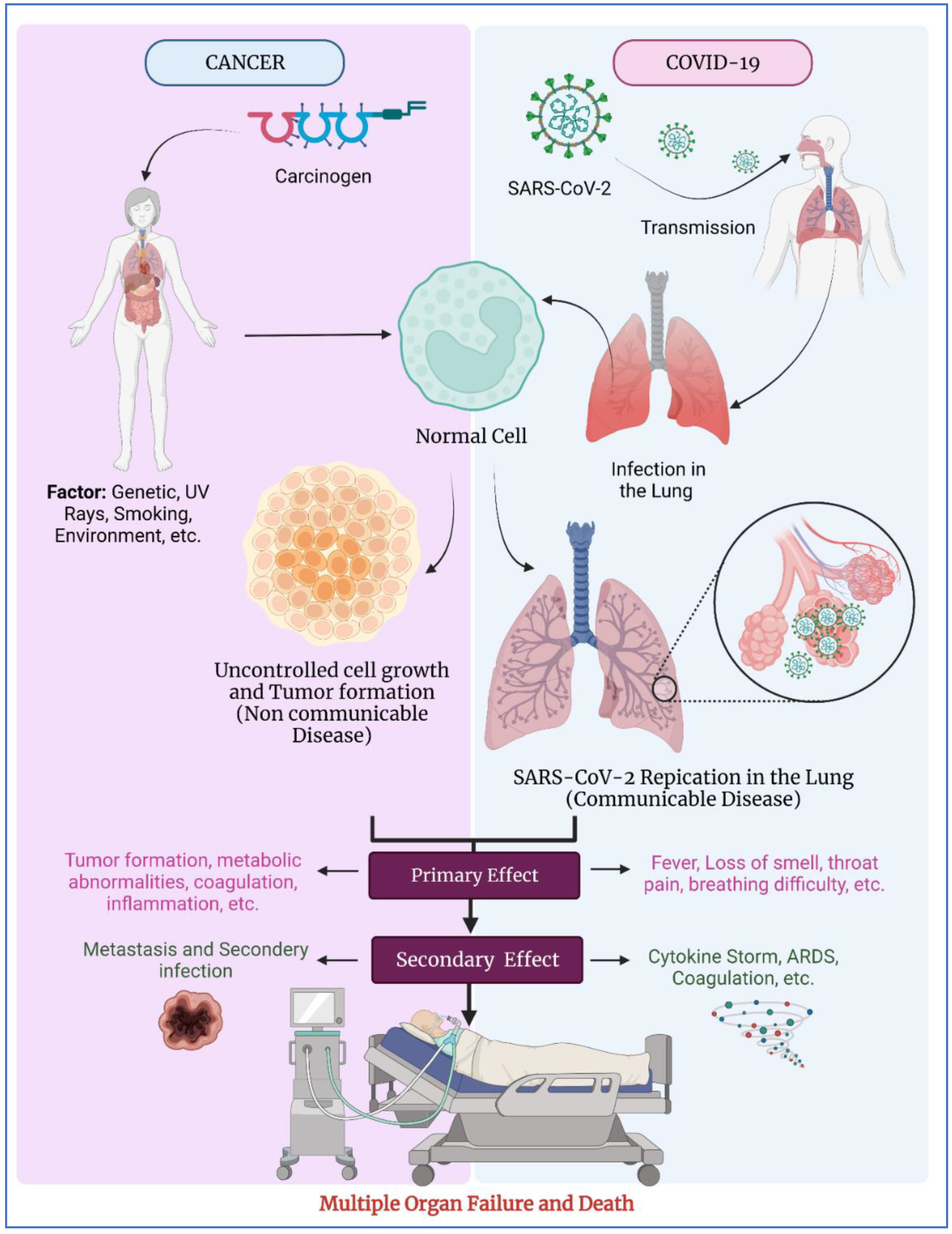
1. Introduction
2. Cancer Diagnostics and COVID-19
3. COVID-19 and Cancer Treatment
4. COVID-19 Vaccine and Cancer
5. Vaccines and Their Ongoing Clinical Trials
6. Discussion
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavda, V.P.; Feehan, J.; Apostolopoulos, V. A Veterinary Vaccine for SARS-CoV-2: The First COVID-19 Vaccine for Animals. Vaccines 2021, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Kapadia, C.; Soni, S.; Prajapati, R.; Chauhan, S.C.; Yallapu, M.M.; Apostolopoulos, V. A Global Picture: Therapeutic Perspectives for COVID-19. Immunotherapy 2022, 14, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Cancer Services Disrupted by up to 50% in All Countries Reporting: A Deadly Impact of COVID-19. Available online: https://www.who.int/europe/news/item/03-02-2022-cancer-services-disrupted-by-up-to-50-in-all-countries-reporting-a-deadly-impact-of-covid-19 (accessed on 24 September 2022).
- Pramesh, C.S.; Chinnaswamy, G.; Sengar, M.; Ranganathan, P.; Badwe, R. COVID-19 and Cancer Care in India. Nat. Cancer 2021, 2, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus. Available online: https://www.who.int/health-topics/coronavirus (accessed on 24 September 2022).
- Sinha, S.; Kundu, C.N. Cancer and COVID-19: Why Are Cancer Patients More Susceptible to COVID-19? Med. Oncol. 2021, 38, 101. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Niknam, Z.; Mohammadi Amirabad, L.; Amiri-Dashatan, N.; Koushki, M.; Nemati, M.; Danesh Pouya, F.; Rezaei-Tavirani, M.; Rasmi, Y.; Tayebi, L. Molecular Pathways Involved in COVID-19 and Potential Pathway-Based Therapeutic Targets. Biomed. Pharm. 2022, 145, 112420. [Google Scholar] [CrossRef]
- Zhang, W.-N.; Li, X.-P.; Wang, P.-F.; Zhu, L.; Xiao, X.-H.; Dai, Y.-J. Comprehensive Analysis of the Novel Omicron Receptor AXL in Cancers. Comput. Struct. Biotechnol. J. 2022, 20, 3304–3312. [Google Scholar] [CrossRef]
- Chavda, V.P. Nanotherapeutics and Nanobiotechnology. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–13. [Google Scholar]
- Chen, R.-P.; Chavda, V.P.; Patel, A.B.; Chen, Z.-S. Phytochemical Delivery Through Transferosome (Phytosome): An Advanced Transdermal Drug Delivery for Complementary Medicines. Front. Pharm. 2022, 13, 850862. [Google Scholar] [CrossRef]
- Chavda, V.P. Chapter 4 Nanobased Nano Drug Delivery A Comprehensive Review. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 69–92. [Google Scholar]
- Chavda, V.P.; Vihol, D.; Mehta, B.; Shah, D.; Patel, M.; Vora, L.K.; Pereira-Silva, M.; Paiva-Santos, A.C. Phytochemical-Loaded Liposomes for Anticancer Therapy: An Updated Review. Nanomedicine 2022, 17, 547–568. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.-X.; Chen, Z.-S.; Hou, K. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Chavda, V.P.; Ertas, Y.N.; Walhekar, V.; Modh, D.; Doshi, A.; Shah, N.; Anand, K.; Chhabria, M. Advanced Computational Methodologies Used in the Discovery of New Natural Anticancer Compounds. Front. Pharm. 2021, 12, 702611. [Google Scholar] [CrossRef]
- Bora, V.R.; Patel, B.M. The Deadly Duo of COVID-19 and Cancer! Front. Mol. Biosci. 2021, 8, 643004. [Google Scholar] [CrossRef]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Cancer and SARS-CoV-2 Infection: Diagnostic and Therapeutic Challenges. Cancers 2020, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Hall, G.; Denaxas, S.; Chang, W.H.; Katsoulis, M.; Williams, B.; Pillay, D.; Noursadeghi, M.; et al. Estimated Impact of the COVID-19 Pandemic on Cancer Services and Excess 1-Year Mortality in People with Cancer and Multimorbidity: Near Real-Time Data on Cancer Care, Cancer Deaths and a Population-Based Cohort Study. Bmj Open 2020, 10, e043828. [Google Scholar] [CrossRef]
- Englum, B.R.; Prasad, N.K.; Lake, R.E.; Mayorga-Carlin, M.; Turner, D.J.; Siddiqui, T.; Sorkin, J.D.; Lal, B.K. Impact of the COVID-19 Pandemic on Diagnosis of New Cancers: A National Multicenter Study of the Veterans Affairs Healthcare System. Cancer 2022, 128, 1048–1056. [Google Scholar] [CrossRef]
- Gallagher, A. Breast Cancer Diagnosis & Treatment Before vs. During COVID-19 Pandemic. Oncol. Times 2022, 44, 27. [Google Scholar]
- Lee, K.A.; Ma, W.; Sikavi, D.R.; Drew, D.A.; Nguyen, L.H.; Bowyer, R.C.E.; Cardoso, M.J.; Fall, T.; Freidin, M.B.; Gomez, M.; et al. Cancer and Risk of COVID-19 Through a General Community Survey. Oncologist 2021, 26, e182–e185. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.-Y.; Kwon, D.H.; Glover, M.J.; Henry, S.; Wood, D.; Rubin, D.L.; Koshkin, V.S.; Schapira, L.; Shah, S.A. Changes in Cancer Management Due to COVID-19 Illness in Patients with Cancer in Northern California. Jco Oncol. Pr. 2021, 17, e377–e385. [Google Scholar] [CrossRef]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in Immunocompromised Patients: A Systematic Review of Cancer, Hematopoietic Cell and Solid Organ Transplant Patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Vaccine Effectiveness against COVID-19 Breakthrough Infections in Patients with Cancer (UKCCEP): A Population-Based Test-Negative Case-Control Study—The Lancet Oncology. Available online: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(22)00202-9/fulltext (accessed on 24 September 2022).
- Liu, A.P.Y.; Lam, G.K.S.; Chan, W.Y.K.; Chow, T.T.W.; Cheung, J.; Wong, S.C.Y.; Leung, W.; Lee, P.P.W.; Cheng, F.W.T.; Chan, G.C.F. SARS-CoV-2 Infection in Children Undergoing Oncologic Treatment in Hong Kong: A Population-Based Cohort during the Omicron Wave. Pediatric Blood Cancer 2022, e29894. [Google Scholar] [CrossRef]
- Al-Shamsi, H.O.; Alhazzani, W.; Alhuraiji, A.; Coomes, E.A.; Chemaly, R.F.; Almuhanna, M.; Wolff, R.A.; Ibrahim, N.K.; Chua, M.L.K.; Hotte, S.J.; et al. A Practical Approach to the Management of Cancer Patients During the Novel Coronavirus Disease 2019 (COVID-19) Pandemic: An International Collaborative Group. Oncologist 2020, 25, e936–e945. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, N.; Jethava, Y.; Vikas, P. Repurposing Anti-Cancer Drugs for COVID-19 Treatment. Drug Des. Dev. 2020, 14, 5045–5058. [Google Scholar] [CrossRef]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug Repurposing Approach to Fight COVID-19. Pharm. Rep. Pr 2020, 72, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Oft, J.; Dorff, T.; Pal, S.; Agarwal, N.; Figlin, R.A.; Posadas, E.M.; Freedland, S.J.; Gong, J. COVID-19 and Androgen-Targeted Therapy for Prostate Cancer Patients. Endocr. Relat. Cancer 2020, 27, R281–R292. [Google Scholar] [CrossRef]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral Damage: The Impact on Outcomes from Cancer Surgery of the COVID-19 Pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- COVID-19: Considerations in Patients with Cancer—UpToDate. Available online: https://www.uptodate.com/contents/covid-19-considerations-in-patients-with-cancer#H1193654967 (accessed on 24 September 2022).
- Fligor, S.C.; Wang, S.; Allar, B.G.; Tsikis, S.T.; Ore, A.S.; Whitlock, A.E.; Calvillo-Ortiz, R.; Arndt, K.R.; Gangadharan, S.P.; Callery, M.P. Gastrointestinal Malignancies and the COVID-19 Pandemic: Evidence-Based Triage to Surgery. J. Gastrointest Surg. 2020, 24, 2357–2373. [Google Scholar] [CrossRef]
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired Immunogenicity of BNT162b2 Anti-SARS-CoV-2 Vaccine in Patients Treated for Solid Tumors. Ann. Oncol. 2021, 32, 1053–1055. [Google Scholar] [CrossRef]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. MRNA Booster Immunization Elicits Potent Neutralizing Serum Activity against the SARS-CoV-2 Omicron Variant. Nat. Med. 2022, 28, 477–480. [Google Scholar] [CrossRef]
- Chavda, V.P.; Prajapati, R.; Lathigara, D.; Nagar, B.; Kukadiya, J.; Redwan, E.M.; Uversky, V.N.; Kher, M.N.; Patel, R. Therapeutic Monoclonal Antibodies for COVID-19 Management: An Update. Expert Opin. Biol. Ther. 2022, 22, 763–780. [Google Scholar] [CrossRef]
- Barrière, J.; Zalcman, G.; Fignon, L.; Peiffer-Smadja, N.; Audigier-Valette, C.; Carles, M. Omicron Variant: A Clear and Present Danger for Patients with Cancer. Eur. J. Cancer 2022, 165, 25–26. [Google Scholar] [CrossRef]
- Bagheri, G.; Thiede, B.; Hejazi, B.; Schlenczek, O.; Bodenschatz, E. An Upper Bound on One-to-One Exposure to Infectious Human Respiratory Particles. Proc. Natl. Acad. Sci. USA 2021, 118, e2110117118. [Google Scholar] [CrossRef]
- COVID-19 Vaccines in Patients with Cancer: Immunogenicity, Efficacy and Safety|Nature Reviews Clinical Oncology. Available online: https://www.nature.com/articles/s41571-022-00610-8 (accessed on 24 September 2022).
- Chavda, V.P.; Yao, Q.; Vora, L.K.; Apostolopoulos, V.; Patel, C.A.; Bezbaruah, R.; Patel, A.B.; Chen, Z.-S. Fast-Track Development of Vaccines for SARS-CoV-2: The Shots That Saved the World. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Apostolopoulos, V. COVID-19 Vaccine Design and Vaccination Strategy for Emerging Variants. Expert Rev. Vaccines 2022, 21, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Apostolopoulos, V. Is Booster Dose Strategy Sufficient for Omicron Variant of SARS-CoV-2? Vaccines 2022, 10, 367. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Global Impact of Delta plus Variant and Vaccination. Expert Rev. Vaccines 2022, 21, 597–600. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Omicron Variant (B.1.1.529) of SARS-CoV-2: Threat for the Elderly? Maturitas 2022, 158, P78–P81. [Google Scholar] [CrossRef]
- Slomski, A. Most Fully Vaccinated Patients With Cancer Have SARS-CoV-2 Antibodies. JAMA 2021, 326, 800. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Zhang, T.; Wang, A.Z.; Li, Z. COVID-19 Vaccines for Patients with Cancer: Benefits Likely Outweigh Risks. J. Hematol. Oncol. 2021, 14, 38. [Google Scholar] [CrossRef]
- Han, H.J.; Nwagwu, C.; Anyim, O.; Ekweremadu, C.; Kim, S. COVID-19 and Cancer: From Basic Mechanisms to Vaccine Development Using Nanotechnology. Int. Immunopharmacol. 2021, 90, 107247. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Just a Third of Blood Cancer Patients Had Antibodies against Delta Variant after Two Vaccine Doses, Study Finds. BMJ 2021, 375, n2623. [Google Scholar] [CrossRef]
- Obeid, M.; Suffiotti, M.; Pellaton, C.; Bouchaab, H.; Cairoli, A.; Salvadé, V.; Stevenel, C.; Hottinger, R.; Pythoud, C.; Coutechier, L.; et al. Humoral Responses Against Variants of Concern by COVID-19 MRNA Vaccines in Immunocompromised Patients. Jama Oncol. 2022, 8, e220446. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and Immunogenicity of One versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients with Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Lee, M.; Quinn, R.; Pradhan, K.; Fedorov, K.; Levitz, D.; Fromowitz, A.; Thakkar, A.; Shapiro, L.C.; Kabarriti, R.; Ruiz, R.E.; et al. Impact of COVID-19 on Case Fatality Rate of Patients with Cancer during the Omicron Wave. Cancer Cell 2022, 40, 343–345. [Google Scholar] [CrossRef]
- Bakouny, Z.; Labaki, C.; Bhalla, S.; Schmidt, A.L.; Steinharter, J.A.; Cocco, J.; Tremblay, D.A.; Awad, M.M.; Kessler, A.; Haddad, R.I.; et al. Oncology Clinical Trial Disruption during the COVID-19 Pandemic: A COVID-19 and Cancer Outcomes Study. Ann. Oncol. 2022, 33, 836–844. [Google Scholar] [CrossRef]
- Jazieh, A.R.; Akbulut, H.; Curigliano, G.; Rogado, A.; Alsharm, A.A.; Razis, E.D.; Mula-Hussain, L.; Errihani, H.; Khattak, A.; De Guzman, R.B.; et al. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. Jco Glob. Oncol. 2020, 6, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Perez, J.L.; Lockhart, S.P.; Hariharan, S.; Kitchin, N.; Bailey, R.; Liau, K.; Lagkadinou, E.; Türeci, Ö.; Şahin, U.; et al. Efficacy and Safety of the BNT162b2 MRNA COVID-19 Vaccine in Participants with a History of Cancer: Subgroup Analysis of a Global Phase 3 Randomized Clinical Trial. Vaccine 2022, 40, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The Impact of the COVID-19 Pandemic on Cancer Care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.D.; Catto, J.W.F.; Finelli, A.; Glaser, A.W.; Gore, J.L.; Loeb, S.; Morgan, T.M.; Morgans, A.K.; Mottet, N.; Neal, R.; et al. The Impact of the COVID-19 Pandemic on Genitourinary Cancer Care: Re-Envisioning the Future. Eur. Urol. 2020, 78, 731–742. [Google Scholar] [CrossRef]
- Wang, H.; Elsheikh, M.; Gilmour, K.; Cohen, V.; Sagoo, M.S.; Damato, B.; Anguita, R.; Heimann, H.; Hussain, R.; Cauchi, P.; et al. Impact of COVID-19 Pandemic on Eye Cancer Care in United Kingdom. Br. J. Cancer 2021, 124, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Marcum, M.; Kurtzweil, N.; Vollmer, C.; Schmid, L.; Vollmer, A.; Kastl, A.; Acker, K.; Gulati, S.; Grover, P.; Herzog, T.J.; et al. COVID-19 Pandemic and Impact on Cancer Clinical Trials: An Academic Medical Center Perspective. Cancer Med. 2020, 9, 6141–6146. [Google Scholar] [CrossRef]
- Sessa, C.; Cortes, J.; Conte, P.; Cardoso, F.; Choueiri, T.; Dummer, R.; Lorusso, P.; Ottmann, O.; Ryll, B.; Mok, T.; et al. The Impact of COVID-19 on Cancer Care and Oncology Clinical Research: An Experts’ Perspective. Esmo Open 2022, 7, 100339. [Google Scholar] [CrossRef]
- SARS-CoV-2 Omicron Variant in Cancer Patients: An Insight into the Vaccine Booster Debate|Future Oncology. Available online: https://www.futuremedicine.com/doi/10.2217/fon-2022-0024 (accessed on 24 September 2022).
- COVID-19 Vaccination and Breakthrough Infections in Patients with Cancer—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0923753421048808 (accessed on 24 September 2022).
- Ranganathan, P.; Sengar, M.; Chinnaswamy, G.; Agrawal, G.; Arumugham, R.; Bhatt, R.; Bilimagga, R.; Chakrabarti, J.; Chandrasekharan, A.; Chaturvedi, H.K.; et al. Impact of COVID-19 on Cancer Care in India: A Cohort Study. Lancet Oncol. 2021, 22, 970–976. [Google Scholar] [CrossRef]
- Ingram, S.A.; Caston, N.E.; Andrews, C.J.; England, R.; Williams, C.; Azuero, A.; Gallagher, K.D.; Angove, R.; Anderson, E.; Balch, A.J.; et al. Hesitancy and Malignancy: Vaccine Hesitancy among Individuals with Cancer. JCO 2021, 39, 148. [Google Scholar] [CrossRef]
- Ahmad Malik, J.; Ahmed, S.; Shinde, M.; Almermesh, M.H.S.; Alghamdi, S.; Hussain, A.; Anwar, S. The Impact of COVID-19 On Comorbidities: A Review Of Recent Updates For Combating It. Saudi J. Biol. Sci. 2022, 29, 3586–3599. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Vihol, D.R. COVAX-19Ⓡ Vaccine: Completely Blocks Virus Transmission to Non-Immune Individuals. Clin. Complementary Med. Pharmacol. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal Vaccines for SARS-CoV-2: From Challenges to Potential in COVID-19 Management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Athalye, M.; Parikh, P.K.; Chhipa, A.S.; Patel, S.; Apostolopoulos, V. Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses 2022, 14, 759. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vihol, D.; Vaghasiya, D.D.; Ahmed, K.M.S.B.; Trivedi, K.U.; Dave, D.J. Herbal Remedies, Nutraceuticals, and Dietary Supplements for COVID-19 Management: An Update. Clin. Complementary Med. Pharmacol. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Huang, Z.; Chavda, V.P.; Vora, L.K.; Gajjar, N.; Apostolopoulos, V.; Shah, N.; Chen, Z.-S. 2-Deoxy-D-Glucose and Its Derivatives for the COVID-19 Treatment: An Update. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Hanuma Kumar Ghali, E.N.; Yallapu, M.M.; Apostolopoulos, V. Therapeutics to Tackle Omicron Outbreak. Immunotherapy 2022, 14, 833–838. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Ping, F.-F.; Chen, Z.-S. An Impact of COVID-19 on Cancer Care: An Update. Vaccines 2022, 10, 2072. https://doi.org/10.3390/vaccines10122072
Chavda VP, Ping F-F, Chen Z-S. An Impact of COVID-19 on Cancer Care: An Update. Vaccines. 2022; 10(12):2072. https://doi.org/10.3390/vaccines10122072
Chicago/Turabian StyleChavda, Vivek P., Feng-Feng Ping, and Zhe-Sheng Chen. 2022. "An Impact of COVID-19 on Cancer Care: An Update" Vaccines 10, no. 12: 2072. https://doi.org/10.3390/vaccines10122072
APA StyleChavda, V. P., Ping, F.-F., & Chen, Z.-S. (2022). An Impact of COVID-19 on Cancer Care: An Update. Vaccines, 10(12), 2072. https://doi.org/10.3390/vaccines10122072







