Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Population
2.2. Data Sources
3. Exposure and Outcomes
4. Covariates
5. Statistical Analysis
6. Results
6.1. Study Population
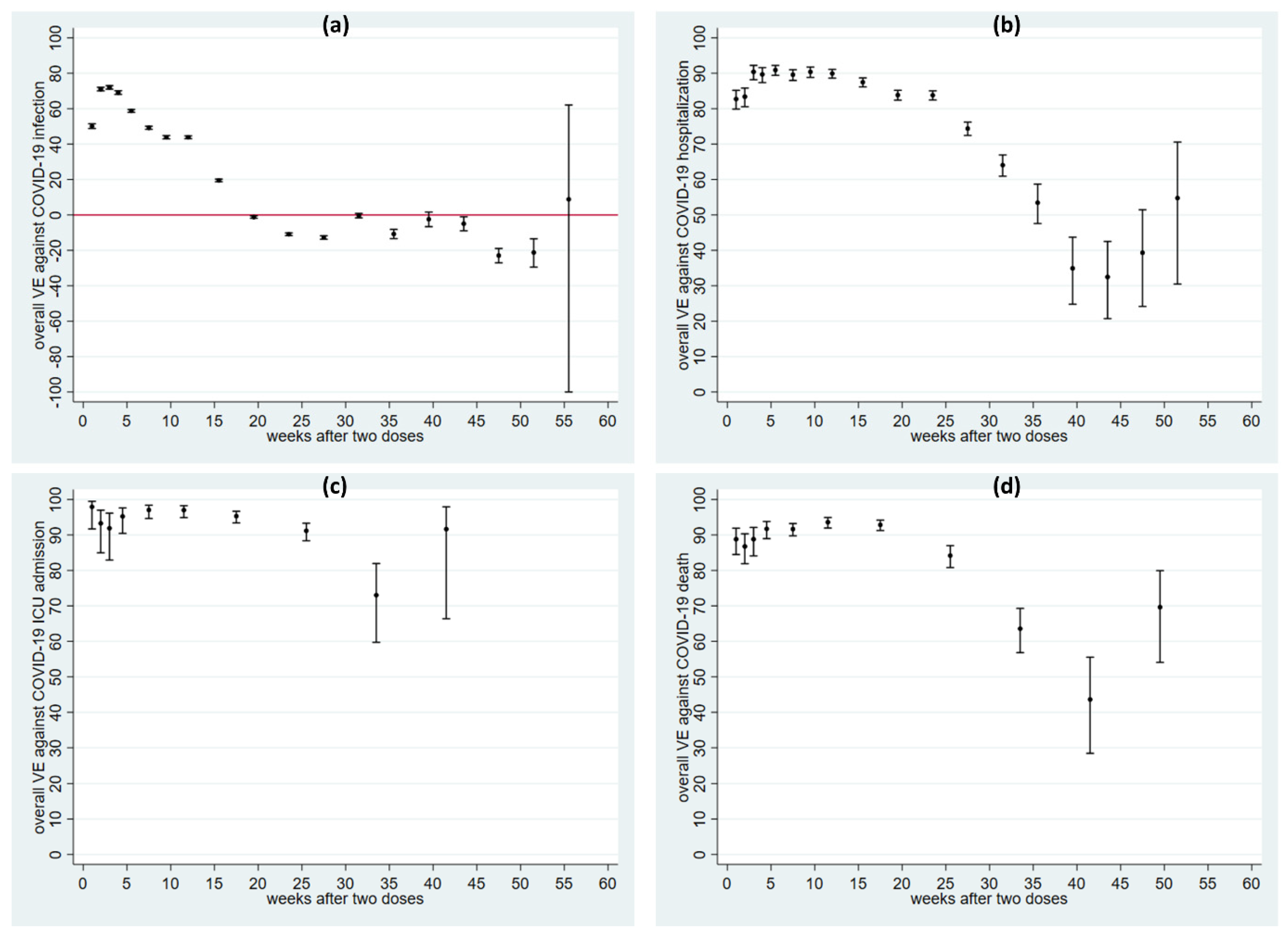
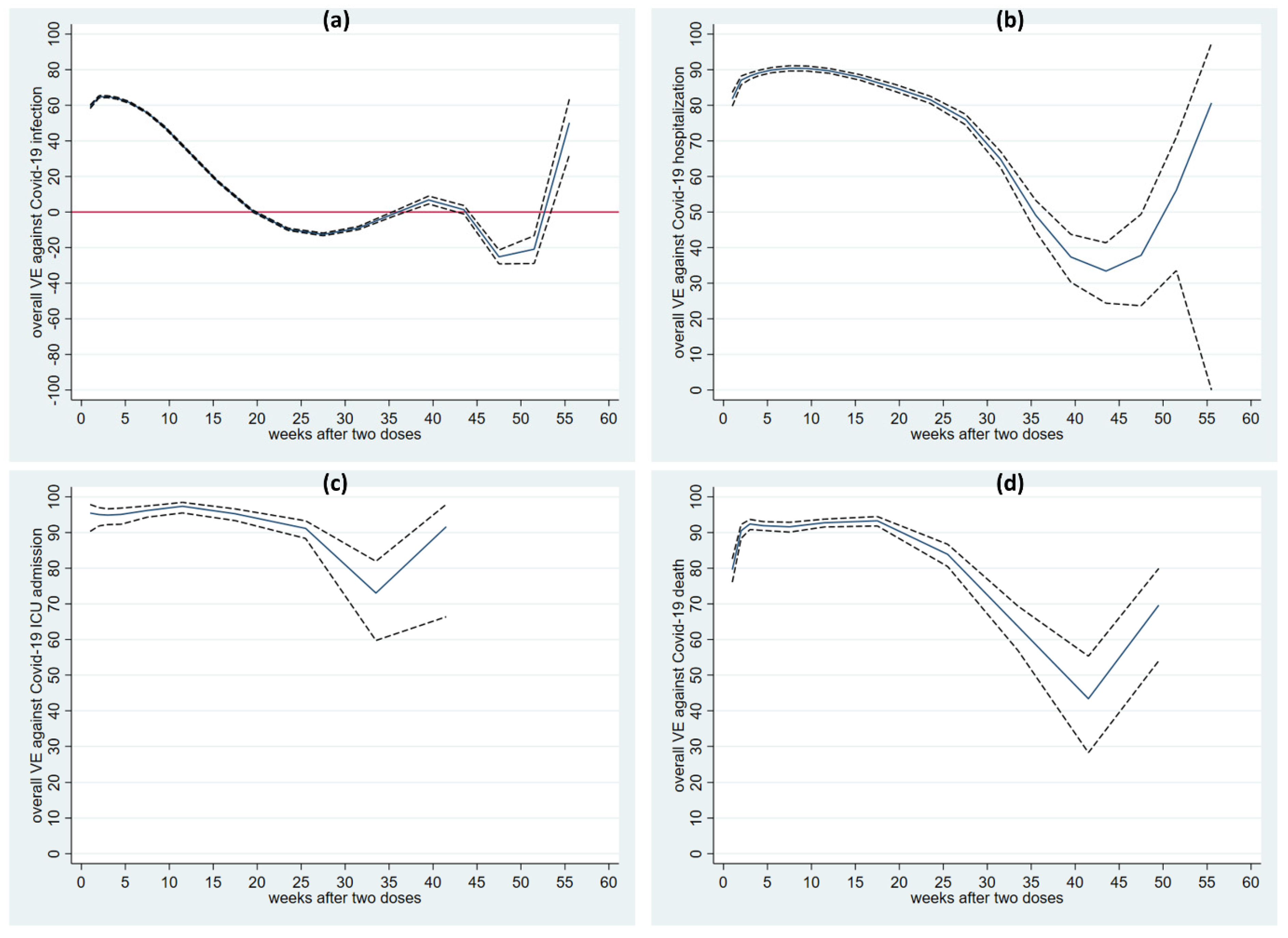
6.2. VE during a 13-Month Follow-Up Period
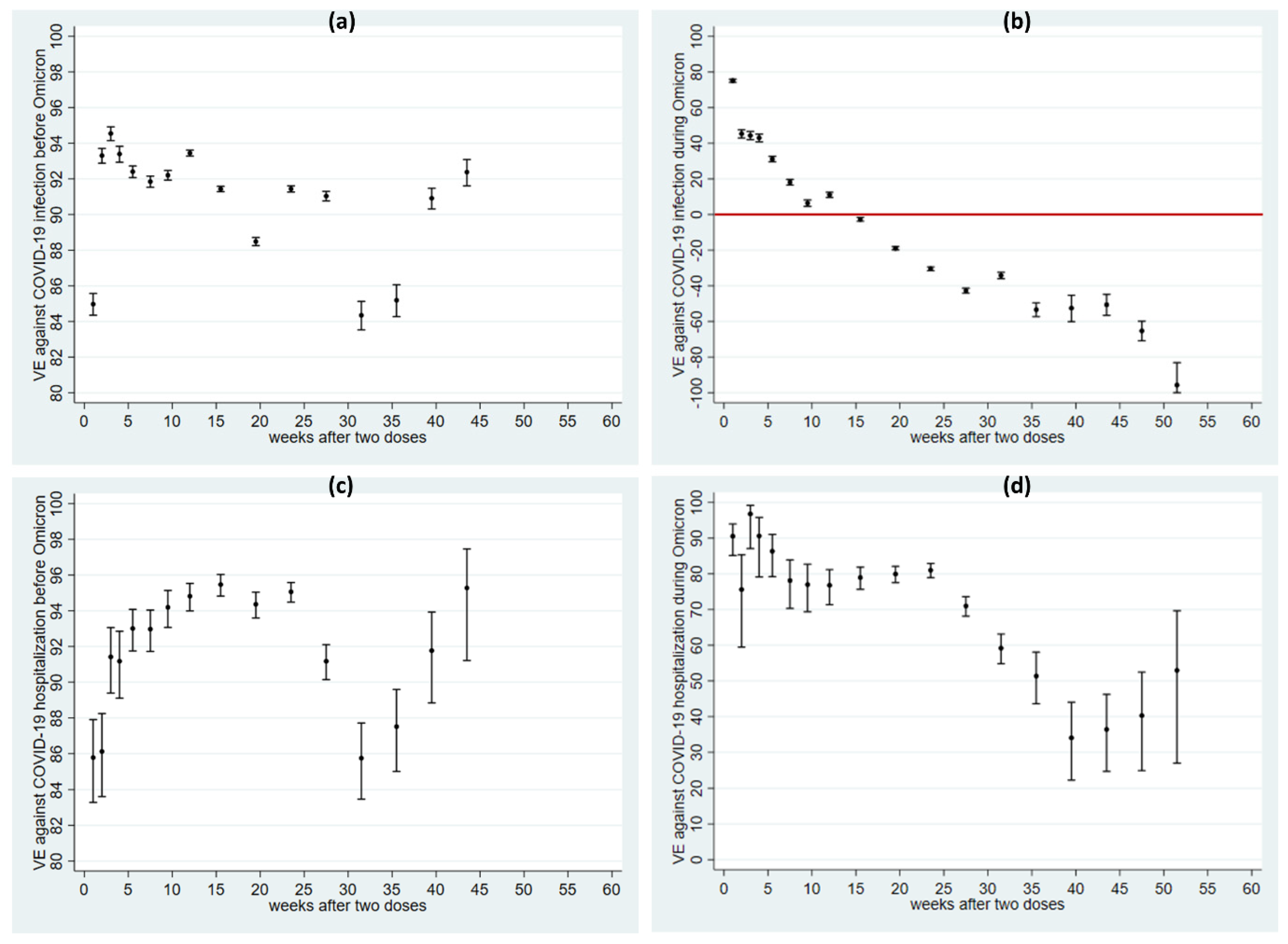
6.3. VE after Two Doses before and after the Emergence of Omicron
6.4. VE after Two Doses by Age, Sex, and Vaccine Type
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Guo, P.; Zhang, X.; Yu, Z.; Zhang, W.; Sun, H. SARS-CoV-2 vaccine candidates in rapid development. Hum. Vaccines Immunother. 2021, 17, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Phan, T. Genetic diversity and evolution of SARS-CoV-2. Infect. Genet. Evol. 2020, 81, 104260. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 20 August 2022).
- European Centre for Disease Prevention and Control. Epidemiological Update: Omicron Variant of Concern (VOC)—Data as of 16 December 2021 (12:00). 2021. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-omicron-data-16-december (accessed on 20 August 2022).
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.; et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. arXiv 2021. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Suah, J.L.; Tng, B.H.; Tok, P.S.K.; Husin, M.; Thevananthan, T.; Peariasamy, K.M.; Sivasampu, S. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against Delta and Omicron SARS-CoV-2 infection. Emerg. Microbes Infect. 2022, 11, 1343–1345. [Google Scholar] [CrossRef]
- Ljung, R.; Sundström, A.; Grünewald, M.; Backman, C.; Feltelius, N.; Gedeborg, R.; Zethelius, B. The profile of the COVID-19 VACcination register SAFEty study in Sweden (CoVacSafe-SE). Ups. J. Med. Sci. 2021, 126. [Google Scholar] [CrossRef]
- Nyberg, F.; Franzén, S.; Lindh, M.; Vanfleteren, L.; Hammar, N.; Wettermark, B.; Sundström, J.; Santosa, A.; Björck, S.; Gisslén, M. Swedish COVID-19 Investigation for Future Insights—A Population Epidemiology Approach Using Register Linkage (SCIFI-PEARL). Clin. Epidemiol. 2021, 13, 649–659. [Google Scholar] [CrossRef]
- Vecka 52, 2021—Statistik om förekomst av misstänkta fall av SARS-CoV-2 virusvarianten omicron—Folkhälsomyndigheten. Available online: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/sars-cov-2-virusvarianter-av-sarskild-betydelse/statistik-om-forekomst-av-misstankta-fall-av-sars-cov-2-virusvarianten-omikron/vecka-52-2021/ (accessed on 23 August 2022).
- Zaki, N.; Mohamed, E.A. The estimations of the COVID-19 incubation period: A scoping reviews of the literature. J. Infect. Public Health 2021, 14, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kleinbaum, D.G.; Klein, M. Survival Analysis: A Self-Learning Text; Springer: New York, NY, USA, 2012; Volume 3. [Google Scholar]
- Lin, D.-Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.-K.; Moore, Z.; Zeng, D. Effectiveness of COVID-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: A retrospective, total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Horne, E.M.F.; Hulme, W.J.; Keogh, R.H.; Palmer, T.M.; Williamson, E.J.; Parker, E.P.K.; Green, A.; Walker, V.; Walker, A.J.; Curtis, H.; et al. Waning effectiveness of BNT162b2 and ChAdOx1 COVID-19 vaccines over six months since second dose: OpenSAFELY cohort study using linked electronic health records. BMJ 2022, 378, e071249. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Toffa, S.; Sachdeva, R.; Gallagher, E.; Groves, N.; O’Connell, A.-M.; Chand, M.; Ramsay, M.; et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect. Dis. 2022, 22, 931–933. [Google Scholar] [CrossRef]
- Chua, H.; Feng, S.; Lewnard, J.A.; Sullivan, S.G.; Blyth, C.C.; Lipsitch, M.; Cowling, B.J. The use of test-negative controls to monitor vaccine effectiveness: A systematic review of methodology. Epidemiology 2020, 31, 43–64. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Katikireddi, S.V.; Cerqueira-Silva, T.; Vasileiou, E.; Robertson, C.; Amele, S.; Pan, J.; Taylor, B.; Boaventura, V.; Werneck, G.L.; Flores-Ortiz, R.; et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: A retrospective, population-based cohort study in Scotland and Brazil. Lancet 2022, 399, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Kahn, F. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 Omicron BA.1 and BA.2 subvariants—Surveillance results from southern Sweden, December 2021 to March 2022. Eurosurveillance 2022, 27, 2200322. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Ostropolets, A.; Hripcsak, G. COVID-19 vaccination effectiveness rates by week and sources of bias: A retrospective cohort study. BMJ open 2022, 12, e061126. [Google Scholar] [CrossRef]
| Entire Population | Vaccine Uptake | COVID-19 Outcome Events | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated with at Least One Dose | Vaccinated with at Least Two Doses | Vaccinated with More Than Two Doses | Infection | Hospitalization | ICU | Death | |||||
| Characteristics | Count | Count | % | Count | % | Count | % | Count | Count | Count | Count |
| All residents ≥ 12 year | 9,153,456 | 7,750,175 | 84.7 | 7,492,231 | 81.9 | 4,069,838 | 44.5 | 2,002,024 | 81,488 | 8167 | 17,408 |
| Age group | |||||||||||
| 12–17 y | 729,379 | 524,970 | 72.0 | 451,781 | 61.9 | 462 | 0.1 | 172,493 | 476 | 26 | 1 |
| 18–39 y | 2,919,192 | 2,317,509 | 79.4 | 2,201,250 | 75.4 | 487,513 | 16.7 | 827,836 | 9388 | 601 | 76 |
| 40–59 y | 2,636,496 | 2,304,475 | 87.4 | 2,262,200 | 85.8 | 1,289,901 | 48.9 | 719,142 | 20,626 | 2432 | 563 |
| 60–64 y | 576,885 | 526,344 | 91.2 | 521,098 | 90.3 | 430,858 | 74.7 | 98,341 | 7226 | 1147 | 460 |
| 65–79 y | 1,613,620 | 1,496,463 | 92.7 | 1,483,929 | 92.0 | 1,352,311 | 83.8 | 126,937 | 23,392 | 3355 | 4309 |
| ≥80 y | 677,884 | 580,414 | 85.6 | 571,973 | 84.4 | 508,793 | 75.1 | 57,275 | 20,380 | 606 | 11,999 |
| Sex | |||||||||||
| Males | 4,593,274 | 3,827,301 | 83.3 | 3,689,578 | 80.3 | 1,914,129 | 41.7 | 955,270 | 45,558 | 5704 | 9538 |
| Females | 4,560,182 | 3,922,874 | 86.0 | 3,802,653 | 83.4 | 2,155,709 | 47.3 | 1,046,754 | 35,930 | 2463 | 7870 |
| Country of birth | |||||||||||
| Sweden | 7,173,976 | 6,375,825 | 88.9 | 6,201,225 | 86.4 | 3,557,726 | 49.6 | 1,561,900 | 53,760 | 4941 | 13,810 |
| Other countries | 1,979,480 | 1,374,350 | 69.4 | 1,291,006 | 65.2 | 512,112 | 25.9 | 440,124 | 27,728 | 3226 | 3598 |
| Health care workers | |||||||||||
| Yes | 1,765,714 | 1,548,251 | 87.7 | 1,504,469 | 85.2 | 806,464 | 45.7 | 540,620 | 12,198 | 1304 | 414 |
| No | 7,387,742 | 6,201,924 | 83.9 | 5,987,762 | 81.0 | 3,263,374 | 44.2 | 1,461,404 | 69,290 | 6863 | 16,994 |
| Education | |||||||||||
| Primary | 1,624,747 | 1,330,160 | 81.9 | 1,276,567 | 78.6 | 711,662 | 43.8 | 292,496 | 23,732 | 2288 | 7389 |
| Secondary | 3,515,542 | 3,037,726 | 86.4 | 2,961,176 | 84.2 | 1,729,812 | 49.2 | 771,477 | 33,412 | 3545 | 6450 |
| Tertiary | 3,033,764 | 2,728,483 | 89.9 | 2,684,092 | 88.5 | 1,586,958 | 52.3 | 731,879 | 21,109 | 2037 | 2978 |
| Unknown | 979,403 | 653,806 | 66.8 | 570,396 | 58.2 | 41,406 | 4.2 | 206,172 | 3235 | 297 | 591 |
| Income (a) | |||||||||||
| Low | 2,843,443 | 2,151,668 | 75.7 | 2,055,470 | 72.3 | 1,067,553 | 37.5 | 137,7828 | 101,658 | 7885 | 13,811 |
| Medium | 2,843,745 | 2,512,100 | 88.3 | 2,452,190 | 86.2 | 1,317,623 | 46.3 | 2,079,536 | 68,123 | 6560 | 5159 |
| High | 2,872,082 | 2,653,320 | 92.4 | 2,617,388 | 91.1 | 1,684,159 | 58.6 | 2,171,200 | 61,814 | 6675 | 2617 |
| Unknown | 624,186 | 433,087 | 69.4 | 367,183 | 58.8 | 503 | 0.1 | 330,659 | 794 | 51 | 3 |
| Marital status | |||||||||||
| Married | 3,408,329 | 3,054,335 | 89.6 | 3,005,911 | 88.2 | 2,096,079 | 61.5 | 738,892 | 37,793 | 4355 | 6123 |
| Unmarried | 5,732,710 | 4,693,822 | 81.9 | 4,484,570 | 78.2 | 1,973,458 | 34.4 | 1,262,593 | 43,666 | 3810 | 11,284 |
| Unknown | 12,417 | 2018 | 16.3 | 1750 | 14.1 | 301 | 2.4 | 539 | 29 | 2 | 1 |
| Special care facilities | |||||||||||
| No | 9,065,928 | 7,693,339 | 84.9 | 7,437,607 | 82.0 | 4,028,541 | 44.4 | 1,983,797 | 78,662 | 8135 | 11,832 |
| Yes | 87,528 | 56,836 | 64.9 | 54,624 | 62.4 | 41,297 | 47.2 | 18,227 | 2826 | 32 | 5576 |
| Home care service | |||||||||||
| No | 8,903,449 | 7,559,894 | 84.9 | 7,307,304 | 82.1 | 3,922,014 | 44.1 | 1,964,729 | 68,803 | 7824 | 8818 |
| Yes | 250,007 | 190,281 | 76.1 | 184,927 | 74.0 | 147,824 | 59.1 | 37,295 | 12,685 | 343 | 8590 |
| Prior comorbidities and treatments (b) | |||||||||||
| Cardiovascular disease | |||||||||||
| No | 8,478,793 | 7,162,241 | 84.5 | 6,914,482 | 81.6 | 3,594,586 | 42.4 | 1,911,159 | 61,569 | 6762 | 9537 |
| Yes | 674,663 | 587,934 | 87.1 | 577,749 | 85.6 | 475,252 | 70.4 | 90,865 | 19,919 | 1405 | 7871 |
| Stroke | |||||||||||
| No | 9,058,839 | 7,670,273 | 84.7 | 7,413,794 | 81.8 | 4,004,535 | 44.2 | 1,989,901 | 78,182 | 7987 | 15,800 |
| Yes | 94,617 | 79,902 | 84.4 | 78,437 | 82.9 | 65,303 | 69.0 | 12,123 | 3306 | 180 | 1608 |
| Hypertension | |||||||||||
| No | 7,113,536 | 5,892,738 | 82.8 | 5,657,425 | 79.5 | 2,513,924 | 35.3 | 1,743,515 | 38,670 | 3918 | 4184 |
| Yes | 2,039,920 | 1,857,437 | 91.1 | 1,834,806 | 89.9 | 1,555,914 | 76.3 | 258,509 | 42,818 | 4249 | 13,224 |
| Diabetes | |||||||||||
| No | 8,610,137 | 7,266,301 | 84.4 | 7,015,964 | 81.5 | 3,684,709 | 42.8 | 1,923,342 | 64,901 | 6204 | 12,690 |
| Yes | 543,319 | 483,874 | 89.1 | 476,267 | 87.7 | 385,129 | 70.9 | 78,682 | 16,587 | 1963 | 4718 |
| Obstructive respiratory diseases | |||||||||||
| No | 8,314,536 | 7,006,607 | 84.3 | 6,769,169 | 81.4 | 3,601,311 | 43.3 | 1,824,514 | 65,536 | 6648 | 13,652 |
| Yes | 838,920 | 743,568 | 88.6 | 723,062 | 86.2 | 468,527 | 55.8 | 177,510 | 15,952 | 1519 | 3756 |
| Chronic kidney diseases | |||||||||||
| No | 9,055,450 | 7,671,976 | 84.7 | 7,415,874 | 81.9 | 4,008,312 | 44.3 | 1,987,063 | 75,642 | 7731 | 14,937 |
| Yes | 98,006 | 78,199 | 79.8 | 76,357 | 77.9 | 61,526 | 62.8 | 14,961 | 5846 | 436 | 2471 |
| Obesity | |||||||||||
| No | 8,981,258 | 7,604,699 | 84.7 | 7,352,735 | 81.9 | 3,992,828 | 44.5 | 1,957,435 | 77,704 | 7678 | 16,835 |
| Yes | 172,198 | 145,476 | 84.5 | 139,496 | 81.0 | 77,010 | 44.7 | 44,589 | 3784 | 489 | 573 |
| Autoimmune diseases | |||||||||||
| No | 8,943,975 | 7,564,864 | 84.6 | 7,310,314 | 81.7 | 3,927,986 | 43.9 | 1,967,287 | 75,473 | 7635 | 15,460 |
| Yes | 209,481 | 185,311 | 88.5 | 181,971 | 86.9 | 141,852 | 67.7 | 34,737 | 6015 | 532 | 1948 |
| Dementia | |||||||||||
| No | 9,097,600 | 7,710,590 | 84.8 | 7,453,854 | 81.9 | 4,039,271 | 44.4 | 1,991,197 | 79,069 | 8149 | 14,309 |
| Yes | 55,856 | 39,585 | 70.9 | 38,377 | 68.7 | 30,567 | 54.7 | 10,827 | 2419 | 18 | 3099 |
| Psychiatric conditions | |||||||||||
| No | 7,411,418 | 6,240,821 | 84.2 | 6,025,166 | 81.3 | 3,120,897 | 42.1 | 1,666,204 | 53,403 | 5898 | 7771 |
| Yes | 1,742,038 | 1,509,354 | 86.6 | 1,467,065 | 84.2 | 948,941 | 54.5 | 335,820 | 28,085 | 2269 | 9637 |
| Cancer | |||||||||||
| No | 8,705,114 | 7,352,319 | 84.5 | 7,099,348 | 81.6 | 3,732,517 | 42.9 | 1,948,418 | 71,208 | 7429 | 13,814 |
| Yes | 448,342 | 397,856 | 88.7 | 392,883 | 87.6 | 337,321 | 75.2 | 53,606 | 10,280 | 738 | 3594 |
| Vaccine Uptake | Vaccine Type | ||||||
|---|---|---|---|---|---|---|---|
| Two Doses | Homologous BNT162b2 | Homologous mRNA-1273 | Homologous AZD1222 | ||||
| Characteristics | Count | Count | % | Count | % | Count | % |
| All residents ≥ 12 year | 7,492,231 | 5,858,168 | 78.2 | 894,487 | 11.9 | 595,039 | 7.9 |
| Age group | |||||||
| 12–17 y | 451,781 | 446,576 | 7.6 | 30,129 | 3.4 | 1 | 0.0 |
| 18–39 y | 2,201,250 | 1,739,396 | 29.7 | 356,327 | 39.8 | 29,772 | 5.0 |
| 40–59 y | 2,262,200 | 1,859,436 | 31.7 | 298,996 | 33.4 | 41,381 | 7.0 |
| 60–64 y | 521,098 | 456,742 | 7.8 | 38,203 | 4.3 | 12,063 | 2.0 |
| 65–79 y | 1,483,929 | 883,888 | 15.1 | 106,293 | 11.9 | 478,493 | 80.4 |
| ≥80 y | 571,973 | 472,130 | 8.1 | 64,539 | 7.2 | 33,329 | 5.6 |
| Sex | |||||||
| Males | 3,689,578 | 2,910,935 | 49.7 | 455,604 | 50.9 | 281,162 | 47.3 |
| Females | 3,802,653 | 2,947,233 | 50.3 | 438,883 | 49.1 | 313,877 | 52.7 |
| Country of birth | |||||||
| Sweden | 6,201,225 | 4,827,821 | 82.4 | 724,205 | 81.0 | 524,892 | 88.2 |
| Other countries | 1,291,006 | 1,030,347 | 17.6 | 170,282 | 19.0 | 70,147 | 11.8 |
| Health care workers | |||||||
| Yes | 1,504,469 | 1,112,637 | 19.0 | 179,500 | 20.1 | 119,297 | 20.0 |
| No | 5,987,762 | 4,745,531 | 81.0 | 714,987 | 79.9 | 475,742 | 80.0 |
| Education | |||||||
| Primary | 1,276,567 | 971,760 | 16.6 | 162,404 | 18.2 | 121,539 | 20.4 |
| Secondary | 2,961,176 | 2,270,684 | 38.8 | 357,753 | 40.0 | 261,433 | 43.9 |
| Tertiary | 2,684,092 | 2,075,266 | 35.4 | 326,770 | 36.5 | 207,945 | 34.9 |
| Unknown | 570,396 | 540,458 | 9.2 | 47,560 | 5.3 | 4122 | 0.7 |
| Income (a) | |||||||
| Low | 2,055,470 | 1,583,045 | 27.0 | 266,250 | 29.8 | 166,084 | 27.9 |
| Medium | 2,452,190 | 1,865,049 | 31.8 | 313,698 | 35.1 | 203,912 | 34.3 |
| High | 2,617,388 | 2,026,669 | 34.6 | 300,081 | 33.5 | 225,007 | 37.8 |
| Unknown | 367,183 | 383,405 | 6.5 | 14,458 | 1.6 | 36 | 0.0 |
| Marital status | |||||||
| Married | 3,005,911 | 2,276,394 | 38.9 | 330,761 | 37.0 | 339,868 | 57.1 |
| Unmarried | 4,484,570 | 3,580,270 | 61.1 | 563,482 | 63.0 | 255,135 | 42.9 |
| Unknown | 1750 | 1504 | 0.0 | 244 | 0.0 | 36 | 0.0 |
| Special care facilities | |||||||
| No | 7,437,607 | 5,804,580 | 99.1 | 893,989 | 99.9 | 594,881 | 100.0 |
| Yes | 54,624 | 53,588 | 0.9 | 498 | 0.1 | 158 | 0.0 |
| Home care service | |||||||
| No | 7,307,304 | 5,692,424 | 97.2 | 880,716 | 98.5 | 590,566 | 99.2 |
| Yes | 184,927 | 165,744 | 2.8 | 13,771 | 1.5 | 4473 | 0.8 |
| Prior comorbidities and treatments (b) | |||||||
| Cardiovascular disease | |||||||
| No | 6914,482 | 5,428,674 | 92.7 | 837,515 | 93.6 | 510,530 | 85.8 |
| Yes | 577,749 | 429,494 | 7.3 | 56,972 | 6.4 | 84,509 | 14.2 |
| Stroke | |||||||
| No | 7,413,794 | 5,797,064 | 99.0 | 887,348 | 99.2 | 585,524 | 98.4 |
| Yes | 78,437 | 61,104 | 1.0 | 7139 | 0.8 | 9515 | 1.6 |
| Hypertension | |||||||
| No | 5,657,425 | 4,526,433 | 77.3 | 719,071 | 80.4 | 293,171 | 49.3 |
| Yes | 1,834,806 | 1,331,735 | 22.7 | 175,416 | 19.6 | 301,868 | 50.7 |
| Diabetes | |||||||
| No | 7,015,964 | 5,509,183 | 94.0 | 846,257 | 94.6 | 5224,51 | 87.8 |
| Yes | 476,267 | 348,985 | 6.0 | 48,230 | 5.4 | 72,588 | 12.2 |
| Obstructive respiratory diseases | |||||||
| No | 6,769,169 | 5,302,461 | 90.5 | 814,549 | 91.1 | 521,747 | 87.7 |
| Yes | 723,062 | 555,707 | 9.5 | 79,938 | 8.9 | 73,292 | 12.3 |
| Chronic kidney diseases | |||||||
| No | 7,415,874 | 5,799,854 | 99.0 | 885,461 | 99.0 | 586,798 | 98.6 |
| Yes | 76,357 | 58,314 | 1.0 | 9026 | 1.0 | 8241 | 1.4 |
| Obesity | |||||||
| No | 7352,,735 | 5,748,820 | 98.1 | 877,508 | 98.1 | 585,194 | 98.3 |
| Yes | 139,496 | 109,348 | 1.9 | 16,979 | 1.9 | 9845 | 1.7 |
| Autoimmune diseases | |||||||
| No | 7,310,314 | 5,721,375 | 97.7 | 875,715 | 97.9 | 571,667 | 96.1 |
| Yes | 181,971 | 136,793 | 2.3 | 18,772 | 2.1 | 23,372 | 3.9 |
| Dementia | |||||||
| No | 7,453,854 | 5,823,453 | 99.4 | 892,704 | 99.8 | 593,332 | 99.7 |
| Yes | 38,377 | 34,715 | 0.6 | 1783 | 0.2 | 1707 | 0.3 |
| Psychiatric conditions | |||||||
| No | 6,025,166 | 4,729,802 | 80.7 | 727,596 | 81.3 | 460,745 | 77.4 |
| Yes | 1,467,065 | 1,128,366 | 19.3 | 166,891 | 18.7 | 134,294 | 22.6 |
| Cancer | |||||||
| No | 7,099,348 | 5,576,635 | 95.2 | 856,102 | 95.7 | 527,112 | 88.6 |
| Yes | 392,883 | 281,533 | 4.8 | 38,385 | 4.3 | 67,927 | 11.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, H.; Kirui, B.; Santosa, A.; Gisslén, M.; Leach, S.; Wettermark, B.; Vanfleteren, L.E.G.W.; Nyberg, F. Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population. Vaccines 2022, 10, 2074. https://doi.org/10.3390/vaccines10122074
Xu Y, Li H, Kirui B, Santosa A, Gisslén M, Leach S, Wettermark B, Vanfleteren LEGW, Nyberg F. Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population. Vaccines. 2022; 10(12):2074. https://doi.org/10.3390/vaccines10122074
Chicago/Turabian StyleXu, Yiyi, Huiqi Li, Brian Kirui, Ailiana Santosa, Magnus Gisslén, Susannah Leach, Björn Wettermark, Lowie E. G. W. Vanfleteren, and Fredrik Nyberg. 2022. "Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population" Vaccines 10, no. 12: 2074. https://doi.org/10.3390/vaccines10122074
APA StyleXu, Y., Li, H., Kirui, B., Santosa, A., Gisslén, M., Leach, S., Wettermark, B., Vanfleteren, L. E. G. W., & Nyberg, F. (2022). Effectiveness of COVID-19 Vaccines over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population. Vaccines, 10(12), 2074. https://doi.org/10.3390/vaccines10122074





