Action Mechanisms and Scientific Rationale of Using Nasal Vaccine (HeberNasvac) for the Treatment of Chronic Hepatitis B
Abstract
1. Introduction
2. Nasvac: A Novel Vaccine for Chronic Hepatitis B Treatment
3. Rationale of Using the Nasal Route of Immunization
3.1. Association of the i.n. Immunization to Immune Activation and HBV Clearance
3.2. Unique Properties of the Nasal Route in Animal Models of HBV Immunotolerance
3.3. Long-Term Follow-Up of CHB Patients Treated with Nasvac by Nasal Route Only
3.4. Nasal Administration of Nasvac in the Setting of Inactive Carriers
4. On the Rationale of Combining NUCs and Therapeutic Vaccination
4.1. Vaccination under NUCs: Pros and Cons
4.2. Nasvac Vaccination and NUCs Cessation
4.3. Immunotherapy under NUCs: Immunological Perspective
4.4. NUCs Stopping Rules: Learning from Nature
4.5. Therapeutic Avenues Using Nasvac in the Setting of Patients with NUCs
5. On the Immunomodulatory Properties of Nasvac Antigens
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Hepatitis B. Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 24 October 2022).
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Cuban Regulatory Agency (CECMED). HeberNasvac: Recombinant Therapeutic Vaccine against the Chronic Infection by the Hepatitis B Virus. Summary of Product Characteristics. 2019. Available online: https://www.cecmed.cu/sites/default/files/adjuntos/rcp/biologicos/rcphebernasvac0.pdf (accessed on 24 October 2022).
- Akbar, S.M.F.; Yoshida, O.; Chen, S.; Cesar, A.J.; Abe, M.; Matsuura, B.; Hiasa, Y.; Onji, M. Immune modulator and antiviral potential of dendritic cells pulsed with both hepatitis B surface Ag and core antigen for treating chronic HBV infection. Antivir. Ther. 2010, 15, 887–895. [Google Scholar] [CrossRef]
- Lobaina, Y.; Hardtke, S.; Wedemeyer, H.; Aguilar, J.C.; Schlaphoff, V. In vitro stimulation with HBV therapeutic vaccine candidate Nasvac activates B and T cells from chronic hepatitis B patients and healthy donors. Mol. Immunol. 2015, 63, 320–327. [Google Scholar] [CrossRef]
- Pentón-Arias, E.; Aguilar-Rubido, J.C. Cuban prophylactic and therapeutic vaccines for controlling hepatitis B. MEDICC Rev. 2021, 23, 21–29. [Google Scholar] [CrossRef]
- Al-Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Uddin, M.H.; Khan, M.S.; Rahman, S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol. Int. 2013, 7, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; Yoshida, O.; Hiasa, Y.; Onji, M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS ONE 2018, 13, e0201236. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, O.; Akbar, S.M.F.; Imai, Y.; Sanada, T.; Tsukiyama-Kohara, K.; Miyazaki, T.; Kamishita, T.; Miyake, T.; Tokumoto, Y.; Hikita, H.; et al. Intranasal therapeutic vaccine containing HBsAg and HBcAg for patients with chronic hepatitis B; 18 months follow-up results of phase IIa clinical study. Hepatol. Res. 2022, 1–12. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Hui, A.J.; Sukeepaisarnjaroen, W.; Tangkijvanich, P.; Su, W.W.; Nieto, G.E.; Gineste, P.; Nitcheu, J.; Crabé, S.; Stepien, S.; et al. Therapeutic vaccination of chronic hepatitis B patients with ABX203 (NASVAC) to prevent relapse after stopping NUCs: Contrasting timing rebound between tenofovir and entecavir. J. Hepatol. 2017, 66, S101. [Google Scholar] [CrossRef]
- Aguilar, J.C. On the future of therapeutic vaccination in chronic hepatitis B. Future Sci. OA 2019, 5, FSO426. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, M.; Crabe, S.; Lobaina, Y.; Guillen, G.; Aguilar, J.C.; Michel, M.L. Nasal route favors the induction of CD4+ T cell responses in the liver of HBV-carrier mice immunized with a recombinant hepatitis B surface-and core-based therapeutic vaccine. Antivir. Res. 2018, 153, 23–32. [Google Scholar] [CrossRef]
- Trujillo, H.; Blanco, A.; Garcia, D.; Freyre, F.; Aguiar, J.; Lobaina, Y.; Aguilar, J.C. Optimization of a therapeutic vaccine candidate by studying routes, immunization schedules and antigen doses in HBsAg-positive transgenic mice. Euroasian J. Hepato-Gastroenterol. 2014, 4, 70–78. [Google Scholar] [CrossRef]
- Almeida, F.M.F.; Blanco, A.; Trujillo, H.; Hernández, D.; García, D.; Alba, J.S.; Lopez, M.; Merino, N.; Lobaina, Y.; Rubido, J.C.A. Dynamic of immune response induced in hepatitis B surface antigen-transgenic mice immunized with a novel therapeutic formulation. Euroasian J. Hepato-Gastroenterol. 2016, 6, 25–30. [Google Scholar] [CrossRef]
- WIPO IP Portal Patentscope. WO2021213558A2. Viral Nucleoproteins and Formulations Containing Same. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021213558 (accessed on 8 November 2022).
- Fleites, Y.A.; Aguiar, J.; Cinza, Z.; Bequet, M.; Marrero, E.; Vizcaíno, M.; Esquivel, I.; Diaz, M.; Sin-Mayor, A.; Garcia, M.; et al. HeberNasvac, a therapeutic vaccine for chronic hepatitis B, stimulates local and systemic markers of innate immunity: Potential use in SARS-CoV-2 post-exposure prophylaxis. Euroasian J. Hepatogastroenterol. 2021, 11, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Fernández, G.; Sanchez, A.L.; Jerez, E.; Anillo, L.E.; Freyre, F.; Aguiar, J.A.; Leon, Y.; Cinza, Z.; Dias, P.; Figueroa, N.; et al. Five-year follow-up of chronic hepatitis B patients immunized by nasal route with the therapeutic vaccine HeberNasvac. Euroasian J. Hepato-Gastroenterol. 2018, 8, 133–139. [Google Scholar] [CrossRef]
- Lok, A.S.; Pan, C.Q.; Han, S.H.B.; Trinh, H.N.; Fessel, W.J.; Rodell, T.; Massetto, B.; Lin, L.; Gaggar, A.; Subramanian, G.M.; et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2016, 65, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, H.; Kahi, S.; Chazallon, C.; Bourgine, M.; Varaut, A.; Buffet, C.; Godon, O.; Meritet, J.F.; Saïdi, Y.; Michel, M.L.; et al. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: A randomised trial–ANRS HB02 VAC-ADN. Gut 2015, 64, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Vandepapeliere, P.; Lau, G.K.; Leroux-Roels, G.; Horsmans, Y.; Gane, E.; Tawandeee, T.; bin Merican, M.I.; Wing, K.M.; Trepo, C.; Cooksley, G.; et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: A randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 2007, 25, 8585–8597. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.L.; Deng, Q.; Mancini-Bourgine, M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: Perspectives and challenges. J. Hepatol. 2011, 54, 1286–1296. [Google Scholar] [CrossRef]
- Boni, C.; Bertoletti, A.; Penna, A.; Cavalli, A.; Pilli, M.; Urbani, S.; Scognamiglio, P.; Boehme, R.; Panebianco, R.; Fiaccadori, F.; et al. Lamivudine treatment can restore T-cell responsiveness in chronic hepatitis. B. J. Clin. Invest. 1998, 102, 968–975. [Google Scholar] [CrossRef]
- Boni, C.; Penna, A.; Bertoletti, A.; Lamonaca, V.; Rapti, I.; Missale, G.; Pilli, M.; Urbani, S.; Cavalli, A.; Cerioni, S.; et al. Transient restoration of anti-viral T-cell responses induced by lamivudine therapy in chronic hepatitis B. J. Hepatol. 2003, 39, 595–605. [Google Scholar] [CrossRef]
- Papatheodoridis, G.; Vlachogiannakos, I.; Cholongitas, E.; Wursthorn, K.; Thomadakis, C.; Touloumi, G.; Petersen, J. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology 2016, 63, 1481–1492. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Van de Klundert, M.; Michel, M.L. The International Liver Congress™, Amsterdam, the Netherlands 2017. Biotecnol. Apl. 2017, 34, 2511–2521. [Google Scholar]
- Pan, C.Q.; Afdhal, N.H.; Ankoma-Sey, V.; Bae, H.; Curry, M.P.; Dieterich, D.; Frazier, L.; Frick, A.; Hann, H.W.; Kim, W.R.; et al. First-line therapies for hepatitis B in the United States: A 3-year prospective and multicenter real-world study after approval of tenofovir alefenamide. Hepatol. Commun. 2022, 6, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Wang, H.; Zheng, Q.H.; Zheng, X.W.; Fan, J.N.; Ding, Y.L.; Niu, J.L. Comparative efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naive chronic hepatitis B: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0224773. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.M.; Lim, J.K.; Pan, C.Q.; Nguyen, M.H.; Ray Kim, W.; Mannalithara, A.; Trinh, H.; Chu, D.; Tran, T.; et al. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US—The ENUMERATE study. Aliment. Pharm 2016, 43, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Knolle, P.A. Staying local-antigen presentation in the liver. Curr. Opin. Immunol. 2016, 40, 36–42. [Google Scholar] [CrossRef]
- Krawitt, E.L.; Zannier, A.; Chossegros, P.; Gerard, F.; Chevallier, M.; Mutin, M.; Trepo, C.; Touraine, J.L. Expression of HLA antigens and T cell infiltrates in chronic viral hepatitis: A comparison of biopsy and fine-needle aspiration findings. J. Hepatol. 1991, 12, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Van den Oord, J.J.; de Vos, R.; Desmet, V.J. In situ distribution of major histocompatibility complex products and viral antigens in chronic hepatitis B virus infection: Evidence that HBc-containing hepatocytes may express HLA-DR antigens. Hepatology 1986, 6, 981–989. [Google Scholar] [CrossRef]
- Uzun, Y.; Bozkaya, H.; Erden, E.; Cinar, K.; Idilman, R.; Yurdaydin, C.; Uzunalimoglu, O. Hepatitis B core antigen expression pattern reflects the response to anti-viral treatment. J. Gastroenterol. Hepatol. 2006, 21, 977–981. [Google Scholar] [CrossRef]
- Buti, M.; Tsai, N.; Petersen, J.; Flisiak, R.; Gurel, S.; Krastev, Z.; Aguilar Schall, R.; Flaherty, J.F.; Martins, E.B.; Charuworn, P.; et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig. Dis. Sci. 2015, 60, 1457–1464. [Google Scholar] [CrossRef]
- Hadziyannis, S.J.; Sevastianos, V.; Rapti, I.; Vassilopoulos, D.; Hadziyannis, E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology 2012, 143, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Simon, K.G.; Mauss, S.; Schott, E.; Heyne, R.; Klass, D.; Eisenbach, C.; Welzel, T.M.; Zachoval, R.; Felten, G.; et al. Stopping tenofovir disoproxil fumarate (TDF) treatment after long-term virologic suppression in HBeAg-negative CHB: Week 48 interim results from an ongoing randomized, controlled trial (FINITE CHB). J. Hepatol. 2015, 62, S253. [Google Scholar] [CrossRef]
- Honer Zu Siederdissen, C.; Rinker, F.; Maasoumy, B.; Wiegand, S.B.; Filmann, N.; Falk, C.S.; Deterding, K.; Port, K.; Mix, C.; Manns, M.P.; et al. Viral and Host Responses After Stopping Long-term Nucleos(t)ide Analogue Therapy in HBeAg-Negative Chronic Hepatitis B. J. Infect. Dis. 2016, 214, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.C.; Lobaina, Y.; Muzio, V.; García, D.; Pentón, E.; Iglesias, E.; Pichardo, D.; Urquiza, D.; Rodriguez, D.; Silva, D.; et al. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol. Cell Biol. 2004, 82, 539–546. [Google Scholar] [CrossRef]
- Zhu, Q.; Egelston, C.; Gagnon, S.; Sui, Y.; Belyakov, I.M.; Klinman, D.M.; Berzofsky, J.A. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J. Clin. Invest. 2010, 120, 607–616. [Google Scholar] [CrossRef]
- Zhu, Q.; Egelston, C.; Vivekanandhan, A.; Uematsu, S.; Akira, S.; Klinman, D.M.; Belyakov, I.M.; Berzofsky, J.A. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: Implications for vaccines. Proc. Natl. Acad. Sci. USA 2008, 105, 16260–16265. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.; Chen, X. Host innate immunity against hepatitis viruses and viral immune evasion. Front. Microbiol. 2021, 12, 740464. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Pentón, E.; Akbar, S.M.F. Innate Immune Stimulation Should not be Overlooked in Post-exposure Prophylaxis and Early Therapy for Coronavirus Infections. MEDICC Rev. 2022, 24, 70–75. [Google Scholar] [CrossRef]
- Cuban Registry of Clinical Trials (RPCEC00000326-En; RPCEC00000353-En; RPCEC00000356-En; RPCEC00000345-En and RPCEC00000382-En). Available online: https://rpcec.sld.cu/trials/ (accessed on 8 November 2022).
- Akbar, S.M.F.; Mahtab, M.A.; Aguilar, J.C.; Uddin, M.H.; Khan, S.I.; Yoshida, O.; Pentón, E.; Guillén, G.; Hiasa, Y. Repurposing NASVAC, A Hepatitis B Therapeutic Vaccine, for Pre-and Post-exposure Prophylaxis of SARS-CoV-2 Infection. J. Antivir. Antiretrovir. 2021, 13, 1–7. [Google Scholar] [CrossRef]
- Kumaki, Y.; Salazar, A.M.; Wandersee, M.K.; Barnard, D.L. Prophylactic and therapeutic intranasal administration with an immunomo-dulator, Hiltonol® (Poly IC:LC), in a lethal SARS-CoV-infected BALB/c mouse model. Antivir. Res. 2017, 139, 1–12. [Google Scholar] [CrossRef]
- Zhao, J.; Wohlford-Lenane, C.; Zhao, J.; Fleming, E.; Lane, T.E.; McCray, P.B., Jr.; Perlman, S. Intranasal treatment with poly(I•C) protects aged mice from lethal respiratory virus infections. J. Virol. 2012, 86, 11416–11424. [Google Scholar] [CrossRef] [PubMed]
- Sariol, C.A.; Martínez, M.I.; Rivera, F.; Rodríguez, I.V.; Pantoja, P.; Abel, K.; Arana, T.; Giavedoni, L.; Hodara, V.; White, L.J.; et al. Decreased dengue replication and an increased anti-viral humoral response with the use of combined Toll-like receptor 3 and 7/8 agonists in macaques. PLoS ONE 2011, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.M.; Xu, Y.Y.; Chen, L.; Li, X.Y.; Qin, J.; Shen, Y. TLR3 expression correlates with apoptosis, proliferation and angiogenesis in hepatocellular carcinoma and predicts prognosis. BMC Cancer 2015, 15, 245. [Google Scholar] [CrossRef]
- Hao, Y.; Gu, Z.; Yu, Z.; Schomann, T.; Sayedipour, S.; Aguilar, J.C.; Ten Dijke, P.; Cruz, L.J. Photodynamic Therapy in Combination with the Hepatitis B Core Virus-like Particles (HBc VLPs) to Prime Anticancer Immunity for Colorectal Cancer Treatment. Cancers 2022, 14, 2724. [Google Scholar] [CrossRef]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained immunity: A tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef]
- Akbar, S.M.F.; Al Mahtab, M.; Aguilar, J.C.; Yoshida, O.; Penton, E.; Gerardo, G.N.; Hiasa, Y. Sustained Antiviral and Liver Protection by a Nasal Therapeutic Vaccine (NASVAC, Containing Both HBsAg and HBcAg) in Patients with Chronic Hepatitis B: 2-Year Follow-Up of Phase III Clinical Trial. Pathogens 2021, 10, 1440. [Google Scholar] [CrossRef]
- Akbar, S.M.F.; Al Mahtab, M.; Aguilar, J.C.; Yoshida, O.; Khan, S.; Penton, E.; Gerardo, G.N.; Hiasa, Y. The Safety and Efficacy of a Therapeutic Vaccine for Chronic Hepatitis B: A Follow-Up Study of Phase III Clinical Trial. Vaccines 2021, 10, 45. [Google Scholar] [CrossRef]
| Ref. | Nasvac Study (Main Mechanisms of Action) | Pharmacological Results Unveiling the Action Mechanisms |
|---|---|---|
| [4] | Study of dendritic cells pulsed with both hepatitis B surface and core antigens (in vitro activation of APCs and T-cell response). | High levels of anti-HBs, HBsAg-specific and HBcAg-specific T-cells, and CTLs detected in the spleen and liver of HBV TM immunized with HBsAg-/HBcAg-pulsed DCs compared with those immunized with other vaccine formulations (p < 0.05). HBsAg-/HBcAg-pulsed human blood DCs also induced HBsAg- and HBcAg-specific proliferation of autologous T-cells from CHB patients. |
| [5] | In vitro stimulation effect of Nasvac on B- and T-cells from chronic hepatitis B patients and healthy donors (in vitro activation of B- and T-cells). | B-cell activation markers increase in CHB patients after Nasvac in vitro stimulation. B-cell activation, but not exhaustion, is induced in cells from CHB patients after Nasvac in vitro stimulation. An increase in CD25 on CD4+ and CD8+ T-cells and CD69 on CD8+ T-cells was observed after in vitro Nasvac stimulation. Nasvac-activated B-cells can in turn stimulate T-cells. B-cells stimulated by Nasvac may become an important APC during CHB immunotherapy. |
| [7,8] | Phase I/II and phase III clinical trials (in vitro APC activation and cytokine secretion pattern). | PBMCs and HBsAg-/HBcAg-pulsed DCs from HBsAg-/HBcAg-vaccinated CHB patients produced significantly higher levels of various cytokines (interleukin 1β (IL-1β), IL-6, IL-8, IL-12, and tumor necrosis factor α (TNF-α)) than those from control unvaccinated CHB patients (p < 0.05) after stimulation with HBsAg/HBcAg in vitro. Associated with HBV suppression in 50% of CHB patients. Generalized and transient increase of ALT at Week 12 associated with HBV control. |
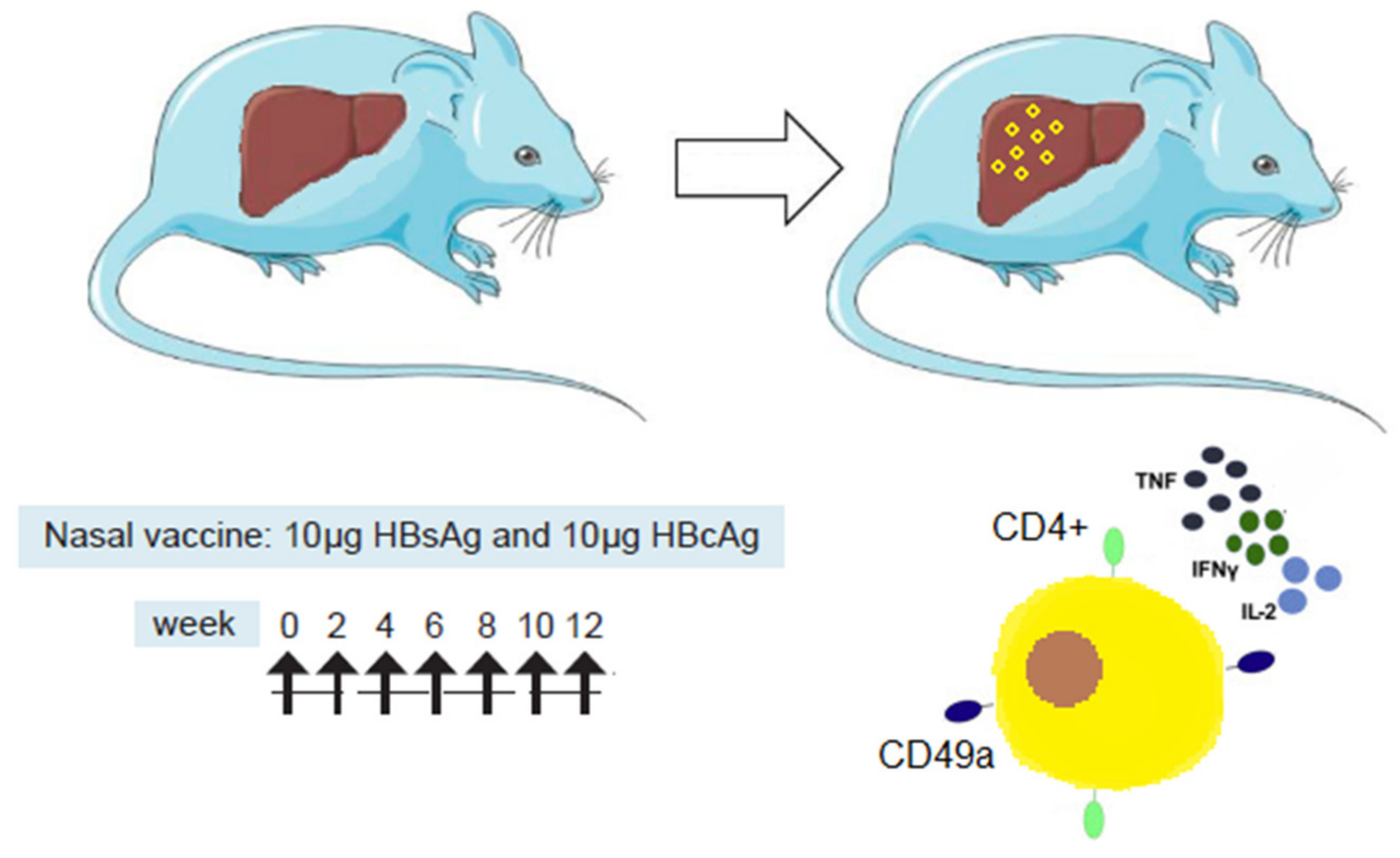
| [12] | Study of i.n. and s.c. routes of immunization in HBV-carrier mice following a schedule of 7 doses (multifunctional T-cell response in the liver and spleen after i.n. immunization), | The i.n. route was the most efficacious at inducing cellular immune responses, in particular CD4+ T-cells in spleen and in the liver. Multifunctional CD4+ T-cells, secreting IFN-γ, IL-2, and TNF-α, were detected in the liver only after i.n. immunization. High frequencies of CD4+ T-cells expressing the integrin CD49a in the liver suggest a role of the nasal route in the homing process (Figure 1). |
| [13,14] | Study of different routes of administration, schedules of immunization, and antigen doses in HBsAg-positive transgenic mice (Ab, CD8 T-cell, and Th1 pattern of response in HBsAg tg and naïve mice). | Anti-HBsAg- and HBsAg-specific CD8+ T-cells in a tg mice model (expressing high levels of the homologous antigen in the blood, liver, and multiple organs) are induced by combining i.n. and s.c. immunization compared to the s.c. route alone. The dynamics of immune response induced in hepatitis B surface-antigen-transgenic mice immunized with Nasvac evidenced that a multiple dose schedule is a prerequisite to overcome immunotolerance in HBV-carrier mice’s Th1 pattern of response induced after i.n. administration. |
| [15] | HBcAg effect on HepaRG gene expression of TLR, MyD88/TRIF pathways, MHC class I/II, B7.1/2 proteins, IFNs, and cytokines and antiviral effect in HBV-infected HepaRG cells (HBcAg multi-TLR agonist effect, increasing presentation molecules, IFNs, and cytokines). | Multi-TLR agonist effect in TLR2, TLR3, TLR7, TLR8, and TLR9 cells leading to an increased stimulation of adaptors MyD88; TRIF, NF-kb, and IRF3 and molecules involved in antigen presentation (HLA class I, HLA class II, B7.1; B7.2) as well as IFNα, IFNβ, and other cytokines. Innate immune stimulation triggered a strong antiviral effect in infected HepaRG cells (similar in intensity to Entecavir). |
| [16] | Confirmatory clinical trial: i.n. and s.l. administration of Nasvac in >60-year-olds suspicion of SARS-CoV-2 (multi-agonist TLR expression and HLA molecules in PBMCs). | Nasvac stimulates local (TLR3, TLR,7 and TLR8 in oropharyngeal cavity) and systemic markers of innate immunity (HLA class II expression in monocytes and lymphocytes, new indication for potential use in SARS-CoV-2 post-exposure prophylaxis). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, J.C.; Aguiar, J.A.; Akbar, S.M.F. Action Mechanisms and Scientific Rationale of Using Nasal Vaccine (HeberNasvac) for the Treatment of Chronic Hepatitis B. Vaccines 2022, 10, 2087. https://doi.org/10.3390/vaccines10122087
Aguilar JC, Aguiar JA, Akbar SMF. Action Mechanisms and Scientific Rationale of Using Nasal Vaccine (HeberNasvac) for the Treatment of Chronic Hepatitis B. Vaccines. 2022; 10(12):2087. https://doi.org/10.3390/vaccines10122087
Chicago/Turabian StyleAguilar, Julio Cesar, Jorge Agustin Aguiar, and Sheikh Mohammad Fazle Akbar. 2022. "Action Mechanisms and Scientific Rationale of Using Nasal Vaccine (HeberNasvac) for the Treatment of Chronic Hepatitis B" Vaccines 10, no. 12: 2087. https://doi.org/10.3390/vaccines10122087
APA StyleAguilar, J. C., Aguiar, J. A., & Akbar, S. M. F. (2022). Action Mechanisms and Scientific Rationale of Using Nasal Vaccine (HeberNasvac) for the Treatment of Chronic Hepatitis B. Vaccines, 10(12), 2087. https://doi.org/10.3390/vaccines10122087






