Impact of COVID-19 Vaccination on Heart Rate Variability: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligible Criteria
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Exposure
2.1.4. Types of Controls
2.1.5. Types of Outcome Measures
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Missing Data
2.7. Data Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Methodological Quality Assessment
3.4. Impact of COVID-19 Vaccinations on HRV Parameters
3.4.1. Qualitative Analysis
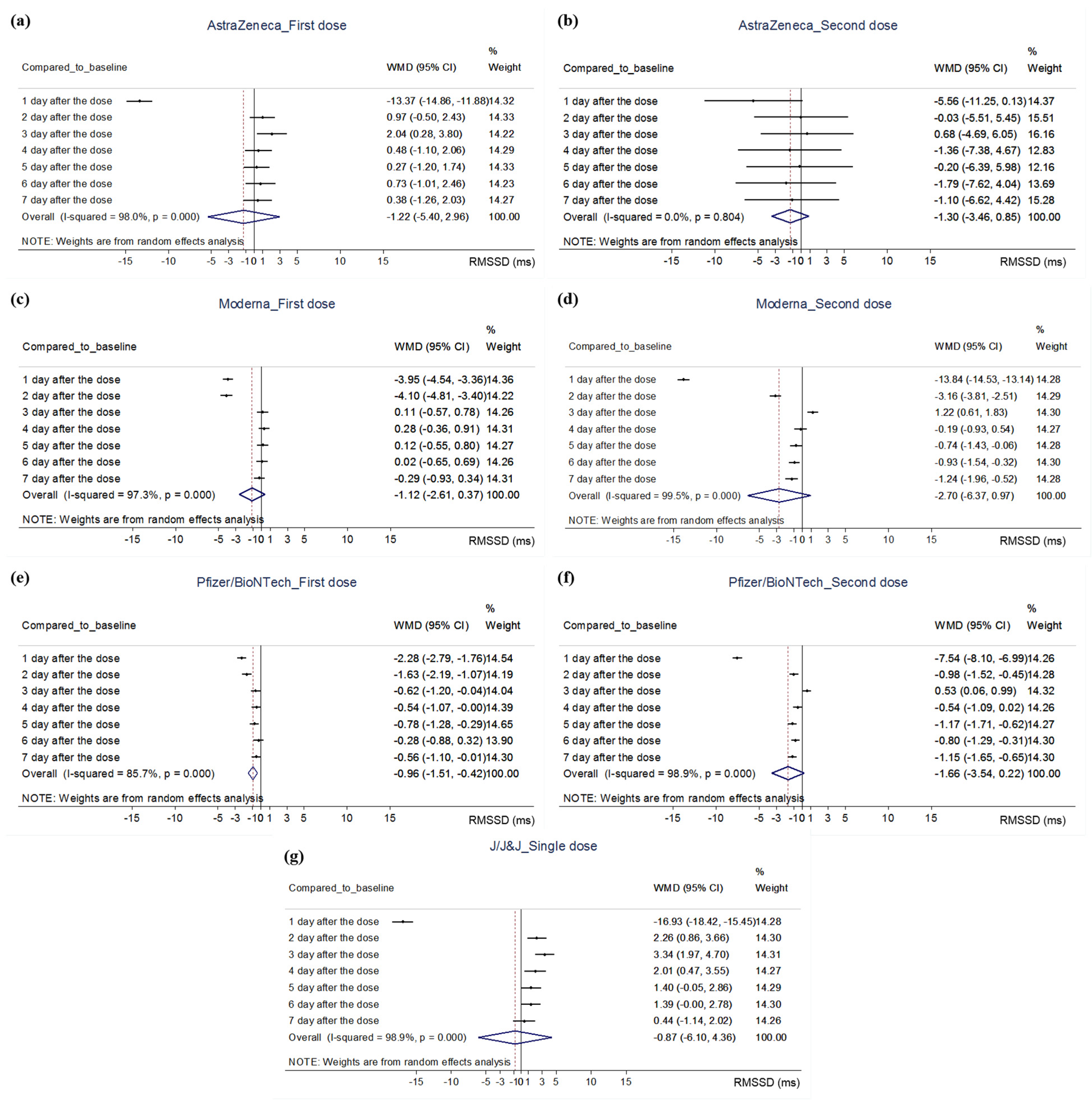
3.4.2. Quantitative Analysis
3.5. Relationship between HRV Parameters and Adverse Events after COVID-19 Vaccinations
4. Discussion
4.1. Findings of This Review
4.2. Clinical Interpretation
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexandridi, M.; Mazej, J.; Palermo, E.; Hiscott, J. The Coronavirus pandemic–2022: Viruses, variants & vaccines. Cytokine Growth Factor Rev. 2022, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fajar, J.K.; Sallam, M.; Soegiarto, G.; Sugiri, Y.J.; Anshory, M.; Wulandari, L.; Kosasih, S.A.P.; Ilmawan, M.; Kusnaeni, K.; Fikri, M.; et al. Global Prevalence and Potential Influencing Factors of COVID-19 Vaccination Hesitancy: A Meta-Analysis. Vaccines 2022, 10, 1356. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, O.V.; Johnson, M.S.; Ebling, S.; Amundsen, O.M.; Halsøy, Ø.; Hoffart, A.; Skjerdingstad, N.; Johnson, S.U. Risk, Trust, and Flawed Assumptions: Vaccine Hesitancy During the COVID-19 Pandemic. Front. Public Health 2021, 9, 700213. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, H.A.; Khedr, A.; Koritala, T.; Bartlett, B.N.; Jain, N.K.; Khan, S.A. A review of adverse effects of COVID-19 vaccines. Infez. Med. 2022, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Kaur, R.; Charan, J.; Bhardwaj, P.; Ambwani, S.R.; Babu, S.; Goyal, J.P.; Haque, M. Analysis of Neurological Adverse Events Reported in VigiBase From COVID-19 Vaccines. Cureus 2022, 14, e21376. [Google Scholar] [CrossRef] [PubMed]
- Hermel, M.; Sweeney, M.; Abud, E.; Luskin, K.; Criado, J.P.; Bonakdar, R.; Gray, J.; Ahern, T. COVID-19 Vaccination Might Induce Postural Orthostatic Tachycardia Syndrome: A Case Report. Vaccines 2022, 10, 991. [Google Scholar] [CrossRef]
- Reddy, S.; Reddy, S.; Arora, M. A Case of Postural Orthostatic Tachycardia Syndrome Secondary to the Messenger RNA COVID-19 Vaccine. Cureus 2021, 13, e14837. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lee, J.; An, J.Y. A case of transient POTS following COVID-19 vaccine. Acta Neurol Belg 2022, 122, 1081–1083. [Google Scholar] [CrossRef]
- Lanman, T.A.; Wu, C.; Cheung, H.; Goyal, N.; Greene, M. Guillain-Barré Syndrome with Rapid Onset and Autonomic Dysfunction Following First Dose of Pfizer-BioNTech COVID-19 Vaccine: A Case Report. Neurohospitalist 2022, 12, 388–390. [Google Scholar] [CrossRef]
- Karimi Galougahi, K. Autonomic dysfunction post-inoculation with ChAdOx1 nCoV-19 vaccine. Eur. Heart J. Case Rep. 2021, 5, ytab472. [Google Scholar] [CrossRef]
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S.; Brinth, L.; Hendrickson, J.E.; Martinez-Lavin, M. Autonomic dysfunction and HPV immunization: An overview. Immunol. Res. 2018, 66, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Barone, L.; Scalone, G.; Pitocco, D.; Sgueglia, G.A.; Mollo, R.; Nerla, R.; Zaccardi, F.; Ghirlanda, G.; Crea, F. Inflammation-related effects of adjuvant influenza A vaccination on platelet activation and cardiac autonomic function. J. Intern. Med. 2011, 269, 118–125. [Google Scholar] [CrossRef]
- Jae, S.Y.; Heffernan, K.S.; Park, S.H.; Jung, S.H.; Yoon, E.S.; Kim, E.J.; Ahn, E.S.; Fernhall, B. Does an acute inflammatory response temporarily attenuate parasympathetic reactivation? Clin. Auton Res. 2010, 20, 229–233. [Google Scholar] [CrossRef]
- Park, J.W.; Yu, S.N.; Chang, S.H.; Ahn, Y.H.; Jeon, M.H. Multisystem Inflammatory Syndrome in an Adult after COVID-19 Vaccination: A Case Report and Literature Review. J. Korean Med. Sci. 2021, 36, e312. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.P.; Koenig, J.; Carnevali, L.; Sgoifo, A.; Jarczok, M.N.; Sternberg, E.M.; Thayer, J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain Behav. Immun. 2019, 80, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hasty, F.; García, G.; Dávila, C.H.; Wittels, S.H.; Hendricks, S.; Chong, S. Heart Rate Variability as a Possible Predictive Marker for Acute Inflammatory Response in COVID-19 Patients. Mil. Med. 2020, 186, e34–e38. [Google Scholar] [CrossRef]
- Asarcikli, L.D.; Hayiroglu, M.; Osken, A.; Keskin, K.; Kolak, Z.; Aksu, T. Heart rate variability and cardiac autonomic functions in post-COVID period. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2022, 63, 715–721. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Presby, D.M.; Capodilupo, E.R. Biometrics from a wearable device reveal temporary effects of COVID-19 vaccines on cardiovascular, respiratory, and sleep physiology. J. Appl. Physiol. 2022, 132, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, C. 7.7. 3.2 Obtaining standard deviations from standard errors and confidence intervals for group means. Cochrane Handb. Syst. Rev. Interv. 2011. Available online: https://handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm (accessed on 4 December 2022).
- Department of Obstetrics and Gynecology at the Chinese University of Hong Kong. Combine Means and SDs into One Group Program. Available online: https://www.obg.cuhk.edu.hk/ResearchSupport/StatTools/CombineMeansSDs_Pgm.php (accessed on 30 October 2022).
- Finsterer, J.; Scorza, F.A.; Scorza, C.A.; Fiorini, A.C. Consider differentials before diagnosing COVID-19 associated polyradiculitis. Eur. J. Transl. Myol. 2022, 32, 10111. [Google Scholar] [CrossRef]
- Finsterer, J. SARS-CoV-2 vaccinations may not only be complicated by GBS but also by distal small fibre neuropathy. J. Neuroimmunol. 2021, 360, 577703. [Google Scholar] [CrossRef]
- Kurtoğlu, E.; Afsin, A.; Aktaş, İ.; Aktürk, E.; Kutlusoy, E.; Çağaşar, Ö. Altered cardiac autonomic function after recovery from COVID-19. Ann. Noninvasive Electrocardiol. Off. J. Int. Soc. Holter Noninvasive Electrocardiol. Inc 2022, 27, e12916. [Google Scholar] [CrossRef] [PubMed]
- Skow, R.J.; Nandadeva, D.; Grotle, A.K.; Stephens, B.Y.; Wright, A.N.; Fadel, P.J. Impact of breakthrough COVID-19 cases during the omicron wave on vascular health and cardiac autonomic function in young adults. Am. J. Physiology. Heart Circ. Physiol. 2022, 323, H59–H64. [Google Scholar] [CrossRef]
- Brakenhoff, T.B.; Franks, B.; Goodale, B.M.; van de Wijgert, J.; Montes, S.; Veen, D.; Fredslund, E.K.; Rispens, T.; Risch, L.; Dowling, A.V.; et al. A prospective, randomized, single-blinded, crossover trial to investigate the effect of a wearable device in addition to a daily symptom diary for the Remote Early Detection of SARS-CoV-2 infections (COVID-RED): A structured summary of a study protocol for a randomized controlled trial. Trials 2021, 22, 694. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Schult, F.; Baehren, C.; Buedding, E.; Mboma, O.; Ahmad-Nejad, P.; Ghebremedhin, B.; Ehrhardt, A.; Wirth, S.; Aydin, M. Viral Infection and Respiratory Exacerbation in Children: Results from a Local German Pediatric Exacerbation Cohort. Viruses 2022, 14, 491. [Google Scholar] [CrossRef]
- Hajduczok, A.G.; DiJoseph, K.M.; Bent, B.; Thorp, A.K.; Mullholand, J.B.; MacKay, S.A.; Barik, S.; Coleman, J.J.; Paules, C.I.; Tinsley, A. Physiologic Response to the Pfizer-BioNTech COVID-19 Vaccine Measured Using Wearable Devices: Prospective Observational Study. JMIR Form. Res. 2021, 5, e28568. [Google Scholar] [CrossRef]
- Gepner, Y.; Mofaz, M.; Oved, S.; Yechezkel, M.; Constantini, K.; Goldstein, N.; Eisenkraft, A.; Shmueli, E.; Yamin, D. Utilizing wearable sensors for continuous and highly-sensitive monitoring of reactions to the BNT162b2 mRNA COVID-19 vaccine. Commun. Med. 2022, 2, 27. [Google Scholar] [CrossRef]
- Mason, A.E.; Kasl, P.; Hartogensis, W.; Natale, J.L.; Dilchert, S.; Dasgupta, S.; Purawat, S.; Chowdhary, A.; Anglo, C.; Veasna, D.; et al. Metrics from Wearable Devices as Candidate Predictors of Antibody Response Following Vaccination against COVID-19: Data from the Second TemPredict Study. Vaccines 2022, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Mofaz, M.; Yechezkel, M.; Guan, G.; Brandeau, M.L.; Patalon, T.; Gazit, S.; Yamin, D.; Shmueli, E. Self-Reported and Physiologic Reactions to Third BNT162b2 mRNA COVID-19 (Booster) Vaccine Dose. Emerg. Infect. Dis. 2022, 28, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Eldokla, A.M.; Numan, M.T. Postural orthostatic tachycardia syndrome after mRNA COVID-19 vaccine. Clin. Auton. Res. 2022, 32, 307–311. [Google Scholar] [CrossRef]
- Inbaraj, G.; Udupa, K.; Vasuki, P.P.; Nalini, A.; Sathyaprabha, T.N. Resting heart rate variability as a diagnostic marker of cardiovascular dysautonomia in postural tachycardia syndrome. J. Basic Clin. Physiol. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- DeGiorgio, C.M.; Miller, P.; Meymandi, S.; Chin, A.; Epps, J.; Gordon, S.; Gornbein, J.; Harper, R.M. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: The SUDEP-7 Inventory. Epilepsy Behav. 2010, 19, 78–81. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Chen, L.Y.; Nazarian, S.; Soliman, E.Z. Reference ranges for short-term heart rate variability measures in individuals free of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Electrocardiol. 2016, 49, 686–690. [Google Scholar] [CrossRef]
- Sampath, V.; Rabinowitz, G.; Shah, M.; Jain, S.; Diamant, Z.; Jesenak, M.; Rabin, R.; Vieths, S.; Agache, I.; Akdis, M.; et al. Vaccines and allergic reactions: The past, the current COVID-19 pandemic, and future perspectives. Allergy 2021, 76, 1640–1660. [Google Scholar] [CrossRef]
- Bellomo, R.G.; Gallenga, C.E.; Caraffa, A.; Tetè, G.; Ronconi, G.; Conti, P. Anaphylaxis is a rare reaction in COVID-19 vaccination. J. Biol. Regul. Homeost Agents 2021, 35, 839–842. [Google Scholar] [CrossRef]
- Souza, H.C.D.; Philbois, S.V.; Veiga, A.C.; Aguilar, B.A. Heart Rate Variability and Cardiovascular Fitness: What We Know so Far. Vasc. Health Risk Manag. 2021, 17, 701–711. [Google Scholar] [CrossRef]
- Bourdillon, N.; Yazdani, S.; Schmitt, L.; Millet, G.P. Effects of COVID-19 lockdown on heart rate variability. PLoS ONE 2020, 15, e0242303. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Stein, P.K. Heart rate variability in risk stratification of cardiac patients. Prog. Cardiovasc. Dis. 2013, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]


| Author (Country) | Study Type | Sample Size (M:F) | Population (Mean Age, Yr) | Type of Vaccinations (Dose) | Assessment Tool for HRV | HRV Parameters (Unit) |
|---|---|---|---|---|---|---|
| Hajduczok 2021 (U.S.) | prospective cohort study | 19 (9:10) | adult (28.8 ± 2.2) | Pfizer-BioNTech COVID-19 vaccine (first and second doses) | commercial wearable sensor on wrist (WHOOP Strap) | 1. RMSSD (change, %) |
| Gepner 2022 (U.S.) | prospective cohort study | 160 (70:90) | adult (median: 40, range: 21–78) | Pfizer-BioNTech COVID-19 vaccine (second dose) | FDA-approved chest-patch sensor | 1. RMSSD (per ten beats, %) |
| Mason 2022 (U.S.) | prospective cohort study | 1. Pfizer-BioNTech: 706 (334:371, others: a participant classified as intersex) 2. Moderna: 366 (166:200) 3. J/J&J: 107 (53:54) | 1. 50.4 ± 11.4 2. 52.8 ± 12.0 3. 49.5 ± 9.9 | Pfizer-BioNTech, Moderna, J/J&J (first and second doses, except for J/J&J) | commercial wearable sensor on finger (Oura Ring) | 1. RMSSD (Spearman’s rank order correlation coefficient) |
| Mofaz 2022 (Israel) | prospective cohort study | 1609 (755:854) | adult (median: 52, range: 18–88) | Pfizer-BioNTech COVID-19 vaccine (first, second, and booster doses) | commercial wearable sensor on wrist (Garmin Vivo-smart 4 smartwatches) | 1. Garmin’s stress level (HRV-based stress indicator) |
| Presby 2022 (U.S.) | retrospective analysis | first dose/second dose 1. AstraZeneca: 3457 (870:2587) / 325 (86:239) 2. J/J&J: 4584 (1416:3168) 3. Moderna: 17,632 (6063:11,569)/16,987 (6025:10,962) 4. Pfizer/BioNTech: 29,366 (10,027:19,339)/27,084 (9614:17,470) | adult (18 or older) | AstraZeneca, J/J&J, Moderna, or Pfizer/BioNTech vaccine (not limited) | commercial wearable sensor on wrist (WHOOP Strap) | 1. RMSSD (ms) |
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hajduczok 2021 | Y | Y | Y | Y | N | Y | Y | Y | Y | NA | Y | Y | N | N |
| Gepner 2022 | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | NR | N | N |
| Mason 2022 | Y | Y | N | Y | N | Y | Y | Y | Y | NA | Y | NR | N | N |
| Mofaz 2022 | Y | Y | Y | Y | N | Y | Y | Y | Y | NA | Y | NR | NA | N |
| Presby 2022 | Y | Y | NR | Y | N | Y | Y | Y | Y | NA | Y | NR | NR | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, C.-Y.; Lee, B. Impact of COVID-19 Vaccination on Heart Rate Variability: A Systematic Review. Vaccines 2022, 10, 2095. https://doi.org/10.3390/vaccines10122095
Kwon C-Y, Lee B. Impact of COVID-19 Vaccination on Heart Rate Variability: A Systematic Review. Vaccines. 2022; 10(12):2095. https://doi.org/10.3390/vaccines10122095
Chicago/Turabian StyleKwon, Chan-Young, and Boram Lee. 2022. "Impact of COVID-19 Vaccination on Heart Rate Variability: A Systematic Review" Vaccines 10, no. 12: 2095. https://doi.org/10.3390/vaccines10122095
APA StyleKwon, C.-Y., & Lee, B. (2022). Impact of COVID-19 Vaccination on Heart Rate Variability: A Systematic Review. Vaccines, 10(12), 2095. https://doi.org/10.3390/vaccines10122095







