The COVID-19 Vaccine and Pregnant Minority Women in the US: Implications for Improving Vaccine Confidence and Uptake
Abstract
:1. Introduction
2. Historical Perspectives on Vaccination during Pregnancy
3. The Historical Impact of the Influenza Vaccine on Pregnancy Care
4. The Historical Impact of the Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis (Tdap) Vaccine on Pregnancy Care
5. The Historical Impact of the Pertussis Vaccine on Pregnancy Care
6. COVID-19 Vaccination Trials for Pregnant and Lactating Women
7. The Safety of the mRNA COVID-19 Vaccines during Pregnancy
8. COVID-19 Morbidity and Mortality in Pregnant Minority Women in the US
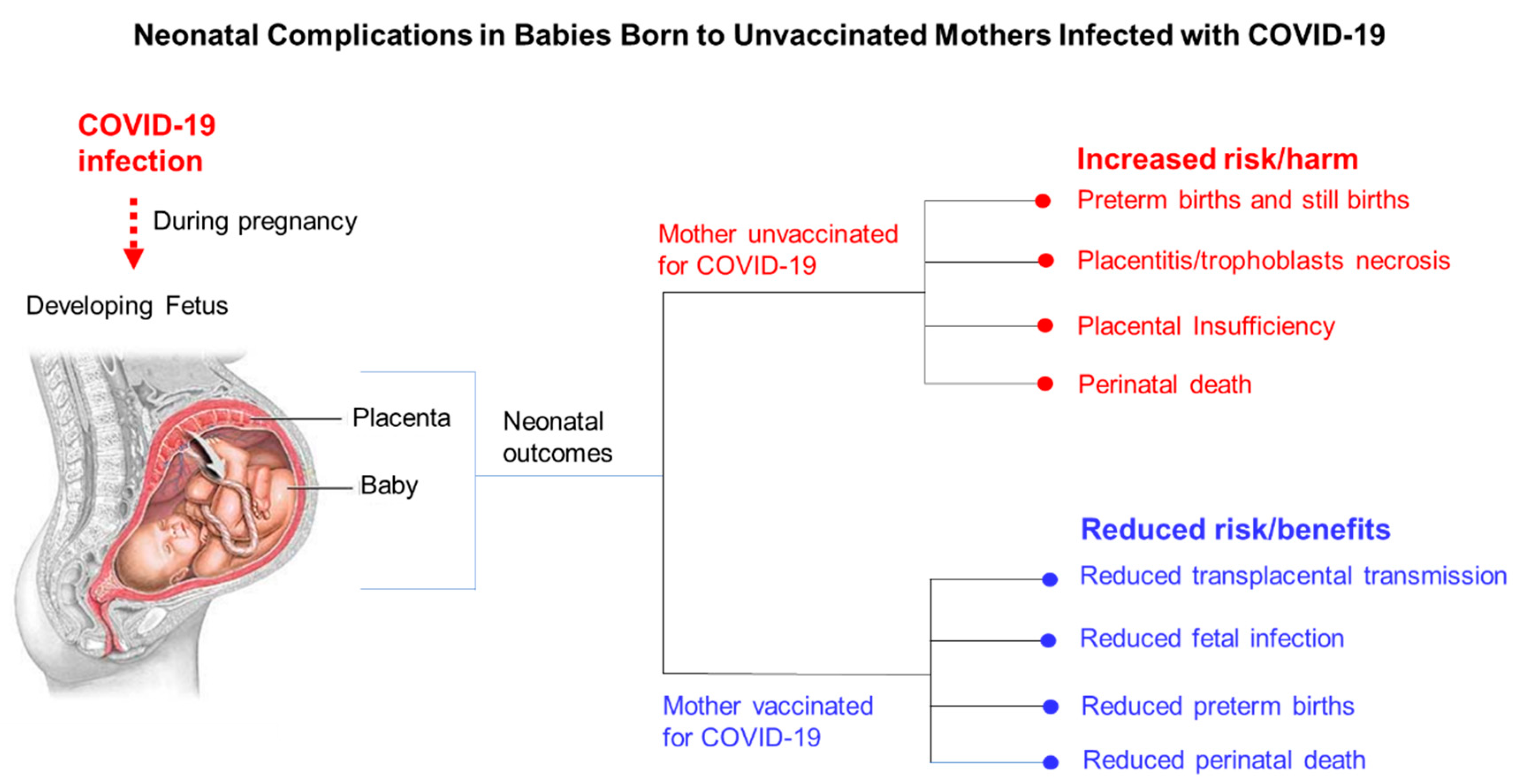
9. Obstetric Complications Associated with Symptomatic COVID-19 in Pregnant Women
10. COVID-19 Vaccine Hesitancy among Pregnant Minority Women
11. Racial Disparities Experienced by Pregnant Minority Women Due to the COVID-19 Pandemic and the Potential Impact on Pregnancy Care
12. Strategies to Improve COVID-19 Vaccine Confidence and Uptake among Pregnant Minority Women in the US
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2022, 2, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ellington, S.; Strid, P.; Tong, V.T.; Woodworth, K.; Galang, R.R.; Zambrano, L.D.; Nahabedian, J.; Anderson, K.; Gilboa, S.M. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, 22 January. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Portilla, R.J.; Sotiriadis, A.; Chatzakis, C.; Torres-Torres, J.; Espino y Sosa, S.; Sandoval-Mandujano, K.; Castro-Bernabe, D.A.; Medina-Jimenez, V.; Monarrez-Martin, J.C.; Figueras, F.; et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: Propensity score matched analysis of a nationwide prospective cohort (COV19Mx). Ultrasound Obstet. Gynecol. 2021, 57, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, M.; Neovius, M.; Saltvedt, S.; Söderling, J.; Pettersson, K.; Brandkvist, C.; Stephansson, O. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA 2020, 324, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Kadiwar, S.; Smith, J.J.; Ledot, S.; Ledot, S.; Johnson, M.; Bianchi, P.; Singh, N.; Montanaro, C.; Gatzoulis, M.; Shah, N.; et al. Were pregnant women more affected by COVID-19 in the second wave of the pandemic? Lancet 2021, 10284, 1539–1540. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.M.; Hughes, B.L.; Swamy, G.K. Coronavirus disease 2019 vaccines in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 2, 100295. [Google Scholar] [CrossRef]
- Molina, R.L.; Tsai, T.C.; Dai, D.; Soto, M.; Rosenthal, N.; Orav, E.J.; Figueroa, J.F. Comparison of Pregnancy and Birth Outcomes Before vs during the COVID-19 Pandemic. JAMA Netw. Open 2022, 8, e2226531. [Google Scholar] [CrossRef]
- Fallach, N.; Segal, Y.; Agassy, J.; Perez, G.; Peretz, A.; Chodick, G.; Gazit, S.; Patalon, T.; Ben Tov, A.; Goldshtein, I. Pregnancy outcomes after SARS-CoV-2 infection by trimester: A large, population-based cohort study. PLoS ONE 2022, 7, e0270893. [Google Scholar] [CrossRef]
- Seasely, A.R.; Blanchard, C.T.; Arora, N.; Battarbee, A.N.; Casey, B.M.; Dionne-Odom, J.; Leal, S.M., Jr.; Moates, D.B.; Sinkey, R.G.; Szychowski, J.M.; et al. CWRH’s COVID-19 Working Group. Maternal and Perinatal Outcomes Associated with the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Obstet. Gynecol. 2022, 2, 262–265. [Google Scholar] [CrossRef]
- Morgan, J.A.; Morgan, J.A.; Biggio, J.R., Jr.; Martin, J.K.; Mussarat, N.; Chawla, H.K.; Puri, P.; Williams, F.B. Maternal outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet. Gynecol. 2022, 139, 107–109. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Olsen, E.O.; Neelam, V.; Lewis, E.L.; Galang, R.R.; Oduyebo, T.; Aveni, K.; Yazdy, M.M.; Harvey, E.; Longcore, N.D.; et al. CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT). Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy—SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 44, 1635–1640. [Google Scholar]
- Rasmussen, S.A.; Jamieson, D.J. Pregnancy, postpartum care, and COVID-19 vaccination in 2021. JAMA 2021, 325, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Information about COVID-19 Vaccines for People Who Are Pregnant or Breastfeeding. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html (accessed on 13 May 2021).
- American College of Obstetricians and Gynecologists. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. 2021. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care (accessed on 16 November 2022).
- The American College of Obstetricians and Gynecologists. Statement of Strong Medical Consensus for Vaccination of Pregnant Individuals Against COVID-19. 2021. Available online: https://www.acog.org/news/news-releases/2021/08/statement-of-strong-medical-consensus-for-vaccination-of-pregnant-individuals-against-covid-19 (accessed on 14 September 2021).
- CDC—Centers for Disease Control and Prevention. COVID-19 Vaccination among Pregnant People Aged 18–49 Years Overall, by Race/Ethnicity, and Date Reported to CDC—Vaccine Safety Datalink, * United States. 2021. Available online: https://covid.cdc.gov/coviddata-tracker/#vaccinations-pregnant-women (accessed on 18 November 2022).
- Edlow, A.G.; Li, J.Z.; Ai-ris, Y.C.; Atyeo, C.; James, K.E.; Boatin, A.A.; Gray, K.J.; Bordt, E.A.; Shook, L.L.; Yonker, L.M. Assessment of maternal and neonatal SARS-CoV2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open 2020, 3, e2030455. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; Weirick, M.E.; et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021, 175, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Nir, O.; Schwartz, A.; Toussia-Cohen, S.; Leibovitch, L.; Strauss, T.; Asraf, K.; Doolman, R.; Sharabi, S.; Cohen, C.; Lustig, Y.; et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am. J. Obstet. Gynecol. 2022, 4, 100492. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.R.; Rai, D.S.; Huang, A.; Flores, C.V.; Lin, C.Y.; Jigmeddagva, U.; Wu, A.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef]
- Prahl, M.; Golan, Y.; Cassidy, A.G.; Matsui, Y.; Li, L.; Alvarenga, B.; Chen, H.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; et al. Evaluation of transplacental transfer of mRNA vaccine products and functional antibodies during pregnancy and infancy. Nat. Commun. 2022, 1, 4422. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E.; et al. Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months—17 States, July 2021-January. MMWR Morb. Mortal. Wkly. Rep. 2022, 7, 264–270. [Google Scholar]
- Centers for Disease Control Maternal Vaccination Coverage. Available online: https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/maternal-vaccination-coverage.html (accessed on 10 August 2017).
- Mackin, D.W.; Walker, S.P. The historical aspects of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 13–22. [Google Scholar] [CrossRef]
- Tan, E.K.; Tan, E.L. Alterations in physiology and anatomy during pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 6, 791–802. [Google Scholar] [CrossRef]
- Nitsch-Osuch, A.; Korzeniewski, K.; Gawlak, M.; Życińska, K.; Wardyn, K.; Kuchar, E. Epidemiological and clinical reasons for vaccination against pertussis and influenza in pregnant women. Adv. Exp. Med. Biol. 2015, 849, 11–21. [Google Scholar] [PubMed]
- Marshall, H.; McMillan, M.; Andrews, R.M.; Macartney, K.; Edwards, K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum. Vaccin. Immunother. 2016, 4, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Walls, T.; Graham, P.; Petousis-Harris, H.; Hill, L.; Austin, N. Infant outcomes after exposure to Tdap vaccine in pregnancy: An observational study. BMJ Open 2016, 1, e009536. [Google Scholar] [CrossRef] [Green Version]
- Baxter, R.; Bartlett, J.; Fireman, B.; Lewis, E.; Klein, N.P. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics 2017, 139, 20164091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.G.; Li, D.-K.; Shifflett, P.; Sokolow, L.Z.; Ferber, J.R.; Kurosky, S.; Bozeman, S.; Reynolds, S.B.; Odouli, R.; Henninger, M.L.; et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: A population-based case-control study during the 2010-2011 and 2011-2012 influenza seasons. Clin. Infect. Dis. 2014, 58, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engmann, C.; Fleming, J.A.; Khan, S.; Innis, B.L.; Smith, J.M.; Hombach, J.; Sobanjo-Ter Meulen, A. Closer and closer? Maternal immunization: Current promise, future horizons. J. Perinatol. 2020, 6, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Faucette, A.N.; Pawlitz, M.D.; Pei, B.; Yao, F.; Chen, K. Immunization of pregnant women: Future of early infant protection. Hum. Vaccin. Immunother. 2015, 11, 2549–2555. [Google Scholar] [CrossRef] [Green Version]
- Woolston, W.J.; Conley, D.O. Epidemic pneumonia (Spanish Influenza) in pregnancy: Effect in one hundred and one cases. J. Am. Med. Assoc. 1918, 23, 1898–1899. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.Q. Influenza occurring in pregnant women: A statistical study of thirteen hundred and fifty cases. J. Am. Med. Assoc. 1919, 14, 978–980. [Google Scholar] [CrossRef]
- Greenberg, M.; Jacobziner, H.; Pakter, J.; Weisl, B.A. Maternal mortality in the epidemic of Asian influenza, New York City. Am. J. Obstet. Gynecol. 1958, 4, 897–902. [Google Scholar] [CrossRef]
- Meiklejohn, G. Viral respiratory disease at lowry air force base in denver, 1952. J. Infect Dis. 1983, 5, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.; Salk, J.E.; Pearson, H.E.; Brown, P.N. Protective effect of vaccination against induced influenza A. J. Clin. Investig. 1945, 4, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Burney, L.E. Influenza immunization: Statement. Publ. Health Rep. 1960, 10, 944. [Google Scholar] [CrossRef]
- Centers for Disease Control Prevention and control of influenza: Recommendations of the advisory committee on immunization practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 1997, 46, 1–25.
- Hulka, J.F. Effectiveness of polyvalent influenza vaccine in pregnancy. Report of a controlled study during an outbreak of asian influenza. Obstet. Gynecol. 1964, 23, 830–837. [Google Scholar]
- Sumaya, C.V.; Gibbs, R.S. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: Reactogenicity and immunogenicity in mother and infant. J. Infect Dis. 1979, 2, 141–146. [Google Scholar] [CrossRef]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 5, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.C.; Katz, J.; Englund, J.A.; Khatry, S.K.; Shrestha, L.; Kuypers, J. Year-round influenza immunisation during pregnancy in Nepal: A phase 4, randomised, placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Flu and Tdap Vaccination Coverage among Pregnant Women—United States, April Centers for Disease Control and Prevention. Updated 7 October 2022. Available online: https://www.cdc.gov/flu/fluvaxview/pregnant-women-apr2021.htm (accessed on 10 January 2022).
- Nowak, G.J.; Sheedy, K.; Bursey, K.; Smith, T.; Basket, M. Promoting influenza vaccination: Insights from a qualitative meta-analysis of 14 years of influenza-related communications research by U.S. Centers for Disease Control and Prevention (CDC). Vaccine 2015, 24, 2741–2756. [Google Scholar] [CrossRef]
- Lindley, M.C.; Kahn, K.E.; Bardenheier, B.H.; D’Angelo, D.V.; Dawood, F.S.; Fink, R.V.; Havers, F.; Skoff, T.H. Vital Signs: Burden and Prevention of Influenza and Pertussis among Pregnant Women and Infants—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 40, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Arditi, B.; Riley, L.E.; Sobhani, N.C.; Norton, M.; D’Alton, M.; Friedman, A.M.; Venkatesh, K.K. Influenza Complicating Delivery Hospitalization and Its Association With Severe Maternal Morbidity in the United States, 2000. Obstet. Gynecol. 2021, 2, 218–227. [Google Scholar] [CrossRef] [PubMed]
- CDC—Centers for Disease Control and Prevention. Flu and Tdap Vaccination Coverage Among Pregnant Women—United States, April 2021. Available online: https://www.cdc.gov/flu/fluvaxview/pregnant-women-apr2021.htm (accessed on 7 October 2021).
- Munoz, F.M.; Bond, N.H.; Maccato, M.; Pinell, P.; Hammill, H.A.; Swamy, G.K.; Walter, E.B.; Jackson, L.A.; Englund, J.A.; Edwards, M.S.; et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: A randomized clinical trial. JAMA 2014, 17, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Kanu, F.A.; Yusuf, N.; Kassogue, M.; Ahmed, B.; Tohme, R.A. Progress Toward Achieving and Sustaining Maternal and Neonatal Tetanus Elimination—Worldwide, 2000. MMWR Morb. Mortal. Wkly. Rep. 2022, 11, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Budhathoki, S.; Manandhar, S. Tetanus. Kathmandu Univ. Med. J. 2009, 27, 315–322. [Google Scholar] [CrossRef]
- Roper, M.H.; Vandelaer, J.H.; Gasse, F.L. Maternal and neonatal tetanus. Lancet 2007, 370, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Surendran, N. Neonatal vaccination: Challenges and intervention strategies. Neonatology 2016, 3, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Thwaites, C.L.; Loan, H.T. Eradication of tetanus. Br. Med. Bull. 2015, 116, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Thwaites, C.L.; Beeching, N.J.; Newton, C.R. Maternal and neonatal tetanus. Lancet 2015, 385, 362–370. [Google Scholar] [CrossRef] [Green Version]
- WHO. Maternal and Neonatal Tetanus (MNT) Elimination. Available online: http://www.who.int/immunization/diseases/MNTE_initiative/en (accessed on 4 April 2020).
- Cherry, J.D. Epidemic pertussis in 2012–the resurgence of a vaccine-preventable disease. N. Engl. J. Med. 2012, 9, 785–787. [Google Scholar] [CrossRef]
- Rohani, P.; Drake, J.M. The decline and resurgence of pertussis in the US. Epidemics 2011, 3, 183–188. [Google Scholar] [CrossRef]
- Chow, M.Y.; Khandaker, G.; McIntyre, P. Global childhood deaths from pertussis: A historical review. Clin. Infect. Dis. 2016, 4, S134–S141. [Google Scholar] [CrossRef] [Green Version]
- Update on immunization and pregnancy: Tetanus, diphtheria, and pertussis vaccination. Committee Opinion No. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017, 130, e153. [CrossRef] [PubMed]
- Academy of Breastfeeding Medicine Considerations for COVID-19 Vaccination in Lactation. Available online: https://abm.memberclicks.net/abm-statement-considerationsfor-covid-19-vaccination-in-lactation (accessed on 5 June 2021).
- Kotlar, B.; Gerson, E.; Petrillo, S.; Langer, A.; Tiemeier, H. The impact of the COVID-19 pandemic on maternal and perinatal health: A scoping review. Reprod. Health. 2021, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Galang, R.R.; Newton, S.M.; Woodworth, K.R.; Griffin, I.; Oduyebo, T.; Sancken, C.L.; Olsen, E.O.; Aveni, K.; Wingate, H.; Shephard, H.; et al. Centers for Disease Control and Prevention COVID-19 Response Pregnancy and Infant Linked Outcomes Team. Risk Factors for Illness Severity Among Pregnant Women With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection-Surveillance for Emerging Threats to Mothers and Babies Network, 22 State, Local, and Territorial Health Departments, 29 March 2020-5 March. Clin Infect Dis. 2021, 1, S17–S23. [Google Scholar]
- Mullins, E.; Hudak, M.L.; Banerjee, J.; Getzlaff, T.; Townson, J.; Barnette, K.; Playle, R.; Perry, A.; Bourne, T.; Lees, C.C.; et al. Pregnancy and neonatal outcomes of COVID-19: Coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obs. Gynecol. 2021, 57, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Van Spall, H.G.; Toren, A.; Kiss, A.; Fowler, R.A. Eligibility criteria of randomized controlledtrials published in high-impact general medical journals: A systematic sampling review. JAMA 2007, 297, 1233–1240. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Van Spall, H.G.C. Exclusion of pregnant and lactating women from COVID-19 vaccine trials: A missed opportunity. Eur. Heart J. 2021, 28, 2724–2726. [Google Scholar] [CrossRef]
- Smith, D.D.; Pippen, J.L.; Adesomo, A.A.; Rood, K.M.; Landon, M.B.; Costantine, M.M. Exclusion of Pregnant Women from Clinical Trials during the Coronavirus Disease 2019 Pandemic: A Review of International Registries. Am. J. Perinatol. 2020, 8, 792–799. [Google Scholar] [CrossRef]
- Garg, I.; Shekhar, R.; Sheikh, A.B.; Pal, S. COVID-19 Vaccine in Pregnant and Lactating Women: A Review of Existing Evidence and Practice Guidelines. Infect. Dis. Rep. 2021, 3, 685–699. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Kaeser, L.; Cernich, A.N. Involving Pregnant Individuals in Clinical Research on COVID-19 Vaccines. JAMA 2021, 325, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.E.; Jamieson, D.J. Inclusion of Pregnant and Lactating Persons in COVID-19 Vaccination Efforts. Ann. Intern. Med. 2021, 174, 701–702. [Google Scholar] [CrossRef] [PubMed]
- HHS Task Force on Research Specific to Pregnant Women and Lactating Women. Available online: https://www.nichd.nih.gov/sites/default/files/2018-09/PRGLAC_Report.pdf (accessed on 5 June 2021).
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 3, 303.e1–303.e17. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Effectiveness and Safety of COVID-19 Vaccine among Pregnant Women in Real-World Studies: A Systematic Review and Meta-Analysis. Vaccines 2022, 2, 246. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. CDC v-safe COVID-19 Pregnancy Registry Team. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 24, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.E.; Declercq, E.R. All-Cause Maternal Mortality in the US Before vs During the COVID-19 Pandemic. JAMA Netw. Open 2022, 6, e2219133. [Google Scholar] [CrossRef]
- Hoyert, D.L. Maternal Mortality Rates in the United States. Health E-Stat. 23 February 2022. Available online: https://www.cdc.gov/nchs/data/hestat/maternal-mortality/2020/E-stat-Maternal-Mortality-Rates-2022.pdf (accessed on 5 May 2022).
- Chinn, J.; Sedighim, S.; Kirby, K.A.; Hohmann, S.; Hameed, A.B.; Jolley, J.; Nguyen, N.T. Characteristics and Outcomes of Women With COVID-19 Giving Birth at US Academic Centers During the COVID-19 Pandemic. JAMA Netw. Open 2021, 8, e2120456. [Google Scholar] [CrossRef]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J. UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population-based cohort study. BMJ 2020, 8, m2107. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 8, 817–826. [Google Scholar] [CrossRef]
- Schwartz, D.A. Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy. Viruses 2022, 3, 458. [Google Scholar] [CrossRef]
- Patanè, L.; Morotti, D.; Giunta, M.R.; Sigismondi, C.; Piccoli, M.G.; Frigerio, L.; Mangili, G.; Arosio, M.; Cornolti, G. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2020, 2, 100145. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, F.; Bugatti, M.; Drera, E.; Tripodo, C.; Sartori, E.; Cancila, V.; Papaccio, M.; Castellani, R.; Casola, S.; Boniotti, M.B.; et al. SARS-CoV-2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine 2020, 59, 102951. [Google Scholar] [CrossRef]
- Sisman, J.; Jaleel, M.A.; Moreno, W.; Rajaram, V.; Collins, R.R.J.; Savani, R.C.; Rakheja, D.; Evans, A.S. Intrauterine Transmission of SARS-COV-2 Infection in a Preterm Infant. Pediatr Infect. Dis. J. 2020, 9, e265–e267. [Google Scholar] [CrossRef] [PubMed]
- Pulinx, B.; Kieffer, D.; Michiels, I.; Petermans, S.; Strybol, D.; Delvaux, S.; Baldewijns, M.; Raymaekers, M.; Cartuyvels, R.; Maurissen, W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2441–2445. [Google Scholar] [CrossRef] [PubMed]
- Theiler, R.N.; Wick, M.; Mehta, R.; Weaver, A.L.; Virk, A.; Swift, M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 6, 100467. [Google Scholar] [CrossRef]
- Fazio, N.D.; Delogu, G.; Bertozzi, G.; Fineschi, V.; Frati, P. SARS-CoV2 Vaccination Adverse Events Trend in Italy: A Retrospective Interpretation of the Last Year (December 2020–September 2021). Vaccines 2022, 2, 216. [Google Scholar] [CrossRef]
- MacDonald, N.E. SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 34, 4161–4164. [Google Scholar] [CrossRef]
- Bhattacharya, O.; Siddiquea, B.N.; Shetty, A.; Afroz, A.; Billah, B. COVID-19 vaccine hesitancy among pregnant women: A systematic review and meta-analysis. BMJ Open 2022, 8, e061477. [Google Scholar] [CrossRef]
- Hildreth, J.E.K.; Alcendor, D.J. Targeting COVID-19 Vaccine Hesitancy in Minority Populations in the US: Implications for Herd Immunity. Vaccines 2021, 5, 489. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: An overview. Hum. Vaccin. Immunother. 2013, 8, 1763–1773. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, M.K.; Mehl, R.; Costantine, M.M.; Johnson, A.; Cohen, J.; Summerfield, T.L.; Landon, M.B.; Rood, K.M.; Venkatesh, K.K. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: A cross-sectional study. BJOG 2022, 8, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Battarbee, A.N.; Stockwell, M.S.; Varner, M.; Newes-Adeyi, G.; Daugherty, M.; Gyamfi-Bannerman, C.; Tita, A.T.; Vorwaller, K.; Vargas, C.; Subramaniam, A.; et al. Attitudes Toward COVID-19 Illness and COVID-19 Vaccination among Pregnant Women: A Cross-Sectional Multicenter Study during August-December. Am. J. Perinatol. 2022, 1, 75–83. [Google Scholar]
- Germann, K.; Kiefer, M.K.; Rood, K.M.; Mehl, R.; Wu, J.; Pandit, R.; Lynch, C.D.; Landon, M.B.; Grobman, W.A.; Costantine, M.M.; et al. Association of initial COVID-19 vaccine hesitancy with subsequent vaccination among pregnant and postpartum individuals. BJOG 2022, 8, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Erchick, D.J.; Agarwal, S.; Kaysin, A.; Gibson, D.G.; Labrique, A.B. Changes in prenatal care and vaccine willingness among pregnant women during the COVID-19 pandemic. BMC Pregnancy Childbirth 2022, 1, 558. [Google Scholar] [CrossRef] [PubMed]
- Ross-Davie, M.; Brodrick, A.; Randall, W.; Kerrigan, A.; McSherry, M. Labour and birth. Best Pract. Res. Clin. Obstet Gynaecol. 2021, 73, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, E. Shattering the Infertility Myth: What We Know about COVID-19 Vaccines and Pregnancy. STAT. Available online: https://www.statnews.com/2021/03/25/infertility-myth-covid-19-vaccines-pregnancy/ (accessed on 13 May 2021).
- Yancy, C.W. COVID-19 and African Americans. JAMA 2020, 19, 1891. [Google Scholar] [CrossRef]
- Giurgescu, C.; Misra, D.P.; Sealy-Jefferson, S.; Caldwell, C.H.; Templin, T.N.; Slaughter-Acey, J.C.; Osypuk, T.L. The impact of neighborhood quality, perceived stress, and social support on depressive symptoms during pregnancy in African American women. Soc. Sci. Med. 2015, 130, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Huldani, H.; Kamal Abdelbasset, W.; Abdalkareem Jasim, S.; Suksatan, W.; Turki Jalil, A.; Thangavelu, L.; Fakri Mustafa, Y.; Karami, M. Intimate partner violence against pregnant women during the COVID-19 pandemic: A systematic review and meta-analysis. Women Health 2022, 6, 556–564. [Google Scholar] [CrossRef]
- Gu, T.; Mack, J.A.; Salvatore, M.; Prabhu Sankar, S.; Valley, T.S.; Singh, K.; Nallamothu, B.K.; Kheterpal, S.; Lisabeth, L.; Fritsche, L.G.; et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw. Open 2020, 10, e2025197. [Google Scholar] [CrossRef]
- Coughlin, C.G.; Sandel, M.; Stewart, A.M. Homelessness, Children, and COVID-19: A Looming Crisis. Pediatrics 2020, 2, e20201408. [Google Scholar] [CrossRef]
- Artiga, S.; Garfield, R.; Orgera, K. Communities of Color at Higher Risk for Health and Economic Challenges Due to COVID-19. Available online: https://www.kff.org/coronavirus-covid-19/issue-brief/communities-of-color-at-higher-risk-for-health-and-economic-challenges-due-to-covid-19/ (accessed on 7 April 2020).
- Hung, P.; Henning-Smith, C.E.; Casey, M.M.; Kozhimannil, K.B. Access to obstetric services in rural counties still declining, with 9 percent losing services, 2004. Health Affairs 2017, 9, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Novoa, C.; Hamm, K.; Phadke, S. Eliminating Racial Disparities in Maternal and Infant Mortality: A Comprehensive Policy Blueprint. Center for American Progress. 2019. Available online: https://www.americanprogress.org/issues/women/reports/2019/05/02/469186/eliminating-racial-disparities-maternal-infant-mortality/ (accessed on 2 May 2019).
- Wolf, E.R.; Donahue, E.; Sabo, R.T.; Nelson, B.B.; Krist, A.H. Barriers to attendance of prenatal and well-child visits. Acad. Pediatrics 2020, S1876–S2859, 30635. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Meghani, M.; Pingali, C.; Crane, B.; Naleway, A.; Weintraub, E.; Kenigsberg, T.A.; Lamias, M.J.; Irving, S.A.; Kauffman, T.L.; et al. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy—Eight Integrated Health Care Organizations, United States, 14 December 2020–8 May 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 24, 895–899. [Google Scholar] [CrossRef]
- CDC—Centers for Disease Control and Prevention. 12 COVID-19 Vaccination Strategies for Your Community. Available online: https://www.cdc.gov/vaccines/covid-19/vaccinate-with-confidence/community.html (accessed on 3 November 2021).
- Schuster, M.; Eskola, J.; Duclos, P. the SAGE Working Group on Vaccine Hesitancy. Review of vaccine hesitancy: Rationale, remit and methods. Vaccine 2015, 34, 4157–4160. [Google Scholar] [CrossRef] [PubMed]
- Cockerham, W.C. Health lifestyle theory and the convergence of agency and structure. J. Health Soc. Behav. 2005, 1, 51–67. [Google Scholar] [CrossRef] [Green Version]
- White, R.M. Driving Miss Evers’ Boys to the Historical Tuskegee Study of Untreated Syphilis. J. Natl. Med. Assoc. 2019, 4, 371–382. [Google Scholar] [CrossRef]
- Cobb, W.M. The Tuskegee syphilis study. J. Natl. Med. Assoc. 1973, 4, 345–348. [Google Scholar]
- Henrietta, L. Science must right a historical wrong. Nature 2020, 7823, 7. [Google Scholar]
- Lehner, L.; Gribi, J.; Hoffmann, K.; Paul, K.T.; Kutalek, R. Beyond the “information deficit model”—Understanding vaccine-hesitant attitudes of midwives in Austria: A qualitative study. BMC Public Health 2021, 1, 1671. [Google Scholar] [CrossRef]
- Fadda, M.; Suggs, L.S.; Albanese, E. Willingness to vaccinate against Covid-19: A qualitative study involving older adults from Southern Switzerland. Vaccine X 2021, 8, 100108. [Google Scholar] [CrossRef]
- Streefland, P.; Chowdhury, A.M.R.; Ramos-Jimenez, P. Patterns of vaccination acceptance. Soc. Sci. Med. 1999, 12, 1705–1716. [Google Scholar] [CrossRef]
- Hobson-West, P. Understanding vaccination resistance: Moving beyond risk. Health Risk Soc. 2003, 3, 273–283. [Google Scholar] [CrossRef]
- Wigham, S.; Ternent, L.; Bryant, A.; Robalino, S.; Sniehotta, F.F.; Adams, J. Parental financial incentives for increasing preschool vaccination uptake: Systematic review. Pediatrics 2014, 4, e1117–e1128. [Google Scholar] [CrossRef] [Green Version]
- Cairns, G.; MacDonald, L.; Angus, K.; Walker, L.; Cairns-Haylor, T.; Bowdler, T. Systematic Literature Review of the Evidence for Effective National Immunisation Schedule Promotional Communications; ECDC—European Centre for Disease Prevention and Control: Stockholm, Sweden, 2012; p. 89. [Google Scholar]
- Oyo-Ita, A.; Nwachukwu, C.E.; Oringanje, C.; Meremikwu, M.M. Interventions for improving coverage of child immunization in low- and middle-income countries. Cochrane Database Syst. Rev. 2011, 7, CD008145. [Google Scholar]
- Fu, L.Y.; Bonhomme, L.A.; Cooper, S.C.; Joseph, J.G.; Zimet, G.D. Educational interventions to increase HPV vaccination acceptance: A systematic review. Vaccine 2014, 17, 1901–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, J.; Synnot, A.; Ryan, R.; Hill, S.; Horey, D.; Willis, N. Face to face interventions for informing or educating parents about early childhood vaccination. Cochrane Database Syst. Rev. 2013, 5, CD010038. [Google Scholar] [CrossRef]
- Odone, A.; Ferrari, A.; Spagnoli, F.; Visciarelli, S.; Shefer, A.; Pasquarella, C.; Signorelli, C. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum. Vaccin. Immunother. 2015, 1, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Community Preventive Service Task Force. Guide to Community Preventive Services. Increasing Appropriate Vaccination. Available online: http://www.thecommunityguide.org/vaccines/index.html (accessed on 14 August 2021).
- Leask, J.; Willaby, H.W.; Kaufman, J. The big picture in addressing vaccine hesitancy. Hum. Vaccin. Immunother. 2014, 9, 1–3. [Google Scholar] [CrossRef]
- Anderson, E.L. Recommended solutions to the barriers to immunization in children and adults. Mo Med. 2014, 4, 344–348. [Google Scholar]


| Challenges | Solutions |
|---|---|
| Safety concerns and side effects for themselves and for their babies | Peer to peer communications to improve vaccine confidence and uptake |
| Distrust of medical providers and the government | Town hall meetings with pregnant minority women and medical providers of vaccines of the same race and ethnicity |
| Misinformation about the COVID-19 vaccine effects on fertility | Open discussions on social media platforms with medical providers and pregnant women to discuss vaccine safety regarding fertility |
| Unaware of the benefits of being vaccinated for COVID-19 during pregnancy | Community engagement health forums with pregnant women and OBGYN medical providers |
| Fear due to lack of research on the vaccines and its potential harm specific to minority communities | Community based focus groups with vaccinated and unvaccinated pregnant women that includes OBGYN medical providers providing culturally competent information |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcendor, D.J.; Matthews-Juarez, P.; Smoot, D.; Hildreth, J.E.K.; Tabatabai, M.; Wilus, D.; Brown, K.Y.; Juarez, P.D. The COVID-19 Vaccine and Pregnant Minority Women in the US: Implications for Improving Vaccine Confidence and Uptake. Vaccines 2022, 10, 2122. https://doi.org/10.3390/vaccines10122122
Alcendor DJ, Matthews-Juarez P, Smoot D, Hildreth JEK, Tabatabai M, Wilus D, Brown KY, Juarez PD. The COVID-19 Vaccine and Pregnant Minority Women in the US: Implications for Improving Vaccine Confidence and Uptake. Vaccines. 2022; 10(12):2122. https://doi.org/10.3390/vaccines10122122
Chicago/Turabian StyleAlcendor, Donald J., Patricia Matthews-Juarez, Duane Smoot, James E. K. Hildreth, Mohammad Tabatabai, Derek Wilus, Katherine Y. Brown, and Paul D. Juarez. 2022. "The COVID-19 Vaccine and Pregnant Minority Women in the US: Implications for Improving Vaccine Confidence and Uptake" Vaccines 10, no. 12: 2122. https://doi.org/10.3390/vaccines10122122
APA StyleAlcendor, D. J., Matthews-Juarez, P., Smoot, D., Hildreth, J. E. K., Tabatabai, M., Wilus, D., Brown, K. Y., & Juarez, P. D. (2022). The COVID-19 Vaccine and Pregnant Minority Women in the US: Implications for Improving Vaccine Confidence and Uptake. Vaccines, 10(12), 2122. https://doi.org/10.3390/vaccines10122122






