Therapeutic Potentials of Immunometabolomic Modulations Induced by Tuberculosis Vaccination
Abstract
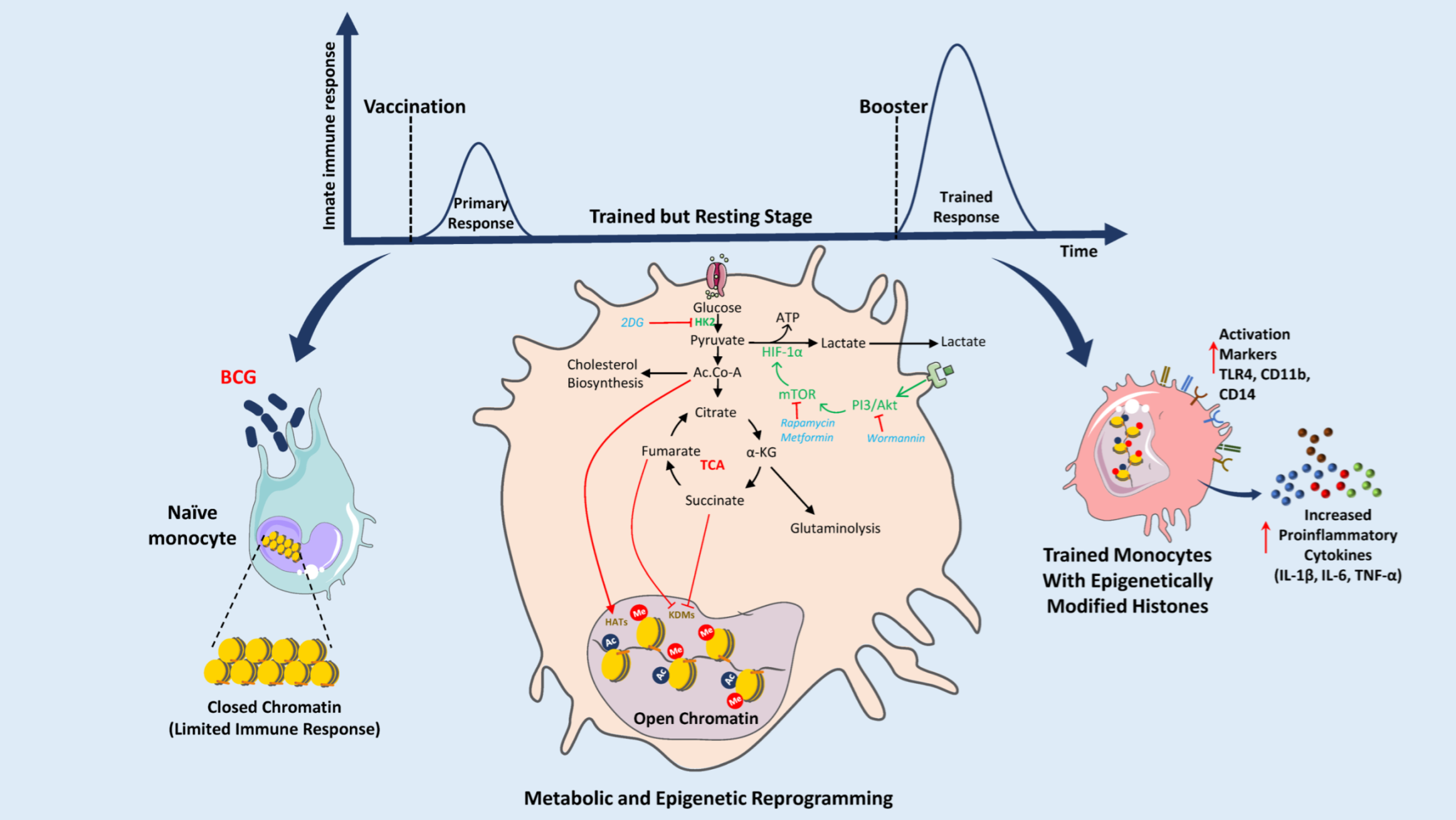
1. Introduction
| Metabolic Modulator | Sample Type | Metabolomics Technique Used | Affected Metabolites or Pathways | References |
|---|---|---|---|---|
| Mtb Infection | Blood serum, plasma, and urine | Gas chromatography-mass spectrometry, Liquid chromatography-mass spectrometry, Flow injection analysis-tandem mass spectrometry | Urea, sphingolipid, sphingosine-1-phosphate, sulfoxymethionine, fatty acid metabolism, sphingomyelins, phosphatidylcholines, lysophosphatidylcholines, amino-acyl tRNA, lysosome pathways, mannose metabolism, pyruvate, citrate, protein digestion pathways, asparagine, aspartate, citrulline, cysteine, lysine, leucine, methylamine, gamma-glutamylglutamine, glutamate, formate, glutamine, histidine, inosine, methionine, tryptophan, kynurenine, lactate, fatty acid beta-oxidation, itaconate, mycolic acids, phthiocerol dimycocerosate, glycerophosphocholine, nicotinate. | [7,8,21,22,23,24,25,26,27] |
| BCG Vaccination | Blood serum | Liquid chromatography-mass spectrometry | Purine biosynthesis, N6-carbomoyltheronyladenosine, glucose metabolism, alpha-ketobutyrate, 1,5-anhydroglucitol, methylguanine, fumarate, glutamate, glutamine, acetyl-CoA, lactate, glucose, nicotinamide adenine dinucleotide, 2-sulfotrehalose, trehalose 6-phosphate, mycobactin, 1-tuberculosynadenosine, conjugate-mycothiolhexadecanoyl-sn-glycero-3-phospho-(1′-myo-inositol), Hypoxanthine, para-aminobenzoic acid, lysophosphatidylcholines, lysophosphatidylethanolamines, sphingolipid metabolism, docosahexaenoic acid. | [9,10,14,28,29] |
2. Mtb: A Smart Pathogen
3. Host Metabolic Adaptation upon Mtb Infection
4. BCG Vaccine: Immunometabolic Reprogramming and Trained Immunity
5. Can BCG Be Used for Other Diseases?
Novel TB Vaccines
| Vaccine Category | Vaccine Candidate | Antigen and Formulation | Latest Clinical Trial Phase (Status) # | NCT Number (References) |
|---|---|---|---|---|
| Recombinant viral vector | Ad5 Ag85A | Ag85A antigen expressed in Adenovirus serotype 5 | I (Completed in 2021) | NCT02337270 [144,145,146] |
| MVA85A | Ag85A antigen expressed in modified Vaccinia virus Ankara | IIa (Completed in 2021) | NCT03681860 [109] | |
| ChAdOx1 85A | Ag85A antigen expressed in Chimpanzee adenovirus | I (Completed in 2021) | NCT03681860 [110,147] | |
| TB/FLU-04L | Ag85A & ESAT-6 antigens expressed in attenuated replication-deficient influenza virus vector | I (Completed in 2015) | NCT02501421 [148] | |
| Viable whole-cell | VPM1002 | Recombinant BCG vaccine | III (Ongoing) | NCT04351685 [149,150] |
| MTBVAC | Attenuated Mtb clinical isolate with ESAT6 & CFP10 and independent genetic deletions of phoP & fadD26 genes | II (Completed in 2022) | NCT03536117 [151,152] | |
| Inactivated whole-cell | RUTI | Polyantigenic liposomal formulation of detoxified, fragmented Mtb | II (Ongoing) | NCT04919239 [153,154,155] |
| Vaccae | Heat-killed M. vaccae | III (Completed in 2017) | NCT01979900 [156] | |
| DAR-901 | Heat killed nontuberculous mycobacteria | II (Completed in 2020) | NCT02712424 [121,122,157,158] | |
| MIP/Immuvac | Whole cell, heat inactivated Mycobacterium indicus pranii | III (Completed in 2012) | NCT00341328 [159] | |
| Protein subunit | AEC/BC02 | Ag85b, ESAT6-CFP10 fusion protein, with BC02 adjuvant | II (Ongoing) | NCT05284812 [128] |
| H56:IC31 | Fusion protein of Ag85B, ESAT-6 and Rv2660c with IC31 adjuvant | II (Ongoing) | NCT03512249 [160,161,162,163] | |
| ID93 + GLA-SE | Fusion protein of Rv1813, Rv2608, Rv3619, and Rv3620 with GLA-SE adjuvant | IIa (Unknown) | NCT03806686 [164,165,166] | |
| M72/AS01E | Fusion protein of Mtb32A and Mtb39A with AS01E adjuvant | II (Ongoing) | NCT04556981 [167,168] |
6. Key Challenges and Recommendations
Future Prospective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L. How COVID is derailing the fight against HIV, TB and malaria. Nature 2021, 597, 314. [Google Scholar] [CrossRef] [PubMed]
- de Vos, M.; Ley, S.D.; Wiggins, K.B.; Derendinger, B.; Dippenaar, A.; Grobbelaar, M.; Reuter, A.; Dolby, T.; Burns, S.; Schito, M.; et al. Bedaquiline Microheteroresistance after Cessation of Tuberculosis Treatment. N. Engl. J. Med. 2019, 380, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H.; et al. WHO’s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Weill, J. Homage to Benjamin Weill-Halle on the 40th anniversary of bcg vaccination. La Presse Med. 1964, 72, 2420–2421. [Google Scholar]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shim, D.; Kim, K.E.S.; Lee, W.; Shin, S.J. Understanding Metabolic Regulation Between Host and Pathogens: New Opportunities for the Development of Improved Therapeutic Strategies Against Mycobacterium tuberculosis Infection. Front. Cell. Infect. Microbiol. 2021, 11, 635335. [Google Scholar] [CrossRef]
- Cho, Y.; Park, Y.; Sim, B.; Kim, J.; Lee, H.; Cho, S.N.; Kang, Y.A.; Lee, S.G. Identification of serum biomarkers for active pulmonary tuberculosis using a targeted metabolomics approach. Sci. Rep. 2020, 10, 3825. [Google Scholar] [CrossRef]
- Diray-Arce, J.; Angelidou, A.; Jensen, K.J.; Conti, M.G.; Kelly, R.S.; Pettengill, M.A.; Liu, M.; van Haren, S.D.; McCulloch, S.D.; Michelloti, G.; et al. Bacille Calmette-Guérin vaccine reprograms human neonatal lipid metabolism in vivo and in vitro. Cell Rep. 2022, 39, 110772. [Google Scholar] [CrossRef]
- Koeken, V.; Qi, C.; Mourits, V.P.; de Bree, L.C.J.; Moorlag, S.; Sonawane, V.; Lemmers, H.; Dijkstra, H.; Joosten, L.A.B.; van Laarhoven, A.; et al. Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS Biol. 2022, 20, e3001765. [Google Scholar] [CrossRef]
- Weiner, J., 3rd; Parida, S.K.; Maertzdorf, J.; Black, G.F.; Repsilber, D.; Telaar, A.; Mohney, R.P.; Arndt-Sullivan, C.; Ganoza, C.A.; Fae, K.C.; et al. Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS ONE 2012, 7, e40221. [Google Scholar] [CrossRef]
- Frediani, J.K.; Jones, D.P.; Tukvadze, N.; Uppal, K.; Sanikidze, E.; Kipiani, M.; Tran, V.T.; Hebbar, G.; Walker, D.I.; Kempker, R.R.; et al. Plasma metabolomics in human pulmonary tuberculosis disease: A pilot study. PLoS ONE 2014, 9, e108854. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, C.J.; Hsiao, Y.H.; Chang, Y.H.; Liu, S.J.; Hsu, H.Y. Therapeutic Effects of BCG Vaccination on Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2020, 2020, 8954125. [Google Scholar] [CrossRef] [PubMed]
- Kuhtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Diray-Arce, J.; Conti, M.G.; Petrova, B.; Kanarek, N.; Angelidou, A.; Levy, O. Integrative Metabolomics to Identify Molecular Signatures of Responses to Vaccines and Infections. Metabolites 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Immunometabolism: Another Road to Sepsis and Its Therapeutic Targeting. Inflammation 2019, 42, 765–788. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- de Carvalho, L.P.; Fischer, S.M.; Marrero, J.; Nathan, C.; Ehrt, S.; Rhee, K.Y. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010, 17, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Siddiqa, A.; Jones, D.P.; Liu, K.; Kempker, R.R.; Nizam, A.; Shah, N.S.; Ismail, N.; Ouma, S.G.; Tukvadze, N.; et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 2020, 5, e137131. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J., 3rd; Maertzdorf, J.; Sutherland, J.S.; Duffy, F.J.; Thompson, E.; Suliman, S.; McEwen, G.; Thiel, B.; Parida, S.K.; Zyla, J.; et al. Metabolite changes in blood predict the onset of tuberculosis. Nat. Commun. 2018, 9, 5208. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Prakash, H. Sphingolipids Are Dual Specific Drug Targets for the Management of Pulmonary Infections: Perspective. Front. Immunol. 2017, 8, 378. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Weiner, J.R.; Maertzdorf, J. Accelerating tuberculosis vaccine trials with diagnostic and prognostic biomarkers. Expert Rev. Vaccines 2017, 16, 845–853. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Banoei, M.M.; Winston, B.W.; Schraufnagel, D.E. Metabolomics: Applications and Promise in Mycobacterial Disease. Ann. Am. Thorac. Soc. 2015, 12, 1278–1287. [Google Scholar] [CrossRef]
- Zhou, A.; Ni, J.; Xu, Z.; Wang, Y.; Lu, S.; Sha, W.; Karakousis, P.C.; Yao, Y.F. Application of (1)h NMR spectroscopy-based metabolomics to sera of tuberculosis patients. J. Proteome Res. 2013, 12, 4642–4649. [Google Scholar] [CrossRef]
- Diaz, C.; Perez Del Palacio, J.; Valero-Guillen, P.L.; Mena Garcia, P.; Perez, I.; Vicente, F.; Martin, C.; Genilloud, O.; Sanchez Pozo, A.; Gonzalo-Asensio, J. Comparative Metabolomics between Mycobacterium tuberculosis and the MTBVAC Vaccine Candidate. ACS Infect. Dis. 2019, 5, 1317–1326. [Google Scholar] [CrossRef]
- Magdalena, D.; Michal, S.; Marta, S.; Magdalena, K.P.; Anna, P.; Magdalena, G.; Rafal, S. Targeted metabolomics analysis of serum and Mycobacterium tuberculosis antigen-stimulated blood cultures of pediatric patients with active and latent tuberculosis. Sci. Rep. 2022, 12, 4131. [Google Scholar] [CrossRef]
- Scriba, T.J.; Coussens, A.K.; Fletcher, H.A. Human Immunology of Tuberculosis. Microbiol. Spectr. 2017, 5, 15. [Google Scholar] [CrossRef]
- Gupta, A. Protective efficacy of Mycobacterium indicus pranii against tuberculosis and underlying local lung immune responses in guinea pig model. Vaccine 2012, 30, 6198–6209. [Google Scholar] [CrossRef] [PubMed]
- Ravesloot-Chávez, M.M.; Van Dis, E.; Stanley, S.A. The Innate Immune Response to Mycobacterium tuberculosis Infection. Annu. Rev. Immunol. 2021, 39, 611–637. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, V.K.; Hunter, R.L.; Jagannath, C. Macrophage heterogeneity and plasticity in tuberculosis. J. Leukoc. Biol. 2019, 106, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Via, L.E.; Deretic, D.; Ulmer, R.J.; Hibler, N.S.; Huber, L.A.; Deretic, V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 1997, 272, 13326–13331. [Google Scholar] [CrossRef] [PubMed]
- Tailleux, L.; Maeda, N.; Nigou, J.; Gicquel, B.; Neyrolles, O. How is the phagocyte lectin keyboard played? Master class lesson by Mycobacterium tuberculosis. Trends Microbiol. 2003, 11, 259–263. [Google Scholar] [CrossRef]
- Vergne, I.; Fratti, R.A.; Hill, P.J.; Chua, J.; Belisle, J.; Deretic, V. Mycobacterium tuberculosis phagosome maturation arrest: Mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell 2004, 15, 751–760. [Google Scholar] [CrossRef]
- Sturgill-Koszycki, S.; Schlesinger, P.H.; Chakraborty, P.; Haddix, P.L.; Collins, H.L.; Fok, A.K.; Allen, R.D.; Gluck, S.L.; Heuser, J.; Russell, D.G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 1994, 263, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Bach, H.; Sun, J.; Hmama, Z.; Av-Gay, Y. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc. Natl. Acad. Sci. USA 2011, 108, 19371–19376. [Google Scholar] [CrossRef]
- Axelrod, S.; Oschkinat, H.; Enders, J.; Schlegel, B.; Brinkmann, V.; Kaufmann, S.H.; Haas, A.; Schaible, U.E. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell. Microbiol. 2008, 10, 1530–1545. [Google Scholar] [CrossRef]
- van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef]
- Houben, D.; Demangel, C.; van Ingen, J.; Perez, J.; Baldeon, L.; Abdallah, A.M.; Caleechurn, L.; Bottai, D.; van Zon, M.; de Punder, K.; et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell. Microbiol. 2012, 14, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.; Bobard, A.; Lippmann, J.; Bitter, W.; Majlessi, L.; Brosch, R.; Enninga, J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012, 8, e1002507. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.; Sayes, F.; Song, O.; Groschel, M.I.; Brodin, P.; Brosch, R.; Majlessi, L. Cytosolic access of Mycobacterium tuberculosis: Critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 2015, 11, e1004650. [Google Scholar] [CrossRef] [PubMed]
- Augenstreich, J.; Arbues, A.; Simeone, R.; Haanappel, E.; Wegener, A.; Sayes, F.; Le Chevalier, F.; Chalut, C.; Malaga, W.; Guilhot, C.; et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol. 2017, 19, e12726. [Google Scholar] [CrossRef]
- Singh, K.H.; Jha, B.; Dwivedy, A.; Choudhary, E.; N, A.G.; Ashraf, A.; Arora, D.; Agarwal, N.; Biswal, B.K. Characterization of a secretory hydrolase from Mycobacterium tuberculosis sheds critical insight into host lipid utilization by M. tuberculosis. J. Biol. Chem. 2017, 292, 11326–11335. [Google Scholar] [CrossRef]
- Dwivedy, A.; Ashraf, A.; Jha, B.; Kumar, D.; Agarwal, N.; Biswal, B.K. De novo histidine biosynthesis protects Mycobacterium tuberculosis from host IFN-gamma mediated histidine starvation. Commun. Biol. 2021, 4, 410. [Google Scholar] [CrossRef]
- Aguilo, J.I.; Alonso, H.; Uranga, S.; Marinova, D.; Arbues, A.; de Martino, A.; Anel, A.; Monzon, M.; Badiola, J.; Pardo, J.; et al. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell. Microbiol. 2013, 15, 1994–2005. [Google Scholar] [CrossRef]
- Dallenga, T.; Repnik, U.; Corleis, B.; Eich, J.; Reimer, R.; Griffiths, G.W.; Schaible, U.E. M. tuberculosis-Induced Necrosis of Infected Neutrophils Promotes Bacterial Growth Following Phagocytosis by Macrophages. Cell Host Microbe 2017, 22, 519–530.e513. [Google Scholar] [CrossRef]
- Lerner, T.R.; Borel, S.; Greenwood, D.J.; Repnik, U.; Russell, M.R.; Herbst, S.; Jones, M.L.; Collinson, L.M.; Griffiths, G.; Gutierrez, M.G. Mycobacterium tuberculosis replicates within necrotic human macrophages. J. Cell Biol. 2017, 216, 583–594. [Google Scholar] [CrossRef]
- Elkington, P.; Lerm, M.; Kapoor, N.; Mahon, R.; Pienaar, E.; Huh, D.; Kaushal, D.; Schlesinger, L.S. In Vitro Granuloma Models of Tuberculosis: Potential and Challenges. J. Infect. Dis. 2019, 219, 1858–1866. [Google Scholar] [CrossRef]
- Batista, L.A.F.; Silva, K.J.S.; da Costa, E.S.L.M.; de Moura, Y.F.; Zucchi, F.C.R. Tuberculosis: A granulomatous disease mediated by epigenetic factors. Tuberculosis 2020, 123, 101943. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.D.; Chiu, C.; Churchyard, G.J.; Esmail, H.; Lewinsohn, D.M.; Gandhi, N.R.; Fennelly, K.P. Tuberculosis Infectiousness and Host Susceptibility. J. Infect. Dis. 2017, 216, S636–S643. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Muniyan, R. Latent tuberculosis: Interaction of virulence factors in Mycobacterium tuberculosis. Mol. Biol. Rep. 2021, 48, 6181–6196. [Google Scholar] [CrossRef] [PubMed]
- Loxton, A.G.; van Rensburg, I.C. FasL regulatory B-cells during Mycobacterium tuberculosis infection and TB disease. J. Mol. Biol. 2021, 433, 166984. [Google Scholar] [CrossRef]
- Marrero, J.; Rhee, K.Y.; Schnappinger, D.; Pethe, K.; Ehrt, S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. USA 2010, 107, 9819–9824. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; VanderVen, B.C.; Fahey, R.J.; Russell, D.G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 2013, 288, 6788–6800. [Google Scholar] [CrossRef]
- Kinsella, R.J.; Fitzpatrick, D.A.; Creevey, C.J.; McInerney, J.O. Fatty acid biosynthesis in Mycobacterium tuberculosis: Lateral gene transfer, adaptive evolution, and gene duplication. Proc. Natl. Acad. Sci. USA 2003, 100, 10320–10325. [Google Scholar] [CrossRef]
- Johnson, T.S.; Munn, D.H. Host indoleamine 2,3-dioxygenase: Contribution to systemic acquired tumor tolerance. Immunol. Investig. 2012, 41, 765–797. [Google Scholar] [CrossRef]
- Garg, S.K.; Volpe, E.; Palmieri, G.; Mattei, M.; Galati, D.; Martino, A.; Piccioni, M.S.; Valente, E.; Bonanno, E.; De Vito, P.; et al. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J. Infect. Dis. 2004, 189, 2129–2138. [Google Scholar] [CrossRef]
- Dara, M.; Acosta, C.D.; Rusovich, V.; Zellweger, J.P.; Centis, R.; Migliori, G.B. Bacille Calmette-Guérin vaccination: The current situation in Europe. Eur. Respir. J. 2014, 43, 24–35. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Rouphael, N.; Del Rio, C.; Santos-Preciado, J.I. Vaccination strategies to prevent tuberculosis in the new millennium: From BCG to new vaccine candidates. Int. J. Infect. Dis. 2006, 10, 93–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pym, A.S.; Brodin, P.; Majlessi, L.; Brosch, R.; Demangel, C.; Williams, A.; Griffiths, K.E.; Marchal, G.; Leclerc, C.; Cole, S.T. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 2003, 9, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Walk, J.; de Bree, L.C.J.; Graumans, W.; Stoter, R.; van Gemert, G.J.; van de Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.C.; Arts, R.J.W.; Behet, M.C.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e105. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Zimmermann, P.; Donath, S.; Perrett, K.P.; Messina, N.L.; Ritz, N.; Netea, M.G.; Flanagan, K.L.; van der Klis, F.R.M.; Curtis, N. The influence of neonatal Bacille Calmette-Guérin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine 2019, 37, 3735–3744. [Google Scholar] [CrossRef]
- Moorlag, S.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Curtis, N.; Sparrow, A.; Ghebreyesus, T.A.; Netea, M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef]
- Brook, B.; Harbeson, D.J.; Shannon, C.P.; Cai, B.; He, D.; Ben-Othman, R.; Francis, F.; Huang, J.; Varankovich, N.; Liu, A.; et al. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci. Transl. Med. 2020, 12, eaax4517. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Fok, E.T.; Davignon, L.; Fanucchi, S.; Mhlanga, M.M. The lncRNA Connection Between Cellular Metabolism and Epigenetics in Trained Immunity. Front. Immunol. 2018, 9, 3184. [Google Scholar] [CrossRef]
- Playdon, M.C.; Ziegler, R.G.; Sampson, J.N.; Stolzenberg-Solomon, R.; Thompson, H.J.; Irwin, M.L.; Mayne, S.T.; Hoover, R.N.; Moore, S.C. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017, 106, 637–649. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Pettengill, M.A.; van Haren, S.D.; Levy, O. Soluble mediators regulating immunity in early life. Front. Immunol. 2014, 5, 457. [Google Scholar] [CrossRef]
- Reinke, S.N.; Walsh, B.H.; Boylan, G.B.; Sykes, B.D.; Kenny, L.C.; Murray, D.M.; Broadhurst, D.I. 1H NMR derived metabolomic profile of neonatal asphyxia in umbilical cord serum: Implications for hypoxic ischemic encephalopathy. J. Proteome Res. 2013, 12, 4230–4239. [Google Scholar] [CrossRef]
- Kan, B.; Michalski, C.; Fu, H.; Au, H.H.T.; Lee, K.; Marchant, E.A.; Cheng, M.F.; Anderson-Baucum, E.; Aharoni-Simon, M.; Tilley, P.; et al. Cellular metabolism constrains innate immune responses in early human ontogeny. Nat. Commun. 2018, 9, 4822. [Google Scholar] [CrossRef]
- Conti, M.G.; Angelidou, A.; Diray-Arce, J.; Smolen, K.K.; Lasky-Su, J.; De Curtis, M.; Levy, O. Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr. Res. 2020, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Shannon, C.P.; Amenyogbe, N.; Bennike, T.B.; Diray-Arce, J.; Idoko, O.T.; Gill, E.E.; Ben-Othman, R.; Pomat, W.S.; van Haren, S.D.; et al. Dynamic molecular changes during the first week of human life follow a robust developmental trajectory. Nat. Commun. 2019, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Petrick, L.M.; Schiffman, C.; Edmands, W.M.B.; Yano, Y.; Perttula, K.; Whitehead, T.; Metayer, C.; Wheelock, C.E.; Arora, M.; Grigoryan, H.; et al. Metabolomics of neonatal blood spots reveal distinct phenotypes of pediatric acute lymphoblastic leukemia and potential effects of early-life nutrition. Cancer Lett. 2019, 452, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Nakaya, H.I.; Subramaniam, S.; Pulendran, B. Systems vaccinology: Enabling rational vaccine design with systems biological approaches. Vaccine 2015, 33, 5294–5301. [Google Scholar] [CrossRef]
- Amenyogbe, N.; Levy, O.; Kollmann, T.R. Systems vaccinology: A promise for the young and the poor. Philos. Trans. R. Soc. Lond B Biol. Sci. 2015, 370, 20140340. [Google Scholar] [CrossRef]
- Angelidou, A.; Diray-Arce, J.; Conti, M.G.; Smolen, K.K.; van Haren, S.D.; Dowling, D.J.; Husson, R.N.; Levy, O. BCG as a Case Study for Precision Vaccine Development: Lessons From Vaccine Heterogeneity, Trained Immunity, and Immune Ontogeny. Front. Microbiol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Roth, A.; Gustafson, P.; Nhaga, A.; Djana, Q.; Poulsen, A.; Garly, M.L.; Jensen, H.; Sodemann, M.; Rodriques, A.; Aaby, P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005, 34, 540–547. [Google Scholar] [CrossRef]
- Garly, M.L.; Martins, C.L.; Balé, C.; Baldé, M.A.; Hedegaard, K.L.; Gustafson, P.; Lisse, I.M.; Whittle, H.C.; Aaby, P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 2003, 21, 2782–2790. [Google Scholar] [CrossRef]
- Kristensen, I.; Aaby, P.; Jensen, H. Routine vaccinations and child survival: Follow up study in Guinea-Bissau, West Africa. BMJ 2000, 321, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, S.; Aaby, P.; Napirna, B.M.; Roth, A.; Ravn, H.; Rodrigues, A.; Whittle, H.; Benn, C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012, 31, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Wout, J.W.; Poell, R.; van Furth, R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992, 36, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef]
- Pereira, L.I.; Dorta, M.L.; Pereira, A.J.; Bastos, R.P.; Oliveira, M.A.; Pinto, S.A.; Galdino, H., Jr.; Mayrink, W.; Barcelos, W.; Toledo, V.P.; et al. Increase of NK cells and proinflammatory monocytes are associated with the clinical improvement of diffuse cutaneous leishmaniasis after immunochemotherapy with BCG/Leishmania antigens. Am. J. Trop. Med. Hyg. 2009, 81, 378–383. [Google Scholar] [CrossRef]
- Fortier, A.H.; Mock, B.A.; Meltzer, M.S.; Nacy, C.A. Mycobacterium bovis BCG-induced protection against cutaneous and systemic Leishmania major infections of mice. Infect. Immun. 1987, 55, 1707–1714. [Google Scholar] [CrossRef]
- Aldovini, A.; Young, R.A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 1991, 351, 479–482. [Google Scholar] [CrossRef]
- Uno-Furuta, S.; Matsuo, K.; Tamaki, S.; Takamura, S.; Kamei, A.; Kuromatsu, I.; Kaito, M.; Matsuura, Y.; Miyamura, T.; Adachi, Y.; et al. Immunization with recombinant Calmette-Guerin bacillus (BCG)-hepatitis C virus (HCV) elicits HCV-specific cytotoxic T lymphocytes in mice. Vaccine 2003, 21, 3149–3156. [Google Scholar] [CrossRef]
- Ristori, G.; Buzzi, M.G.; Sabatini, U.; Giugni, E.; Bastianello, S.; Viselli, F.; Buttinelli, C.; Ruggieri, S.; Colonnese, C.; Pozzilli, C.; et al. Use of Bacille Calmette-Guerin (BCG) in multiple sclerosis. Neurology 1999, 53, 1588–1589. [Google Scholar] [CrossRef]
- Herr, H.W.; Morales, A. History of bacillus Calmette-Guerin and bladder cancer: An immunotherapy success story. J. Urol. 2008, 179, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, N.; Calcinaro, F.; Bradley, B.J.; Bruchim, I.; Vardi, P.; Lafferty, K.J. Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet 1994, 343, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Hopfenspirger, M.T.; Agrawal, D.K. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J. Immunol. 2002, 168, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Kowalewicz-Kulbat, M.; Locht, C. BCG for the prevention and treatment of allergic asthma. Vaccine 2021, 39, 7341–7352. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 2004, 173, 6357–6365. [Google Scholar] [CrossRef]
- Vordermeier, H.M. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 2009, 77, 3364–3373. [Google Scholar] [CrossRef]
- Dean, G. Comparison of the immunogenicity and protection against bovine tuberculosis following immunization by BCG-priming and boosting with adenovirus or protein based vaccines. Vaccine 2014, 32, 1304–1310. [Google Scholar] [CrossRef]
- Metcalfe, H.J. Ag85A-specific CD4+ T cell lines derived after boosting BCG-vaccinated cattle with Ad5-85A possess both mycobacterial growth inhibition and anti-inflammatory properties. Vaccine 2018, 36, 2850–2854. [Google Scholar] [CrossRef]
- Williams, A.; Hatch, G.J.; Clark, S.O.; Gooch, K.E.; Hatch, K.A.; Hall, G.A.; Huygen, K.; Ottenhoff, T.H.; Franken, K.L.; Andersen, P.; et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis 2005, 85, 29–38. [Google Scholar] [CrossRef]
- Stylianou, E. Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine 2015, 33, 6800–6808. [Google Scholar] [CrossRef]
- Hawkridge, T. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J. Infect. Dis. 2008, 198, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Dockrell, H.M. Towards new TB vaccines: What are the challenges? Pathog. Dis. 2016, 74, ftw016. [Google Scholar] [CrossRef] [PubMed]
- Spertini, F. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: A randomised, double-blind, controlled phase I trial. Lancet Respir. Med. 2015, 3, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E. The recombinant Bacille Calmette-Guerin vaccine VPM1002: Ready for clinical efficacy testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Reyrat, J.M.; Berthet, F.X.; Gicquel, B. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis bacillus Calmette-Guerin. Proc. Natl. Acad. Sci. USA 1995, 92, 8768–8772. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.H.; Hart, P.D.; Young, M.R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature 1980, 286, 79–80. [Google Scholar] [CrossRef]
- Gonzalo-Asensio, J. MTBVAC: Attenuating the human pathogen of tuberculosis (TB) toward a promising vaccine against the TB epidemic. Front. Immunol. 2017, 8, 1803. [Google Scholar] [CrossRef]
- Cardona, P.J.; Amat, I. Origin and development of RUTI, a new therapeutic vaccine against Mycobacterium tuberculosis infection. Arch. Bronconeumol. 2006, 42, 25–32. [Google Scholar] [CrossRef]
- Johnson, J.L.; Kamya, R.M.; Okwera, A.; Loughlin, A.M.; Nyole, S.; Hom, D.L.; Wallis, R.S.; Hirsch, C.S.; Wolski, K.; Foulds, J.; et al. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected ugandan adults with newly diagnosed pulmonary tuberculosis. The Uganda-Case Western Reserve University Research Collaboration. J. Infect. Dis. 2000, 181, 1304–1312. [Google Scholar] [CrossRef][Green Version]
- Reyn, C.F. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS 2010, 24, 675–685. [Google Scholar] [CrossRef]
- Reyn, C.F. Safety and immunogenicity of an inactivated whole cell tuberculosis vaccine booster in adults primed with BCG: A randomized, controlled trial of DAR-901. PLoS ONE 2017, 12, e0175215. [Google Scholar]
- Masonou, T. CD4+ T cell cytokine responses to the DAR-901 booster vaccine in BCG-primed adults: A randomized, placebo-controlled trial. PLoS ONE 2019, 14, e0217091. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.R. Altruism, scepticism, and collective decision-making in foreign-born U.S. residents in a tuberculosis vaccine trial. BMC Public Health 2018, 18, 1–12. [Google Scholar]
- Saini, V.; Raghuvanshi, S.; Talwar, G.P.; Ahmed, N.; Khurana, J.P.; Hasnain, S.E.; Tyagi, A.K.; Tyagi, A.K. Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS ONE 2009, 4, e6263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Disabilities in multibacillary leprosy following multidrug therapy with and without immunotherapy with Mycobacterium w antileprosy vaccine. Int. J. Lepr. Other Mycobact. Dis. 1999, 67, 1–9. [Google Scholar]
- Sharma, P. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr. Rev. 2005, 76, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Mycobacterium w vaccine, a useful adjuvant to multidrug therapy in multibacillary leprosy: A report on hospital based immunotherapeutic clinical trials with a follow-up of 1-7 years after treatment. Lepr. Rev. 2000, 71, 179–192. [Google Scholar]
- Lu, J.B.; Chen, B.W.; Wang, G.Z.; Fu, L.L.; Shen, X.B.; Su, C.; Du, W.X.; Yang, L.; Xu, M. Recombinant tuberculosis vaccine AEC/BC02 induces antigen-specific cellular responses in mice and protects guinea pigs in a model of latent infection. J. Microbiol. Immunol. Infect. 2015, 48, 597–603. [Google Scholar] [CrossRef]
- Perez-Martinez, A.P. Conservation in gene encoding Mycobacterium tuberculosis antigen Rv2660 and a high predicted population coverage of H56 multistage vaccine in South Africa. Infect. Genet. Evol. 2017, 55, 244–250. [Google Scholar] [CrossRef]
- Bertholet, S.; Ireton, G.C.; Kahn, M.; Guderian, J.; Mohamath, R.; Stride, N.; Laughlin, E.M.; Baldwin, S.L.; Vedvick, T.S.; Coler, R.N.; et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J. Immunol. 2008, 181, 7948–7957. [Google Scholar] [CrossRef]
- Homolka, S.; Ubben, T.; Niemann, S. High sequence variability of the ppE18 gene of clinical Mycobacterium tuberculosis complex strains potentially impacts effectivity of vaccine candidate M72/AS01E. PLoS ONE 2016, 11, e0152200. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J. A randomized, controlled dose-finding phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J. Clin. Immunol. 2013, 33, 1360–1375. [Google Scholar] [CrossRef]
- Skeiky, Y.A. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect. Immun. 1999, 67, 3998–4007. [Google Scholar] [CrossRef]
- Dillon, D.C. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 1999, 67, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Al-Attiyah, R. In vitro cellular immune responses to complex and newly defined recombinant antigens of Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004, 138, 139–144. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., 3rd; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.A.; Manson, A.L.; Desjardins, C.A.; Abeel, T.; Earl, A.M. Deciphering drug resistance in Mycobacterium tuberculosis using whole-genome sequencing: Progress, promise, and challenges. Genome Med. 2019, 11, 45. [Google Scholar] [CrossRef]
- Garcia, J.I.; Allue-Guardia, A.; Tampi, R.P.; Restrepo, B.I.; Torrelles, J.B. New Developments and Insights in the Improvement of Mycobacterium tuberculosis Vaccines and Diagnostics Within the End TB Strategy. Curr. Epidemiol. Rep. 2021, 8, 33–45. [Google Scholar] [CrossRef]
- Diego-Gonzalez, L.; Crecente-Campo, J.; Paul, M.J.; Singh, M.; Reljic, R.; Alonso, M.J.; Gonzalez-Fernandez, A.; Simon-Vazquez, R. Design of Polymeric Nanocapsules for Intranasal Vaccination against Mycobacterium Tuberculosis: Influence of the Polymeric Shell and Antigen Positioning. Pharmaceutics 2020, 12, 489. [Google Scholar] [CrossRef]
- Gheibi Hayat, S.M.; Darroudi, M. Nanovaccine: A novel approach in immunization. J. Cell. Physiol. 2019, 234, 12530–12536. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Beissert, T.; Perkovic, M.; Vogel, A.; Erbar, S.; Walzer, K.C.; Hempel, T.; Brill, S.; Haefner, E.; Becker, R.; Tureci, O.; et al. A Trans-amplifying RNA Vaccine Strategy for Induction of Potent Protective Immunity. Mol. Ther. 2020, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.L.; Reese, V.A.; Larsen, S.E.; Beebe, E.; Guderian, J.; Orr, M.T.; Fox, C.B.; Reed, S.G.; Coler, R.N. Prophylactic efficacy against Mycobacterium tuberculosis using ID93 and lipid-based adjuvant formulations in the mouse model. PLoS ONE 2021, 16, e0247990. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F.; Xing, Z. Human type 5 adenovirus-based tuberculosis vaccine: Is the respiratory route of delivery the future? Expert Rev. Vaccines 2014, 13, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci. Transl. Med. 2013, 5, 205ra134. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M. Induction of an immune-protective T-cell repertoire with diverse genetic coverage by a novel viral-vectored tuberculosis vaccine in humans. J. Infect. Dis. 2016, 214, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime—MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef]
- Stukova, M.; Khairullin, B.; Bekembaeva, G.; Erofeeva, M.; Shurygina, A.; Pisareva, M.; Buzitskaya, J.; Grudinin, M.; Kassenov, M.; Sandybaev, N. Randomized double-blind placebo-controlled phase I trial of intranasal TB/FLU-04L tuberculosis vaccine in BCG-vaccinated healthy adults aged 18–50 years. In Proceedings of the 4th Global Forum on TB Vaccines, Shanghai, China, 21–24 April 2015; pp. 21–24. [Google Scholar]
- Grode, L. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 2013, 31, 1340–1348. [Google Scholar] [CrossRef]
- Loxton, A.G.; Knaul, J.K.; Grode, L.; Gutschmidt, A.; Meller, C.; Eisele, B.; Johnstone, H.; van der Spuy, G.; Maertzdorf, J.; Kaufmann, S.H. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin. Vaccine Immunol. 2017, 24, e00439-16. [Google Scholar] [CrossRef]
- Tameris, M.; Mearns, H.; Penn-Nicholson, A.; Gregg, Y.; Bilek, N.; Mabwe, S.; Geldenhuys, H.; Shenje, J.; Luabeya, A.K.K.; Murillo, I.; et al. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: A randomised controlled, double-blind dose-escalation trial. Lancet Respir. Med. 2019, 7, 757–770. [Google Scholar] [CrossRef]
- Marinova, D. MTBVAC from discovery to clinical trials in tuberculosis-endemic countries. Expert Rev. Vaccines 2017, 16, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, S.A.; Painter, H.; Zelmer, A.; Smith, S.G.; Seifert, K.; Amat, M.; Cardona, P.J.; Fletcher, H.A. RUTI Vaccination Enhances Inhibition of Mycobacterial Growth ex vivo and Induces a Shift of Monocyte Phenotype in Mice. Front. Immunol. 2019, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, C. Double-blind, randomized, placebo-controlled phase I clinical trial of the therapeutical antituberculous vaccine RUTI. Vaccine 2010, 28, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.J. RUTI: A new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis 2006, 86, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Moran-Mendoza, O.; Marion, S.A.; Elwood, K.; Patrick, D.M.; FitzGerald, J.M. Tuberculin skin test size and risk of tuberculosis development: A large population-based study in contacts. Int. J. Tuberc. Lung Dis. 2007, 11, 1014–1020. [Google Scholar] [PubMed]
- Munseri, P.; Said, J.; Amour, M.; Magohe, A.; Matee, M.; Rees, C.A.; Mackenzie, T.; Tvaroha, S.; Bailey-Kellogg, C.; Maro, I.; et al. DAR-901 vaccine for the prevention of infection with Mycobacterium tuberculosis among BCG-immunized adolescents in Tanzania: A randomized controlled, double-blind phase 2b trial. Vaccine 2020, 38, 7239–7245. [Google Scholar] [CrossRef]
- Lahey, T. Immunogenicity and protective efficacy of the DAR-901 booster vaccine in a murine model of tuberculosis. PLoS ONE 2016, 11, e0168521. [Google Scholar] [CrossRef]
- Patel, N.; Trapathi, S.B. Improved cure rates in pulmonary tuberculosis category II (retreatment) with mycobacterium w. J. Indian Med. Assoc. 2003, 101, 680–682. [Google Scholar]
- Jenum, S.; Tonby, K.; Rueegg, C.S.; Ruhwald, M.; Kristiansen, M.P.; Bang, P.; Olsen, I.C.; Sellaeg, K.; Rostad, K.; Mustafa, T.; et al. A Phase I/II randomized trial of H56:IC31 vaccination and adjunctive cyclooxygenase-2-inhibitor treatment in tuberculosis patients. Nat. Commun. 2021, 12, 6774. [Google Scholar] [CrossRef]
- Bekker, L.G.; Dintwe, O.; Fiore-Gartland, A.; Middelkoop, K.; Hutter, J.; Williams, A.; Randhawa, A.K.; Ruhwald, M.; Kromann, I.; Andersen, P.L.; et al. A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. EClinicalMedicine 2020, 21, 100313. [Google Scholar] [CrossRef]
- Suliman, S. Dose optimization of H56:IC31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. Am. J. Respir. Crit. Care Med. 2019, 199, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Luabeya, A.K. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine 2015, 33, 4130–4140. [Google Scholar] [CrossRef] [PubMed]
- Day, T.A.; Penn-Nicholson, A.; Luabeya, A.K.K.; Fiore-Gartland, A.; Du Plessis, N.; Loxton, A.G.; Vergara, J.; Rolf, T.A.; Reid, T.D.; Toefy, A.; et al. Safety and immunogenicity of the adjunct therapeutic vaccine ID93 + GLA-SE in adults who have completed treatment for tuberculosis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Respir. Med. 2021, 9, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Penn-Nicholson, A.; Tameris, M.; Smit, E.; Day, T.A.; Musvosvi, M.; Jayashankar, L.; Vergara, J.; Mabwe, S.; Bilek, N.; Geldenhuys, H.; et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: A randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir. Med. 2018, 6, 287–298. [Google Scholar] [CrossRef]
- Coler, R.N. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines 2018, 3, 34. [Google Scholar] [CrossRef]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef]
- Meeren, O. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 2018, 379, 1621–1634. [Google Scholar] [CrossRef]
- Jagannath, C.; Bakhru, P. Rapamycin-induced enhancement of vaccine efficacy in mice. Methods Mol. Biol 2012, 821, 295–303. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Weiner, J.; von Reyn, C.F. Novel approaches to tuberculosis vaccine development. Int J. Infect. Dis. 2017, 56, 263–267. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current Challenges in Vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef]
- Miggiels, P.; Wouters, B.; van Westen, G.J.P.; Dubbelman, A.-C.; Hankemeier, T. Novel technologies for metabolomics: More for less. TrAC Trends Anal. Chem. 2019, 120, 115323. [Google Scholar] [CrossRef]
- Petrick, L.M.; Shomron, N. AI/ML-driven advances in untargeted metabolomics and exposomics for biomedical applications. Cell Rep. Phys. Sci. 2022, 3, 100978. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawat, B.S.; Kumar, D.; Soni, V.; Rosenn, E.H. Therapeutic Potentials of Immunometabolomic Modulations Induced by Tuberculosis Vaccination. Vaccines 2022, 10, 2127. https://doi.org/10.3390/vaccines10122127
Rawat BS, Kumar D, Soni V, Rosenn EH. Therapeutic Potentials of Immunometabolomic Modulations Induced by Tuberculosis Vaccination. Vaccines. 2022; 10(12):2127. https://doi.org/10.3390/vaccines10122127
Chicago/Turabian StyleRawat, Bhupendra Singh, Deepak Kumar, Vijay Soni, and Eric H. Rosenn. 2022. "Therapeutic Potentials of Immunometabolomic Modulations Induced by Tuberculosis Vaccination" Vaccines 10, no. 12: 2127. https://doi.org/10.3390/vaccines10122127
APA StyleRawat, B. S., Kumar, D., Soni, V., & Rosenn, E. H. (2022). Therapeutic Potentials of Immunometabolomic Modulations Induced by Tuberculosis Vaccination. Vaccines, 10(12), 2127. https://doi.org/10.3390/vaccines10122127







