Immunostimulating Effect of Inactivated Parapoxvirus Ovis on the Serological Response to Equine Influenza Booster Vaccination
Abstract
1. Introduction
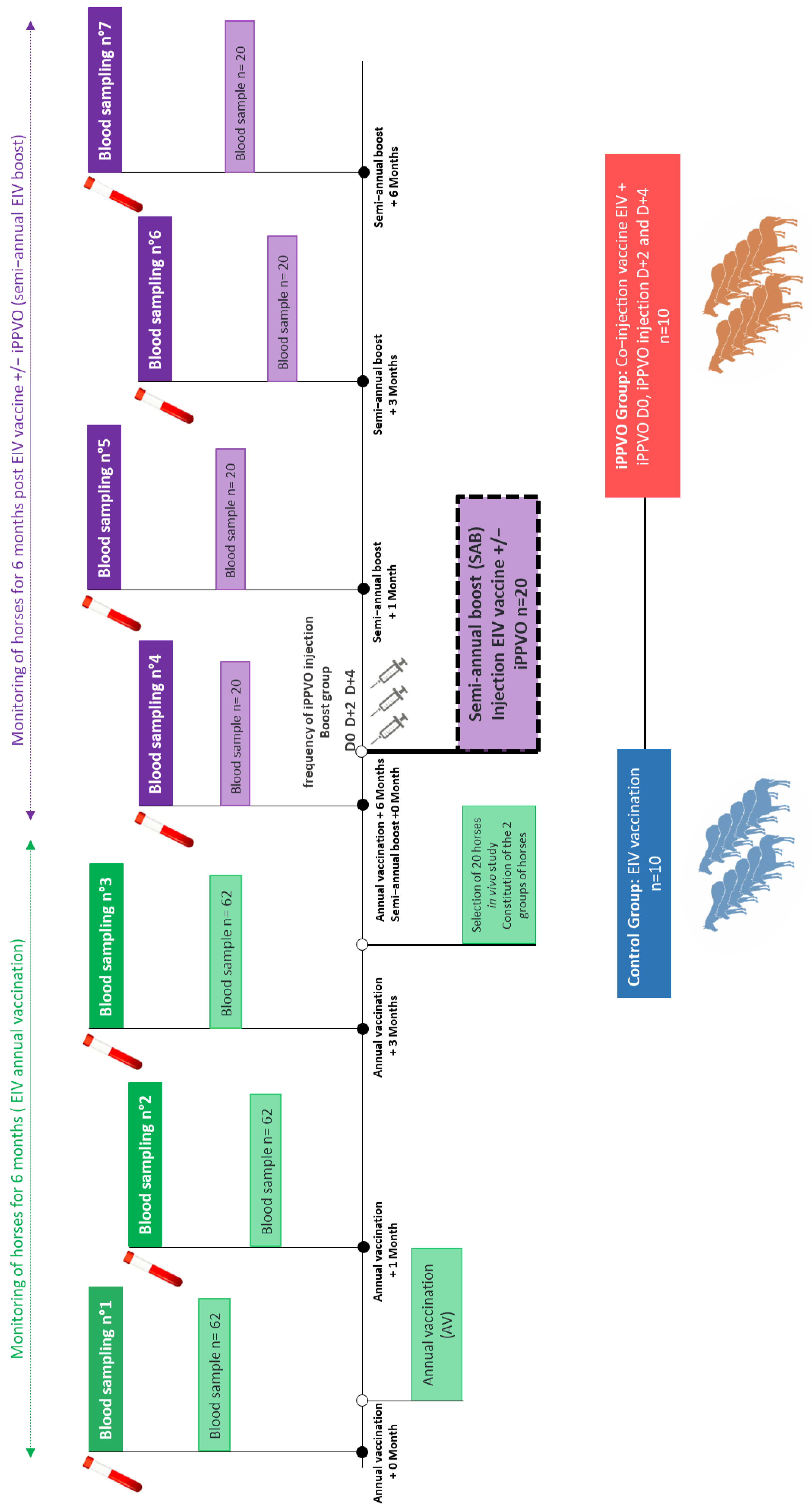
2. Materials and Methods
2.1. Ethical Approval/Animal Welfare
2.2. Horses and Screening Process
2.3. Study Design
2.4. Blood Samples
2.5. Serological Analysis: Single Radial Haemolysis
2.6. Statistical Data Analysis
3. Results
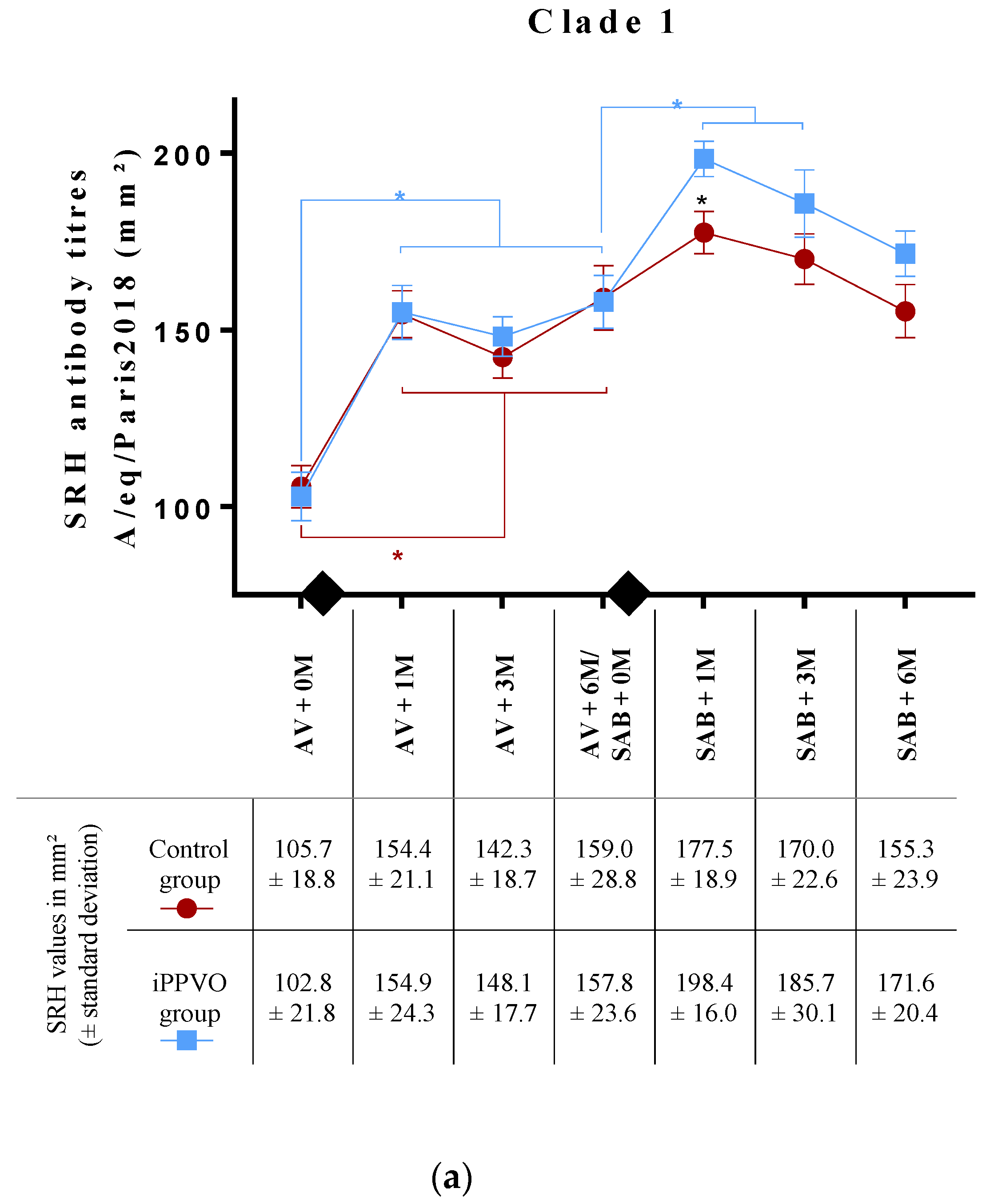
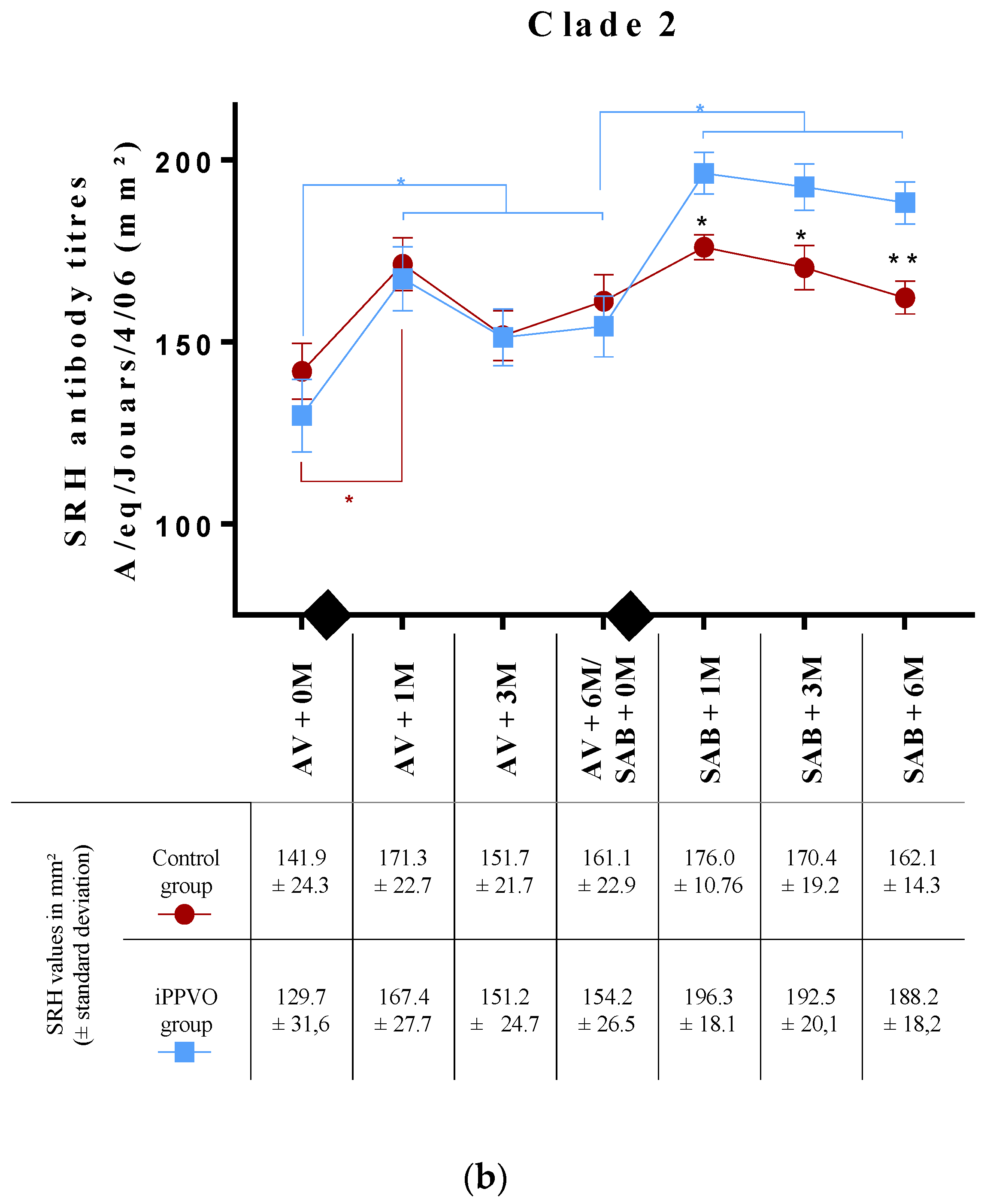
3.1. Antibody Response to Annual EIV Vaccination (Pre-iPPVO Treatment)
3.2. Antibody Response to EIV Vaccination with or without iPPVO as Immunostimulant
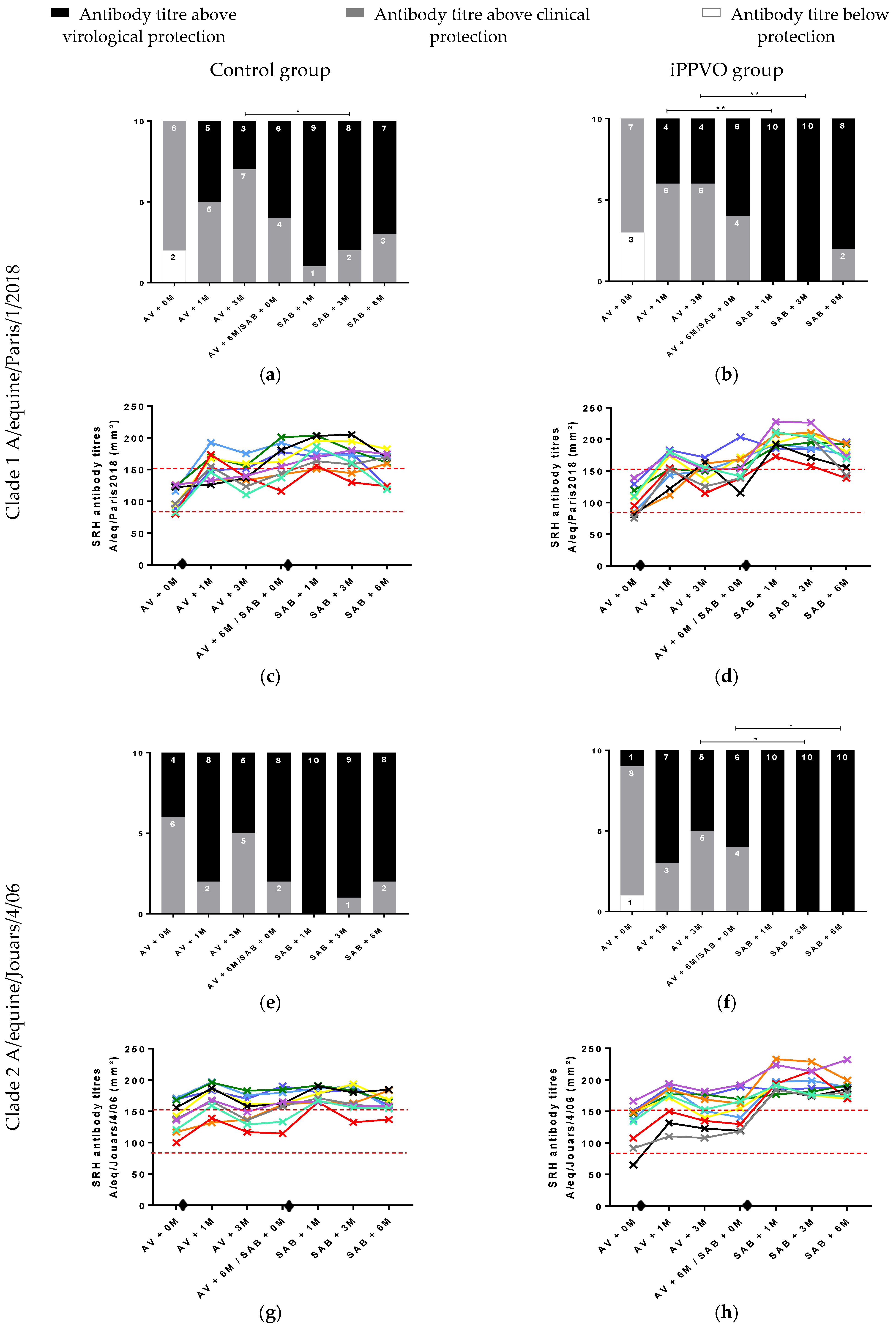
3.3. Individual Antibody Response to EIV Vaccination and iPPVO and Level of Protection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, W.D. Equine Influenza. Vet. Clin. North Am. Equine Pract. 1993, 9, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R. A Systematic Review of Recent Advances in Equine Influenza Vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A. Equine influenza (infection with equine influenza virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE—World Organisation for Animal Health: Paris, France, 2022; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.06.07_EQ_INF.pdf (accessed on 8 December 2022).
- Fougerolle, S.; Fortier, C.; Legrand, L.; Jourdan, M.; Pitel, C.M.; Pronost, S.; Paillot, R. Success and Limitation of Equine Influenza Vaccination: The First Incursion in a Decade of a Florida Clade 1 Equine Influenza Virus That Shakes Protection Despite High Vaccine Coverage. Vaccines 2019, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; El-Hage, C.M. The Use of a Recombinant Canarypox-Based Equine Influenza Vaccine during the 2007 Australian Outbreak: A Systematic Review and Summary. Pathogens 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- The Parliament of the Commonwealth of Australia. Equine Influenza: The August 2007 Outbreak in Australia; Callinan, I.D.F., Ed.; Parliamentary Paper; The Parliament of the Commonwealth of Australia: Canberra, Australia, 2008. [Google Scholar]
- Oladunni, F.S.; Oseni, S.O.; Martinez-Sobrido, L.; Chambers, T.M. Equine Influenza Virus and Vaccines. Viruses 2021, 13, 1657. [Google Scholar] [CrossRef]
- Daly, J.M.; Lai, A.C.; Binns, M.M.; Chambers, T.M.; Barrandeguy, M.; Mumford, J.A. Antigenic and Genetic Evolution of Equine H3N8 Influenza A Viruses. J. Gen. Virol. 1996, 77, 661–671. [Google Scholar] [CrossRef]
- Ziebell, K.-L.; Steinmann, H.; Kretzdorn, D.; Schlapp, T.; Failing, K.; Schmeer, N. The Use of Baypamun N in Crowding Associated Infectious Respiratory Disease: Efficacy of Baypamun N (Freeze Dried Product) in 4–10 Month Old Horses. J. Vet. Med. Ser. B 1997, 44, 529–536. [Google Scholar] [CrossRef]
- Castrucci, G.; Osburn, B.I.; Frigeri, F.; Ferrari, M.; Salvatori, D.; Lo Dico, M.; Barreca, F. The Use of Immunomodulators in the Control of Infectious Bovine Rhinotracheitis. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 163–173. [Google Scholar] [CrossRef]
- Hartmann, K.; Block, A.; Ferk, G.; Vollmar, A.; Goldberg, M.; Lutz, H. Treatment of Feline Leukemia Virus-Infected Cats with Paramunity Inducer. Vet. Immunol. Immunopathol. 1998, 65, 267–275. [Google Scholar] [CrossRef]
- Kyriakis, S.C.; Tzika, E.D.; Lyras, D.N.; Tsinas, A.C.; Saoulidis, K.; Sarris, K. Effect of an Inactivated Parapoxvirus Based Immunomodulator (Baypamun) on Post Weaning Diarrhoea Syndrome and Wasting Pig Syndrome of Piglets. Res. Vet. Sci. 1998, 64, 187–190. [Google Scholar] [CrossRef]
- Paillot, R. A Systematic Review of the Immune-Modulators Parapoxvirus Ovis and Propionibacterium Acnes for the Prevention of Respiratory Disease and Other Infections in the Horse. Vet. Immunol. Immunopathol. 2013, 153, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ons, E.; Van Brussel, L.; Lane, S.; King, V.; Cullinane, A.; Kenna, R.; Lyons, P.; Hammond, T.-A.; Salt, J.; Raue, R. Efficacy of a Parapoxvirus Ovis-Based Immunomodulator against Equine Herpesvirus Type 1 and Streptococcus Equi Equi Infections in Horses. Vet. Microbiol. 2014, 173, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Rush, B.R.; Flaminio, M.J. Immunomodulation in Horses. Vet. Clin. N. Am. Equine Pract. 2000, 16, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Weber, O.; Siegling, A.; Friebe, A.; Limmer, A.; Schlapp, T.; Knolle, P.; Mercer, A.; Schaller, H.; Volk, H.-D. Inactivated Parapoxvirus Ovis (Orf Virus) Has Antiviral Activity against Hepatitis B Virus and Herpes Simplex Virus. J. Gen. Virol. 2003, 84, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Horohov, D.W.; Breathnach, C.C.; Sturgill, T.L.; Rashid, C.; Stiltner, J.L.; Strong, D.; Nieman, N.; Holland, R.E. In Vitro and in Vivo Modulation of the Equine Immune Response by Parapoxvirus Ovis. Equine Vet. J. 2008, 40, 468–472. [Google Scholar] [CrossRef]
- Hue, E.S.; Richard, E.A.; Fortier, C.I.; Fortier, G.D.; Paillot, R.; Raue, R.; Pronost, S.L. Equine PBMC Cytokines Profile after In Vitro α- and γ-EHV Infection: Efficacy of a Parapoxvirus Ovis Based-Immunomodulator Treatment. Vaccines 2017, 5, 28. [Google Scholar] [CrossRef]
- Biuk-Rudan, N.; Šver, L.; Valpotić, I.; Vrbanac, I.; Madić, J.; Župančić, Ž.; Vijtiuk, N.; Pavičić, Ž. Effect of Baypamun Treatment on Aujeszky’s Disease Virus (ADV) Transmission in Pigs. Acta Vet. Brno 2004, 73, 59–68. [Google Scholar] [CrossRef]
- Friebe, A.; Siegling, A.; Friederichs, S.; Volk, H.-D.; Weber, O. Immunomodulatory Effects of Inactivated Parapoxvirus Ovis (ORF Virus) on Human Peripheral Immune Cells: Induction of Cytokine Secretion in Monocytes and Th1-like Cells. J. Virol. 2004, 78, 9400–9411. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to Calculate Sample Size in Animal Studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- AI-Therapy, Statistics for Psychologists, Sample Size Calculator. Available online: https://www.ai-therapy.com/psychology-statistics/sample-size-calculator (accessed on 8 December 2022).
- Morley, P.S.; Hanson, L.K.; Bogdan, J.R.; Townsend, H.G.G.; Appleton, J.A.; Haines, D.M. The Relationship between Single Radial Hemolysis, Hemagglutination Inhibition, and Virus Neutralization Assays Used to Detect Antibodies Specific for Equine Influenza Viruses. Vet. Microbiol. 1995, 45, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.A.; Wood, J. Establishing an Acceptability Threshold for Equine Influenza Vaccines. Dev. Biol. Stand. 1992, 79, 137–146. [Google Scholar] [PubMed]
- OIE. OIE Expert Surveillance Panel on Equine Influenza Vaccine Composition; OIE—World Organisation for Animal Health: Paris, France, 2019. [Google Scholar]
- Lunn, D.P.; Rush, B.R. Immunomodulation: Principles and Mechanisms; IVIS: Denver, CO, USA, 2004. [Google Scholar]
- Paillot, R.; Garrett, D.; Lopez-Alvarez, M.R.; Birand, I.; Montesso, F.; Horspool, L. The Immunity Gap Challenge: Protection against a Recent Florida Clade 2 Equine Influenza Strain. Vaccines 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Reemers, S.; Sonnemans, D.; Horspool, L.; van Bommel, S.; Cao, Q.; van de Zande, S. Determining Equine Influenza Virus Vaccine Efficacy—The Specific Contribution of Strain Versus Other Vaccine Attributes. Vaccines 2020, 8, 501. [Google Scholar] [CrossRef]
- Reeve-Johnson, L. Equine Influenza in Australia. Vet. Rec. 2007, 161, 635. [Google Scholar] [CrossRef]
- Holmes, M.A.; Townsend, H.G.G.; Kohler, A.K.; Hussey, S.; Breathnach, C.; Barnett, C.; Holland, R.; Lunn, D.P. Immune Responses to Commercial Equine Vaccines against Equine Herpesvirus-1, Equine Influenza Virus, Eastern Equine Encephalomyelitis, and Tetanus. Vet. Immunol. Immunopathol. 2006, 111, 67–80. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnet, F.; Paillot, R.; Fortier, C.; Hue, E.S.; Briot, L.; de Geoffroy, F.; Vidalain, P.-O.; Pronost, S. Immunostimulating Effect of Inactivated Parapoxvirus Ovis on the Serological Response to Equine Influenza Booster Vaccination. Vaccines 2022, 10, 2139. https://doi.org/10.3390/vaccines10122139
Carnet F, Paillot R, Fortier C, Hue ES, Briot L, de Geoffroy F, Vidalain P-O, Pronost S. Immunostimulating Effect of Inactivated Parapoxvirus Ovis on the Serological Response to Equine Influenza Booster Vaccination. Vaccines. 2022; 10(12):2139. https://doi.org/10.3390/vaccines10122139
Chicago/Turabian StyleCarnet, Flora, Romain Paillot, Christine Fortier, Erika S. Hue, Laurie Briot, Frédéric de Geoffroy, Pierre-Olivier Vidalain, and Stéphane Pronost. 2022. "Immunostimulating Effect of Inactivated Parapoxvirus Ovis on the Serological Response to Equine Influenza Booster Vaccination" Vaccines 10, no. 12: 2139. https://doi.org/10.3390/vaccines10122139
APA StyleCarnet, F., Paillot, R., Fortier, C., Hue, E. S., Briot, L., de Geoffroy, F., Vidalain, P.-O., & Pronost, S. (2022). Immunostimulating Effect of Inactivated Parapoxvirus Ovis on the Serological Response to Equine Influenza Booster Vaccination. Vaccines, 10(12), 2139. https://doi.org/10.3390/vaccines10122139







