mRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics
Abstract
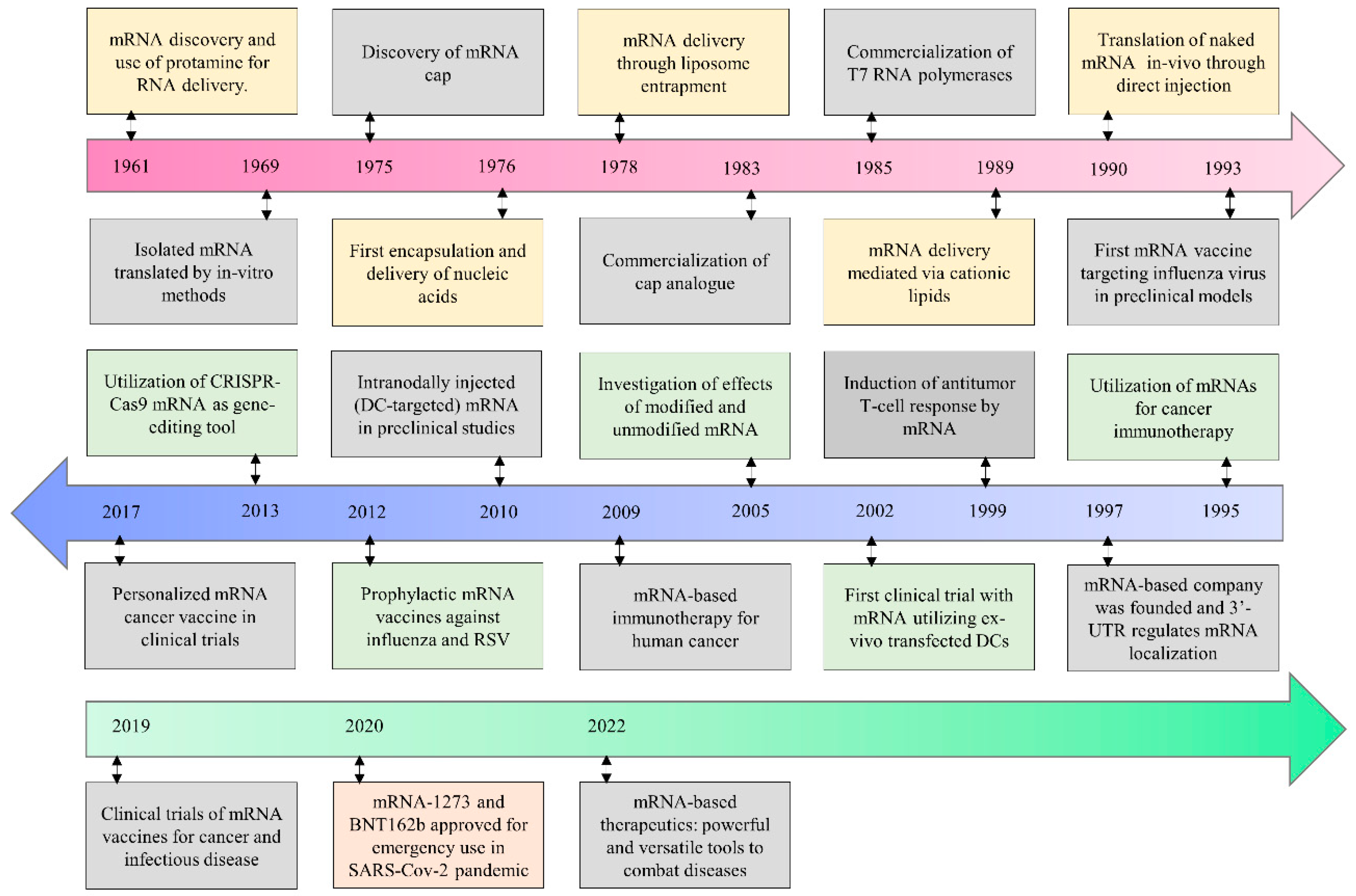
:1. Introduction
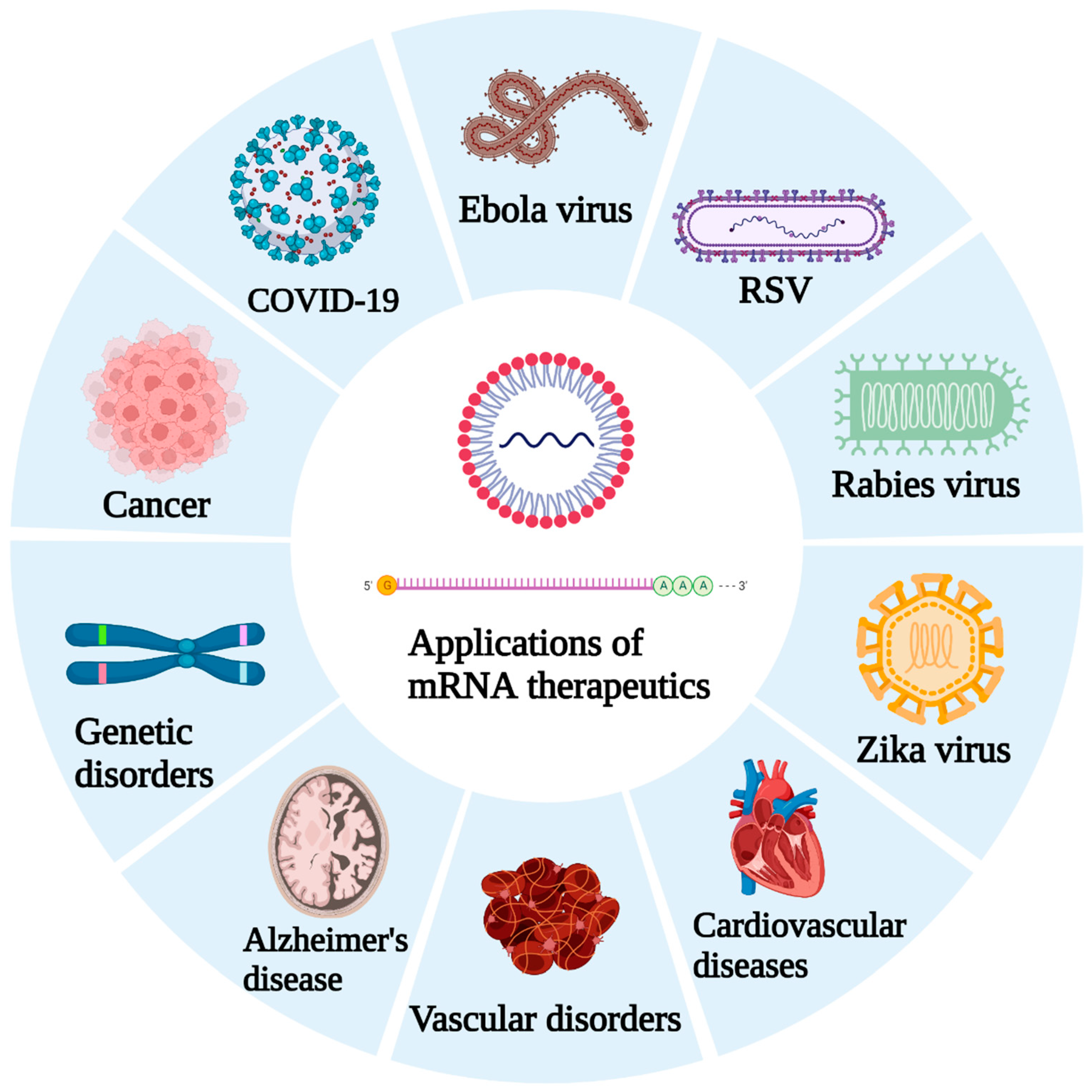
2. Current Status of mRNA Therapeutics
2.1. Bioanalytical Advances in mRNA Platforms
2.2. Recent COVID-19 Vaccines from the mRNA Platform
3. Emphasis Following the COVID-19 Pandemic
4. Carriers for mRNA Delivery
4.1. Lipid-Based Delivery
4.2. Polymer-Based Delivery
4.3. Peptide-Based Delivery
4.4. Exosome-Mediated Delivery
4.5. Cationic Nanoemulsion (CNE)
5. Pulmonary mRNA-Based Vaccines and Therapeutics
6. Pharmaceutical Industry Perspective
7. Quality Check and Characterization
8. Radical Frameshift
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavda, V.P.; Sonak, S.S.; Munshi, N.K.; Dhamade, P.N. Pseudoscience and Fraudulent Products for COVID-19 Management. Environ. Sci. Pollut. Res. Int. 2022, 29, 62887–62912. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Feehan, J.; Apostolopoulos, V. A Veterinary Vaccine for SARS-CoV-2: The First COVID-19 Vaccine for Animals. Vaccines 2021, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Chen, Y.; Dave, J.; Chen, Z.-S.; Chauhan, S.C.; Yallapu, M.M.; Uversky, V.N.; Bezbaruah, R.; Patel, S.; Apostolopoulos, V. COVID-19 and Vaccination: Myths vs Science. Expert Rev. Vaccines 2022, 21, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Chavda, V.P.; Gajjar, N.; Shah, N.; Dave, D.J. Darunavir Ethanolate: Repurposing an Anti-HIV Drug in COVID-19 Treatment. Eur. J. Med. Chem. Rep. 2021, 3, 100013. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. MRNA-Based Therapeutics: Powerful and Versatile Tools to Combat Diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in MRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Chavda, V.P.; Soni, S.; Prajapati, R.; Yallapu, M.M.; Apostolopoulos, V. Reply to the Letter ‘Effectiveness of COVID-19 Vaccines against Omicron Variant’. Immunotherapy 2022, 14, 905–908. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Athalye, M.; Parikh, P.K.; Chhipa, A.S.; Patel, S.; Apostolopoulos, V. Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses 2022, 14, 759. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Is Booster Dose Strategy Sufficient for Omicron Variant of SARS-CoV-2? Vaccines 2022, 10, 367. [Google Scholar] [CrossRef]
- Chavda, V.P.; Hanuma Kumar Ghali, E.N.; Yallapu, M.M.; Apostolopoulos, V. Therapeutics to Tackle Omicron Outbreak. Immunotherapy 2022, 14, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 Variants and Vulnerability at the Global Level. J. Med. Virol. 2022, 94, 2986–3005. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Ertas, Y.N.; Walhekar, V.; Modh, D.; Doshi, A.; Shah, N.; Anand, K.; Chhabria, M. Advanced Computational Methodologies Used in the Discovery of New Natural Anticancer Compounds. Front. Pharmacol. 2021, 12, 702611. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chavda, V.P.; Vora, L.K.; Gajjar, N.; Apostolopoulos, V.; Shah, N.; Chen, Z.-S. 2-Deoxy-D-Glucose and Its Derivatives for the COVID-19 Treatment: An Update. Front. Pharmacol. 2022, 13, 899633. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. MRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Chavda, V.P.; Vora, L.K.; Apostolopoulos, V. Inhalable Vaccines: Can They Help Control Pandemics? Vaccines 2022, 10, 1309. [Google Scholar] [CrossRef]
- Chavda, V.P.; Hossain, M.K.; Beladiya, J.; Apostolopoulos, V. Nucleic Acid Vaccines for COVID-19: A Paradigm Shift in the Vaccine Development Arena. Biologics 2021, 1, 337–356. [Google Scholar] [CrossRef]
- Chavda, V.P.; Pandya, R.; Apostolopoulos, V. DNA Vaccines for SARS-CoV-2: Towards Third Generation Vaccination Era. Expert Rev. Vaccines 2021, 20, 1549–1560. [Google Scholar] [CrossRef]
- He, Q.; Gao, H.; Tan, D.; Zhang, H.; Wang, J.-Z. MRNA Cancer Vaccines: Advances, Trends and Challenges. Acta Pharm. Sin. B 2022, 12, 2969–2989. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. MRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, 6582. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. MRNA Vaccines Manufacturing: Challenges and Bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020, 5, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Shivji, R.; Conocchia, R.; Korakianiti, E.; Jekerle, V. Considerations for the Chemistry, Manufacturing and Controls (CMC)—Quality Package for COVID-19 Vaccines- Interim Lessons Learnt by the European Medicines Agency (EMA). Vaccine 2022, 40, 5539–5541. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 MRNA Vaccines: Platforms and Current Developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef]
- WHO. Relevant WHO Documents for SARS-CoV-2 Vaccines and Other Biologicals; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- FDA. Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization Review Memorandum; FDA: Silver Spring, AR, USA, 2020.
- Walsh, E.E.; Frenck, R.W.J.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Kumar, S.; Zheng, H.; Mahajan, B.; Kozakai, Y.; Morin, M.; Locke, E. Western Blot Assay for Quantitative and Qualitative Antigen Detection in Vaccine Development. Curr. Protoc. Microbiol. 2014, 33, 18.4.1–18.4.11. [Google Scholar] [CrossRef]
- Whitley, J.; Zwolinski, C.; Denis, C.; Maughan, M.; Hayles, L.; Clarke, D.; Snare, M.; Liao, H.; Chiou, S.; Marmura, T.; et al. Development of MRNA Manufacturing for Vaccines and Therapeutics: MRNA Platform Requirements and Development of a Scalable Production Process to Support Early Phase Clinical Trials. Transl. Res. J. Lab. Clin. Med. 2022, 242, 38–55. [Google Scholar] [CrossRef]
- Kremsner, P.; Mann, P.; Bosch, J.; Fendel, R.; Gabor, J.J.; Kreidenweiss, A.; Kroidl, A.; Leroux-Roels, I.; Leroux-Roels, G.; Schindler, C.; et al. Phase 1 Assessment of the Safety and Immunogenicity of an MRNA- Lipid Nanoparticle Vaccine Candidate Against SARS-CoV-2 in Human Volunteers. medRxiv 2020. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-Amplifying RNA Vaccines for Infectious Diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Startups Set off New Wave of MRNA Therapeutics. Nat. Biotechnol. 2021, 39, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Li, C.; Yang, T.; Hu, B.; Zhang, M.; Guo, S.; Xiao, H.; Liang, X.-J.; Huang, Y. The Challenge and Prospect of MRNA Therapeutics Landscape. Biotechnol. Adv. 2020, 40, 107534. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying MRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef]
- Self-Amplifying MRNA Vaccine (SAM). Available online: https://www.creative-biolabs.com/vaccine/self-amplifying-mrna-sam-vaccine-platform.htm (accessed on 2 December 2022).
- Luisi, K.; Morabito, K.M.; Burgomaster, K.E.; Sharma, M.; Kong, W.-P.; Foreman, B.M.; Patel, S.; Fisher, B.; Aleshnick, M.A.; Laliberte, J.; et al. Development of a Potent Zika Virus Vaccine Using Self-Amplifying Messenger RNA. Sci. Adv. 2020, 6, eaba5068. [Google Scholar] [CrossRef]
- Magini, D.; Giovani, C.; Mangiavacchi, S.; Maccari, S.; Cecchi, R.; Ulmer, J.B.; De Gregorio, E.; Geall, A.J.; Brazzoli, M.; Bertholet, S. Self-Amplifying MRNA Vaccines Expressing Multiple Conserved Influenza Antigens Confer Protection against Homologous and Heterosubtypic Viral Challenge. PLoS ONE 2016, 11, e0161193. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.; Xu, M.; He, Q.; Li, C.; Meng, S.; Wang, Y.; Cui, B.; Liang, Z.; Wang, J. COVID-19 Vaccines: Progress and Understanding on Quality Control and Evaluation. Signal Transduct. Target. Ther. 2021, 6, 199. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of MRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle-MRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Malburet, C.; Leclercq, L.; Cotte, J.-F.; Thiebaud, J.; Bazin, E.; Garinot, M.; Cottet, H. Size and Charge Characterization of Lipid Nanoparticles for MRNA Vaccines. Anal. Chem. 2022, 94, 4677–4685. [Google Scholar] [CrossRef]
- Saunders, K.O.; Pardi, N.; Parks, R.; Santra, S.; Mu, Z.; Sutherland, L.; Scearce, R.; Barr, M.; Eaton, A.; Hernandez, G.; et al. Lipid Nanoparticle Encapsulated Nucleoside-Modified MRNA Vaccines Elicit Polyfunctional HIV-1 Antibodies Comparable to Proteins in Nonhuman Primates. NPJ Vaccines 2021, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jeklenec, A.; Langer, R.; Blankschtein, D. MRNA Vaccine Delivery Using Lipid Nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently Neutralizing and Protective Human Antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by MRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [Green Version]
- Labouta, H.I.; Langer, R.; Cullis, P.R.; Merkel, O.M.; Prausnitz, M.R.; Gomaa, Y.; Nogueira, S.S.; Kumeria, T. Role of Drug Delivery Technologies in the Success of COVID-19 Vaccines: A Perspective. Drug Deliv. Transl. Res. 2022, 12, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Tavilani, A.; Abbasi, E.; Kian Ara, F.; Darini, A.; Asefy, Z. COVID-19 Vaccines: Current Evidence and Considerations. Metab. Open 2021, 12, 100124. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. MRNA Based SARS-CoV-2 Vaccine Candidate CVnCoV Induces High Levels of Virus Neutralizing Antibodies and Mediates Protection in Rodents. bioRxiv 2020. [Google Scholar] [CrossRef]
- Nordstr€, P.; Ballin, M.; Nordstr€ Om, A. Effectiveness of a Fourth Dose of MRNA COVID-19 Vaccine against All-Cause Mortality in Long-Term Care Facility Residents and in the Oldest Old: A Nationwide, Retrospective Cohort Study in Sweden. Lancet Reg. Health-Eur. 2022, 21, 100466. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The Socio-Economic Implications of the Coronavirus Pandemic (COVID-19): A Review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A. Hydroxychloroquine in the Prevention of COVID-19 Mortality. Lancet Rheumatol. 2021, 3, e2–e3. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Rane, M.; Solano, J.; Alter, S.; Johnson, H.; Krishnaswamy, S.; Shih, R.; Maki, D.; DeMets, D.L. Updates on Hydroxychloroquine in Prevention and Treatment of COVID-19. Am. J. Med. 2022, 135, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Prajapati, R.; Lathigara, D.; Nagar, B.; Kukadiya, J.; Redwan, E.M.; Uversky, V.N.; Kher, M.N.; Patel, R. Therapeutic Monoclonal Antibodies for COVID-19 Management: An Update. Expert Opin. Biol. Ther. 2022, 22, 763–780. [Google Scholar] [CrossRef]
- Basu, D.; Chavda, V.P.; Mehta, A.A. Therapeutics for COVID-19 and Post COVID-19 Complications: An Update. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100086. [Google Scholar] [CrossRef]
- Bégin, P.; Callum, J.; Jamula, E.; Cook, R.; Heddle, N.M.; Tinmouth, A.; Zeller, M.P.; Beaudoin-Bussières, G.; Amorim, L.; Bazin, R.; et al. Convalescent Plasma for Hospitalized Patients with COVID-19: An Open-Label, Randomized Controlled Trial. Nat. Med. 2021, 27, 2012–2024. [Google Scholar] [CrossRef]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Farahani, F.; Khodadadi, I.; Tayebinia, H. Diagnosis and Treatment of Coronavirus Disease 2019 (COVID-19): Laboratory, PCR, and Chest CT Imaging Findings. Int. J. Surg. 2020, 79, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S. COVID-19 Lessons for Research. Science 2021, 371, 1081. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vihol, D.; Vaghasiya, D.D.; Ahmed, K.M.S.B.; Trivedi, K.U.; Dave, D.J. Herbal Remedies, Nutraceuticals, and Dietary Supplements for COVID-19 Management: An Update. Clin. Complement. Med. Pharmacol. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal Vaccines for SARS-CoV-2: From Challenges to Potential in COVID-19 Management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Vihol, D.R. COVAX-19® Vaccine: Completely Blocks Virus Transmission to Non-Immune Individuals. Clin. Complement. Med. Pharmacol. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Krishnan, A.; Gangadaran, P.; Chavda, V.P.; Jogalekar, M.P.; Muthusamy, R.; Valu, D.; Vadivalagan, C.; Ramani, P.; Laishevtcev, A.; Katari, N.K.; et al. Convalescent Serum-Derived Exosomes: Attractive Niche as COVID-19 Diagnostic Tool and Vehicle for MRNA Delivery. Exp. Biol. Med. 2022, 274, 15353702221092984. [Google Scholar] [CrossRef]
- Coccia, M. Optimal Levels of Vaccination to Reduce COVID-19 Infected Individuals and Deaths: A Global Analysis. Environ. Res. 2022, 204, 112314. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Blanchini, F.; Bruno, R.; Colaneri, P.; Di Filippo, A.; Di Matteo, A.; Colaneri, M. Modelling the COVID-19 Epidemic and Implementation of Population-Wide Interventions in Italy. Nat. Med. 2020, 26, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Girum, T.; Lentiro, K.; Geremew, M.; Migora, B.; Shewamare, S. Global Strategies and Effectiveness for COVID-19 Prevention through Contact Tracing, Screening, Quarantine, and Isolation: A Systematic Review. Trop. Med. Health 2020, 48, 91. [Google Scholar] [CrossRef]
- Coccia, M. Pandemic Prevention: Lessons from COVID-19. Encyclopedia 2021, 1, 433–444. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Guo, C.; Atai, N.A.; Gould, S.J. Exosome-Mediated MRNA Delivery For SARS-CoV-2 Vaccination. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, X.; Huang, H.; Tang, S.; Chai, Y.; Xu, Z.; Li, M.; Chen, X.; Liu, J.; et al. Recent Advances in Exosome-Mediated Nucleic Acid Delivery for Cancer Therapy. J. Nanobiotechnol. 2022, 20, 279. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm. Res. 2021, 38, 473–478. [Google Scholar] [CrossRef]
- Chavda, V.P.; Yao, Q.; Vora, L.K.; Apostolopoulos, V.; Patel, C.A.; Bezbaruah, R.; Patel, A.B.; Chen, Z.-S. Fast-Track Development of Vaccines for SARS-CoV-2: The Shots That Saved the World. Front. Immunol. Online First. 2022, 13. [Google Scholar] [CrossRef]
- Chavda, V.P. Chapter 4—Nanobased Nano Drug Delivery: A Comprehensive Review. In Micro and Nano Technologies; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S.B.T.-A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–92. ISBN 978-0-12-814029-1. [Google Scholar]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Kalyanram, P.; Puri, A.; Gupta, A. Thermotropic Effects of PEGylated Lipids on the Stability of HPPH-Encapsulated Lipid Nanoparticles (LNP). J. Therm. Anal. Calorim. 2022, 147, 6337–6348. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Huang, L.; Liu, T. Development and Delivery Systems of MRNA Vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. MRNA Vaccine: A Potential Therapeutic Strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Granados-Riveron, J.T.; Aquino-Jarquin, G. Engineering of the Current Nucleoside-Modified MRNA-LNP Vaccines against SARS-CoV-2. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 142, 111953. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse Effects of COVID-19 MRNA Vaccines: The Spike Hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic MRNA Delivery. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Son, S.; Nam, J.; Zenkov, I.; Ochyl, L.J.; Xu, Y.; Scheetz, L.; Shi, J.; Farokhzad, O.C.; Moon, J.J. Sugar-Nanocapsules Imprinted with Microbial Molecular Patterns for MRNA Vaccination. Nano Lett. 2020, 20, 1499–1509. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for MRNA Vaccines. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–40. [Google Scholar]
- Grau, M.; Walker, P.R.; Derouazi, M. Mechanistic Insights into the Efficacy of Cell Penetrating Peptide-Based Cancer Vaccines. Cell. Mol. Life Sci. 2018, 75, 2887–2896. [Google Scholar] [CrossRef]
- Jarzebska, N.T.; Mellett, M.; Frei, J.; Kündig, T.M.; Pascolo, S. Protamine-Based Strategies for RNA Transfection. Pharmaceutics 2021, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and Clinical Application of a Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Siewert, C.D.; Haas, H.; Cornet, V.; Nogueira, S.S.; Nawroth, T.; Uebbing, L.; Ziller, A.; Al-Gousous, J.; Radulescu, A.; Schroer, M.A.; et al. Hybrid Biopolymer and Lipid Nanoparticles with Improved Transfection Efficacy for MRNA. Cells 2020, 9, 2034. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Kandimalla, R.; Wallen, M.; Tyagi, N.; Wilcher, S.; Yan, J.; Schultz, D.J.; Spencer, W.; et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021, 505, 58–72. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Delehedde, C.; Even, L.; Midoux, P.; Pichon, C.; Perche, F. Intracellular Routing and Recognition of Lipid-Based MRNA Nanoparticles. Pharmaceutics 2021, 13, 945. [Google Scholar] [CrossRef]
- Aslan, C.; Kiaie, S.; Zolbanin, N.M.; Lotfinejad, P.; Ramezani, R.; Kashanchi, F.; Jafari, R. Exosomes for MRNA Delivery: A Novel Biotherapeutic Strategy with Hurdles and Hope. BMC Biotechnol. 2021, 21, 20. [Google Scholar] [CrossRef]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Singh, P.A.; Baldi, A.; Bajwa, N.; Kumar, R.; et al. A Role for Extracellular Vesicles in SARS-CoV-2 Therapeutics and Prevention. J. Neuroimmune Pharmacol. 2021, 16, 270–288. [Google Scholar] [CrossRef]
- Vivek, P. Chavda, Dhaval Shah. A Review on Novel Emulsification Technique: A Nanoemulsion. Trends Drug Deliv. 2016, 3, 25–34. [Google Scholar]
- Chavda, V.P. Nanotherapeutics and Nanobiotechnology. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–13. [Google Scholar]
- Chavda, V.P.; Shah, D. Chapter 25—Self-Emulsifying Delivery Systems: One Step Ahead in Improving Solubility of Poorly Soluble Drugs. In Micro and Nano Technologies; Ficai, A., Grumezescu, A.M.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 653–718. ISBN 978-0-323-46144-3. [Google Scholar]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Frey, S.E.; Shakib, S.; Chanthavanich, P.; Richmond, P.; Smith, T.; Tantawichien, T.; Kittel, C.; Jaehnig, P.; Mojares, Z.; Verma, B.; et al. Safety and Immunogenicity of MF59-Adjuvanted Cell Culture-Derived A/H5N1 Subunit Influenza Virus Vaccine: Dose-Finding Clinical Trials in Adults and the Elderly. Open Forum Infect. Dis. 2019, 6, ofz107. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W.; et al. A Cationic Nanoemulsion for the Delivery of Next-Generation RNA Vaccines. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.G.; Oladunni, F.S.; Rohaim, M.A.; Whittingham-Dowd, J.; Tollitt, J.; Hodges, M.D.J.; Fathallah, N.; Assas, M.B.; Alhazmi, W.; Almilaibary, A.; et al. Immunogenicity and Protective Efficacy of an Intranasal Live-Attenuated Vaccine against SARS-CoV-2. iScience 2021, 24, 102941. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Casettari, L.; Illum, L. Nasal Vaccination against SARS-CoV-2: Synergistic or Alternative to Intramuscular Vaccines? Int. J. Pharm. 2021, 603, 120686. [Google Scholar] [CrossRef]
- Yusuf, H.; Kett, V. Current Prospects and Future Challenges for Nasal Vaccine Delivery. Hum. Vaccines Immunother. 2016, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early Local Immune Defences in the Respiratory Tract. Nat. Rev. Immunol. 2016, 17, 7–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, C.; Fontana, F.; Cheng, R.; Santos, H.A. Development of Vaccine Formulations: Past, Present, and Future. Drug Deliv. Transl. Res. 2021, 11, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 Vaccines: From Bench to Bed. eBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Dhama, K.; Dhawan, M.; Tiwari, R.; Emran, T.B.; Mitra, S.; Rabaan, A.A.; Alhumaid, S.; Al Alawi, Z.; Al Mutair, A. COVID-19 Intranasal Vaccines: Current Progress, Advantages, Prospects, and Challenges. Hum. Vaccines Immunother. 2022, 18, 2045853. [Google Scholar] [CrossRef]
- Ouranidis, A.; Vavilis, T.; Mandala, E.; Davidopoulou, C.; Stamoula, E.; Markopoulou, C.K.; Karagianni, A.; Kachrimanis, K. MRNA Therapeutic Modalities Design, Formulation and Manufacturing under Pharma 4.0 Principles. Biomedicines 2021, 10, 50. [Google Scholar] [CrossRef]
- Heine, A.; Juranek, S.; Brossart, P. Clinical and Immunological Effects of MRNA Vaccines in Malignant Diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Zhou, S.; Dain, L.; Mei, L.; Zhu, G. Circular RNA: An Emerging Frontier in RNA Therapeutic Targets, RNA Therapeutics, and MRNA Vaccines. J. Control. Release 2022, 348, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, Y.; Wang, X.; Shen, J.; An, W. Advances in Circular RNA and Its Applications. Int. J. Med. Sci. 2022, 19, 975–985. [Google Scholar] [CrossRef]
- Iparraguirre, L.; Prada-Luengo, I.; Regenberg, B.; Otaegui, D. To Be or Not to Be: Circular RNAs or MRNAs From Circular DNAs? Front. Genet. 2019, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Prats, A.-C.; David, F.; Diallo, L.H.; Roussel, E.; Tatin, F.; Garmy-Susini, B.; Lacazette, E. Circular RNA, the Key for Translation. Int. J. Mol. Sci. 2020, 21, 8591. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for Non-Viral Intracellular Delivery of Messenger RNA Therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef]
- Guan, S.; Therapy, J.R.-G. Nanotechnologies in Delivery of MRNA Therapeutics Using Nonviral Vector-Based Delivery Systems. Gene Ther. 2017, 24, 133–143. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of MRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef]
- Karikó, K.; Whitehead, K.; van der Meel, R. What Does the Success of MRNA Vaccines Tell Us about the Future of Biological Therapeutics? Cell Syst. 2021, 12, 757. [Google Scholar] [CrossRef]
- Rouf, N.Z.; Biswas, S.; Tarannum, N.; Oishee, L.M.; Muna, M.M. Demystifying MRNA Vaccines: An Emerging Platform at the Forefront of Cryptic Diseases. RNA Biol. 2022, 19, 386. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of Multivalent MRNA Vaccine Candidates for Seasonal or Pandemic Influenza. NPJ Vaccines 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing MRNA-Vaccine Technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of MRNA-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Study of MRNA-1345 Vaccine Targeting Respiratory Syncytial Virus (RSV) in Adults ≥50 Years of Age—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05330975 (accessed on 11 November 2022).
- Noor, R. MRNA Vaccines as an Efficient Approach for the Rapid and Robust Induction of Host Immunity Against SARS-CoV-2. Sn Compr. Clin. Med. 2022, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-Term Storage of Lipid-like Nanoparticles for MRNA Delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. MRNA-Based Therapeutics—Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of MRNA Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Rele, S. COVID-19 Vaccine Development during Pandemic: Gap Analysis, Opportunities, and Impact on Future Emerging Infectious Disease Development Strategies. Hum. Vaccines Immunother. 2021, 17, 1122–1127. [Google Scholar] [CrossRef]
- Zhang, N.N.; Li, X.F.; Deng, Y.Q.; Zhao, H.; Huang, Y.J.; Yang, G.; Huang, W.J.; Gao, P.; Zhou, C.; Zhang, R.R.; et al. A Thermostable MRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Kuo, H.-C. The Emerging Roles and Functions of Circular RNAs and Their Generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef] [PubMed]
- Gartlan, C.; Tipton, T.; Salguero, F.J.; Sattentau, Q.; Gorringe, A.; Carroll, M.W. Vaccine-Associated Enhanced Disease and Pathogenic Human Coronaviruses. Front. Immunol. 2022, 13, 882972. [Google Scholar] [CrossRef] [PubMed]
- Badger, C.V.; Richardson, J.D.; Dasilva, R.L.; Richards, M.J.; Josleyn, M.D.; Dupuy, L.C.; Hooper, J.W.; Schmaljohn, C.S. Development and Application of a Flow Cytometric Potency Assay for DNA Vaccines. Vaccine 2011, 29, 6728–6735. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, G.; Särnefält, A.; Kumar, A. Considerations for Bioanalytical Characterization and Batch Release of COVID-19 Vaccines. NPJ Vaccines 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Poveda, C.; Biter, A.B.; Bottazzi, M.E.; Strych, U. Establishing Preferred Product Characterization for the Evaluation of RNA Vaccine Antigens. Vaccines 2019, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Sempowski, G.D.; Saunders, K.O.; Acharya, P.; Wiehe, K.J.; Haynes, B.F. Pandemic Preparedness: Developing Vaccines and Therapeutic Antibodies For COVID-19. Cell 2020, 181, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Clem, A.S. Fundamentals of Vaccine Immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- WHO. Guidelines for Assuring the Quality, Safety, and Efficacy of Plasmid DNA Vaccines; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Lou, G.; Anderluzzi, G.; Schmidt, S.T.; Woods, S.; Gallorini, S.; Brazzoli, M.; Giusti, F.; Ferlenghi, I.; Johnson, R.N.; Roberts, C.W.; et al. Delivery of Self-Amplifying MRNA Vaccines by Cationic Lipid Nanoparticles: The Impact of Cationic Lipid Selection. J. Control. Release 2020, 325, 370–379. [Google Scholar] [CrossRef]
- Abramson, A.; Kirtane, A.R.; Shi, Y.; Zhong, G.; Collins, J.E.; Tamang, S.; Ishida, K.; Hayward, A.; Wainer, J.; Rajesh, N.U.; et al. Oral MRNA Delivery Using Capsule-Mediated Gastrointestinal Tissue Injections. Matter 2022, 5, 975–987. [Google Scholar] [CrossRef]


| Disease | Drug Molecule | Target | Remarks | Reference |
|---|---|---|---|---|
| Development of mRNa technology as a therapeutic target | ||||
| Cystic fibrosis | MRT5005 | CFTR gene | Upon inhalation, MRT5005 delivers the mRNA molecules that encode for functional CFTR protein directly into bronchial epithelial cells. In the interim data analysis of phase-I/II, the molecule was found to be safe and tolerable with no serious side effects. | NCT03375047 |
| Melanoma | BNT111 | TAAs (NY-ESO-1, MAGE-A3, tyrosinase, and TPTE) | The molecule is currently in phase-II clinical trials for its use in patients with anti-PD-1-refractory/relapsed unresectable Stage III or IV melanoma in combination with Cemiplimab. Phase-I results have confirmed the safety and dose of this molecule. Some preliminary antitumor responses were also repoted. | NCT04526899 |
| Development of mRNA technology in infectious diseases other than SARS-Cov-2 | ||||
| Zika virus | mRNA-1893 | - | The molecule is currently under phase-II clinical trials; phase-I results exhibited clear neutralizing antibody response. | NCT04917861 |
| Respiratory syncytial virus | mRNA-1345 | Prefusion F glycoprotein | The vaccine is to be administered in adults 60 years and older, along with a seasonal influenza vaccine. | NCT05330975 |
| Rabies | CV7202 | Rabies virus glycoprotein | In the phase-I study, the vaccines were found to be well tolerated and exhibited adequate neutralizing antibody responses. | NCT03713086 |
| Chikungunya | mRNA-1944 | Anti-CK virus mAb | mRNA-1944 is mRNA coated monoclonal antibody CHKV-24. This phase-I trial reported an encouraging safety profile and detectable neutralizing activity. | NCT03829384 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Soni, S.; Vora, L.K.; Soni, S.; Khadela, A.; Ajabiya, J. mRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines 2022, 10, 2150. https://doi.org/10.3390/vaccines10122150
Chavda VP, Soni S, Vora LK, Soni S, Khadela A, Ajabiya J. mRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines. 2022; 10(12):2150. https://doi.org/10.3390/vaccines10122150
Chicago/Turabian StyleChavda, Vivek P., Shailvi Soni, Lalitkumar K. Vora, Shruti Soni, Avinash Khadela, and Jinal Ajabiya. 2022. "mRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics" Vaccines 10, no. 12: 2150. https://doi.org/10.3390/vaccines10122150
APA StyleChavda, V. P., Soni, S., Vora, L. K., Soni, S., Khadela, A., & Ajabiya, J. (2022). mRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines, 10(12), 2150. https://doi.org/10.3390/vaccines10122150








