Epidemiology of Type 3 Poliovirus AFP Cases in Israel between 1973 and 1988: Whole Genome Sequencing of RNA Extracted Directly from Archived Stocks to Avoid Re-Culturing Neurovirulent Wild Poliovirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. AFP Cases and Contacts
“AFP contact sampling is the collection and testing of stool samples from contacts of AFP cases. A contact of an AFP case is defined as a child (preferably younger than five years of age) who likely had direct contact with the AFP case in the week prior to the onset of paralysis and/or in the two-week period after onset of paralysis. …AFP contact sampling is done to increase the sensitivity of the surveillance system to detect circulating polioviruses (wild and/or vaccine-derived) and, during an outbreak, to gain a better understanding of the geographic extent of the transmission.”https://polioeradication.org/tools-and-library/resources-for-polio-eradicators/gpei-tools-protocols-and-guidelines/, last accessed 1 September 2022.
2.2. RNA Extraction from Archived PV3-Positive Isolates for Whole Genomic Sequencing (WGS)
2.3. Whole Genome Sequencing (WGS)
2.4. Phylogenetic Analysis
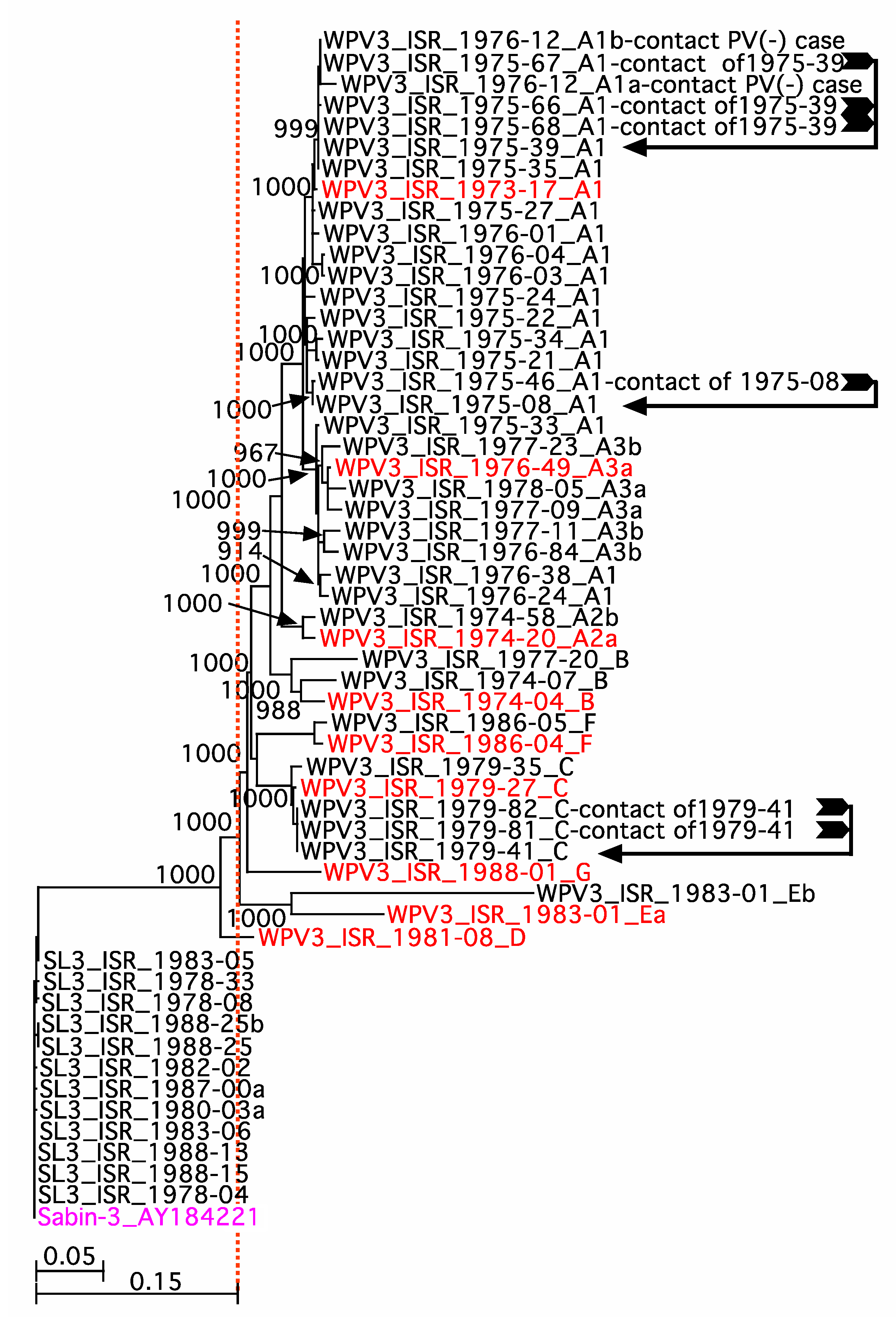
3. Results
4. Discussion
“Testing for polio neutralizing antibodies is not recommended for routine use in the diagnosis of poliomyelitis. It has rarely been useful in clarifying questionable virus diagnoses and requires additional field and laboratory time. Interpreting serum antibody titres is difficult with widespread immunization, and the method does not differentiate between antibodies against wild and vaccine strains.”
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plotkin, S.A.; Vidor, E. Poliovirus vaccine-inactivated. In Vaccines, 5th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Eds.; Saunders/Elsevier: Philadelphia, PA, USA, 2008; pp. 605–629. [Google Scholar]
- Sutter, R.W.; Kew, O.M.; Cochi, S.L. Poliovirus vaccine-live. In Vaccines, 5th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Eds.; Saunders/Elsevier: Philadelphia, PA, USA, 2008; pp. 631–685. [Google Scholar]
- Swartz, T.A. The Epidemiology of Polio in Israel an Historical Perspective; Dyonon Pub. Ltd.: Tel Aviv, Israel, 2008. [Google Scholar]
- Shulman, L.M. Polio and Its Epidemiology. In Encyclopedia of Sustainability Science and Technology Vol. Infectious Diseases; Meyers, R.A., SpringerLink (Online service), Eds.; Springer: New York, NY, USA, 2020; pp. 1–73. [Google Scholar]
- Slater, P.E.; Orenstein, W.A.; Morag, A.; Avni, A.; Handsher, R.; Green, M.S.; Costin, C.; Yarrow, A.; Rishpon, S.; Havkin, O.; et al. Poliomyelitis outbreak in Israel in 1988: A report with two commentaries. Lancet 1990, 335, 1192–1195, discussion 1196–1198. [Google Scholar] [CrossRef]
- GPEI. Global Eradication of Wild Poliovirus Type 2 Declared. 2015. Available online: http://www.polioeradication.org/mediaroom/newsstories/Global-eradication-of-wild-poliovirus-type-2-declared/tabid/526/news/1289/Default.aspx (accessed on 1 September 2016).
- WHO. Two out of Three Wild Poliovirus Strains Eradicated. Global Eradication of Wild Poliovirus Type 3 Declared on World Polio Day. 24 October 2019. Available online: https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wild-poliovirus-strains-eradicated (accessed on 1 March 2021).
- Previsani, N.; Tangermann, R.H.; Tallis, G.; Jafari, H.S. World Health Organization Guidelines for Containment of Poliovirus Following Type-Specific Polio Eradication-Worldwide. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 913–917. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Global Action Plan to Minimize Poliovirus Facility-Associated Risk after Type-Specific Eradication of Wild Polioviruses and Sequential Cessation of Oral Polio Vaccine Use (GAPIII). 2015. Available online: http://www.polioeradication.org/Posteradication/Certification.aspx or https://apps.who.int/iris/handle/10665/208872 (accessed on 30 September 2021).
- Metzker, M.L. Sequencing technologies-the next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laassri, M.; Zagorodnyaya, T.; Hassin-Baer, S.; Handsher, R.; Sofer, D.; Weil, M.; Karagiannis, K.; Simonyan, V.; Chumakov, K.; Shulman, L. Evolution of echovirus 11 in a chronically infected immunodeficient patient. PLoS Pathog. 2018, 14, e1006943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonyan, V.; Mazumder, R. High-Performance Integrated Virtual Environment (HIVE) Tools and Applications for Big Data Analysis. Genes 2014, 5, 957–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagiannis, K.; Simonyan, V.; Chumakov, K.; Mazumder, R. Separation and assembly of deep sequencing data into discrete sub-population genomes. Nucleic Acids Res. 2017, 45, 10989–11003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef] [PubMed]
- Kew, O.M.; Mulders, M.N.; Lipskaya, G.Y.; de Silva, E.; Pallansch, M.A. Molecular Epidemiology of Polioviruses. Semin. Virol. 1995, 6, 401–405. [Google Scholar] [CrossRef]
- Shulman, L.M.; Handsher, R.; Yang, C.F.; Yang, S.J.; Manor, J.; Vonsover, A.; Grossman, Z.; Pallansch, M.; Mendelson, E.; Kew, O.M. Resolution of the pathways of poliovirus type 1 transmission during an outbreak. J. Clin. Microbiol. 2000, 38, 945–952. [Google Scholar] [CrossRef] [PubMed]
- GPEI. Classification and Reporting of Vaccine-Derived Polioviruses (VDPV) GPEI Guidelines. 2016. Available online: http://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf (accessed on 1 January 2022).
- Cello, J.; Paul, A.V.; Wimmer, E. Chemical synthesis of poliovirus cDNA: Generation of infectious virus in the absence of natural template. Science 2002, 297, 1016–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manor, Y.; Blomqvist, S.; Sofer, D.; Alfandari, J.; Halmut, T.; Abramovitz, B.; Mendelson, E.; Shulman, L.M. Advanced environmental surveillance and molecular analyses indicate separate importations rather than endemic circulation of wild type 1 poliovirus in Gaza district in 2002. Appl. Environ. Microbiol. 2007, 73, 5954–5958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, S.; Kumar, N.; Dhanapal, P.; Venkatesan, J.; Kasirajan, A.; Iturriza-Gomara, M.; John, J.; Abraham, A.M.; Grassly, N.C.; Kang, G. Quantity of Vaccine Poliovirus Shed Determines the Titer of the Serum Neutralizing Antibody Response in Indian Children Who Received Oral Vaccine. J. Infect. Dis. 2018, 217, 1395–1398. [Google Scholar] [CrossRef] [PubMed]


| Year | AFP Cases | Total Pop (×105) | Pop ≤ 15 Yrs (×105) | AFP Rate/(×105) ≤15 Yrs | PV(+) AFP Cases | PV3(+) AFP Cases | PV(−) Cases PV3(+) Contacts | PV(+) PV3(−) Cases PV3(+) Contacts |
|---|---|---|---|---|---|---|---|---|
| 1973 | 86 | 3338 | 935 | 9.2 | 26 | 13 | 2 | 3 |
| 1974 | 164 | 3422 | 958 | 17.1 | 69 | 8 | 0 | 0 |
| 1975 | 77 | 3493 | 978 | 7.9 | 38 | 11 | 1 | 2 |
| 1976 | 150 | 3575 | 1001 | 15 | 84 | 9 | 1 | 2 |
| 1977 | 76 | 3653 | 1023 | 7.4 | 25 | 5 | 0 | 0 |
| 1978 | 72 | 3738 | 1047 | 6.9 | 36 | 4 | 0 | 0 |
| 1979 | 77 | 3836 | 1074 | 7.2 | 39 | 4 | 0 | 0 |
| 1980 | 70 | 3922 | 1098 | 6.4 | 40 | 1 | 0 | 0 |
| 1981 | 42 | 3978 | 1114 | 3.8 | 10 | 2 | 0 | 0 |
| 1982 | 32 | 4064 | 1138 | 2.8 | 8 | 2 | 0 | 0 |
| 1983 | 35 | 4119 | 1153 | 3 | 10 | 4 | 0 | 0 |
| 1984 | 22 | 4200 | 1176 | 1.9 | 2 | 0 | 0 | 0 |
| 1985 | 23 | 4266 | 1194 | 1.9 | 6 | 1 | 0 | 0 |
| 1986 | 27 | 4331 | 1213 | 2.2 | 7 | 3 | 0 | 0 |
| 1987 | 12 | 4407 | 1234 | 1 | 4 | 1 | 0 | 0 |
| 1988 | 55 | 4477 | 1254 | 4.4 | 23 | 5 | 0 | 1 |
| Total | 1020 | 427 | 73 | 4 | 8 |
| ID | Number | Description |
|---|---|---|
| AFP Cases | ||
| T2.01 | 1020 | AFP cases investigated between 1973 and 1988 |
| T2.02 | 427 | AFP cases with PV(+) stools |
| T2.03 | 85 | AFP cases with PV3(+) stools from the index case and/or contacts |
| T2.04 | 73 | AFP cases with PV3(+) positive stools |
| T2.05 | 8 | AFP PV(+)/PV3(−) cases with PV3(+) contacts |
| T2.06 | 4 | AFP PV(−) cases with PV3(+) contacts |
| Archived PV Isolates | ||
| T2.07 | 257 | Archived PV isolates (Types 1, 2, and 3) |
| T2.08 | 93 | PV3(+) isolates reported for the index case and/or contacts (isolates from 93 individuals) |
| T2.09 | 71 of the 93 | Archived PV3 isolates (isolates: 70 labeled PV3; 1 labeled PV1) from 58 cases with archived PV3(+) samples from the index case and/or contact |
| Whole Genome Sequences (WGS) | ||
| T2.10 | 55 | PV3(+) isolates with WGS from 52 WPV3(+) cases and 1 WPV1(+) case |
| T2.11 | 53 of the 55 | PV3(+) WGS from 51 WPV3(+) cases and 1 WPV1(+) case |
| T2.12 | 2 of the 55 | WGS available from PV3(-), PV(+) cases |
| T2.13 | 43 of 55 | WPV3(+) WGS from 42 archived isolates from 34 cases |
| T2.14 | 12 of 55 | SL3(+) WGS from 11 archived isolates from 11 cases—one sample contained two isolates |
| T2.15 | 9 of 12 | Isolates from VP3(+) cases (including 2 isolates from one case) |
| T2.16 | 1 of 12 | Isolate from a case with a mixture of PVs |
| T2.17 | 1 of 12 | Isolate from a case with a major incomplete WPV3 WGS component |
| Genetic lineages | ||
| T2.18 | 7 | Lineages (arbitrarily labeled A to G) |
| T2.19 | 23 | Isolates with WGS from Lineage A |
| T2.20 | 15 | Isolates in sub lineage A1 |
| T2.21 | 2 | Isolates in sub lineage A2 |
| T2.22 | 6 | Isolates in sub lineage A3 |
| T2.23 | 8 | SL3 a with recombinant genomes |
| PV3(+) Cases and Contacts a (n = 93T2.08) | PV3(+) Case b (n = 73T2.04) | |
|---|---|---|
| Ethnicity | ||
| Jewish | 23 | 16 |
| Non-Jewish (Arab) | 69 | 56 |
| Non-Jewish (Druse) | 1 | 1 |
| Not listed | 0 | 0 |
| Geographical location | ||
| Israel (all health districts) | 39 | 26 |
| West Bank/E. Jerusalem | 5 | 4 |
| Gaza | 45 | 41 |
| Not listed | 3 | 2 |
| Other (Jordan) | 1 | 0 |
| Gender | ||
| Male | 56 (60%) | 47 (64%) |
| Female | 33 (36%) | 23 (32%) |
| Not recorded/not clear | 4 (4%) | 3 (4%) |
| Description of Archived Sample | Number Archived | Number Sequenced | ||
|---|---|---|---|---|
| Total | WPV3 | SL3 | ||
| Isolates from Cases | ||||
| PV3-positive | 53T2.11 | 43T2.13 | 33 | 10 a |
| PV3-positive and PV1-positive | 2 | 1 | 0 | 1 |
| PV3-positive and PV2-positive | 1 | 1 | 1 | 0 |
| PV3-positive and PV1- and PV2-positive | 2 | 0 | 0 | 0 |
| Isolates from PV3-positive Contacts | ||||
| PV-negative cases | 4 | 1 | 1 | 0 |
| PV1-positive cases | 3 | 0 | 0 | 0 |
| PV2-positive cases | 2 | 0 | 0 | 0 |
| PV3-positive cases | 7 | 6 | 6 | 0 |
| Anomalous result | ||||
| PV3 sequence from a PV1-positive case | 1 | 1 | 1 | 0 |
| 5′UTR | VP3 | VP1 | OPV Doses | Interval between Last OPV and Symptoms | Recombinant | |
|---|---|---|---|---|---|---|
| Nucleotide Substitution | U472C | U2034C | U2493C | |||
| Amino Acid Substitution | n/a | phe91ser | thr6iso | |||
| SL3_ISR-1978-04 | C | phe | iso | 1 | <30 days | No |
| SL3_ISR-1978-08 | U472C | ser | iso | 1 | 18 days | Yes |
| SL3_ISR-1978-33 | U472C | phe | iso | 1 | 1 day | Yes |
| SL3_ISR-1980-03a | U472C | phe | iso | 0 | unvaccinated | Yes |
| SL3_ISR-1982-02 | U472C | ser | iso | 3 | 176 days | Yes |
| SL3_ISR-1983-05 | U472C | ser | iso | 1 | 44 days | Yes |
| SL3_ISR-1983-06 | U472C | phe | iso | 2 | 8 days | Yes |
| SL3_ISR-1987-00 | U472C | phe | iso | 1 | <30 days | No |
| SL3_ISR-1988-13 | U472C | phe | iso | Unk | Years | Yes |
| SL3_ISR-1988-15 | U472C | phe | iso | 2 | 7 days | Yes |
| SL3_ISR-1988-25 | U472C | ser | iso | 1 | 14 days | No |
| OPV Vaccination History | ||||||
|---|---|---|---|---|---|---|
| PV3(+) (n = 85T2.03) | PV3(+) Case a (n = 73T2.04) | PV3(+) Cases with Sequenced Isolates | ||||
| PV3(+) (n = 43T2.13) | WPV3(+) A1 (n = 15T2.20) | WPV3(+) A3 (n = 6T2.22) | SL3(+) (n = 11T2.14) | |||
| Unvaccinated b | 18 b | 17 b | 9 | 3 | 1 | 1 |
| 1 dose OPV | 18 | 13 | 8 | 3 | 0 | 6 c |
| 2 doses OPV d | 8 | 7 | 3 | 0 | 0 | 2 |
| 3 doses OPV e | 23 | 21 | 12 | 9 | 1 | 1 |
| 4 doses of OPV | 12 | 9 | 7 | 0 | 4 | 0 |
| Not recorded or incomplete f | 6 | 6 | 4 | 0 | 0 | 1 |
| Days between last OPV dose and appearance of symptoms | ||||||
| PV3(+) (n = 55T2.10/85T2.03) | PV3(+) case b (n = 43T2.13/73T2.04) | PV3(+) Cases with Sequenced Isolates c | ||||
| PV3(+) (n = 30/43T2.13) | WPV3(+) A1 (n = 12/15T2.20) | WPV3(+) A3 (n = 5/6T2.22) | SL3(+) (n = 6/11T2.14) | |||
| Average | 219 | 244 | 175 | 164 | 243 | 45 |
| Median | 113 | 130 | 131 | 155 | 331 | 16 |
| Minimum | 3 | 7 | 7 | 24 | 24 | 7 |
| Maximum | 2006 | 2006 | 968 | 355 | 421 | 176 |
| Number < 30 days | 19 | 13 | 8 | 2 | 1 | 5 |
| Symptom | PV3(+) (n = 85T2.03) | PV3(+) Cases a (n = 73T2.04) | Sequence Confirmed Cases | ||||
|---|---|---|---|---|---|---|---|
| PV3(+) (n = 43T2.13) | WPV3(+) A1 (n = 15T2.20) | WPV3(+) A3 (n = 6T2.22) | SL3 (n = 10T2.14) b | ||||
| Fever | 14 | 12 | 0 | 0 | 0 | 0 | |
| Rash | 1 | 1 | 0 | 0 | 0 | 0 | |
| Sore throat or problem swallowing | 2 | 2 | 0 | 0 | 0 | 0 | |
| Diarrhea/nausea | 2 | 2 | 0 | 0 | 0 | 1 | |
| Respiratory/bulbar | 4 | 4 | 3 | 1 | 0 | 1 | |
| Meningitis/encephalitis | 3 | 2 | 0 | 0 | 0 | 1 | |
| Unconscious/coma | 2 | 2 | 1 | 1 | 0 | 0 | |
| Death | 3 | 3 | 0 | 0 | 0 | 1 | |
| General paresis | 22 | 21 | 9 | 2 | 1 | 3 | |
| Left leg only | 6 | 6 | 5 | 1 | 1 | 0 | |
| Right leg only | 2 | 2 | 0 | 0 | 0 | 0 | |
| Left and right legs only | 3 | 3 | 3 | 1 | 0 | 1 | |
| Left arm only | 0 | 0 | 0 | 0 | 0 | 0 | |
| Right arm only | 2 | 2 | 0 | 0 | 0 | 0 | |
| Left and right arm only | 0 | 0 | 0 | 0 | 0 | 0 | |
| Arms and legs (any combination) | 7 | 6 | 1 | 0 | 0 | 1 | |
| One leg (not designated) | 2 | 2 | 0 | 0 | 0 | 1 | |
| General paralysis | 56 | 44 | 25 | 12 | 4 | 5 | |
| Left leg only | 23 | 21 | 13 | 6 | 4 | 1 | |
| Right leg only | 6 | 4 | 2 | 1 | 0 | 1 | |
| Left and right legs only | 17 | 15 | 10 | 5 | 0 | 2 | |
| Left arm only | 0 | 0 | 0 | 0 | 0 | 0 | |
| Right arm only | 0 | 0 | 0 | 0 | 0 | 0 | |
| Left and right arm only | 0 | 0 | 0 | 0 | 0 | 0 | |
| Arms and legs (any combination) | 10 | 4 | 0 | 0 | 0 | 0 | |
| Misc (palsy, arthritis, GB, otitis, facialis) | 4 | 4 | 2 | 0 | 0 | 2 | |
| Lineage | PV3 Total (n = 43T2.13) | Positive a or ≥4-Fold Increase in Anti-PV3 IgG | >Anti-PV3 IgG or ≥4-Fold Increase in IgG | Not Done or Not Recorded | ||||
|---|---|---|---|---|---|---|---|---|
| Positive But No Increase a | Sero-Conversion or ≥4-Fold Increase | Total | Anti-PV1 IgG | Anti-PV2 IgG | ||||
| A | 23 | 5 | 9 b | 11 b | 2 | 1 | 6 | |
| A1 | 15 | 5 | 6 b | 11 b | 2 | 0 | 1 | |
| A2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |
| A3 | 6 | 0 | 3 | 3 | 0 | 1 | 3 | |
| B | 3 | 1 | 2 | 3 | 1 c | 0 | 0 | |
| C | 3 | 0 | 3 | 3 | 0 | 0 | 0 | |
| D | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| E | 1 | 0 | 1 | 1 | 0 | 0 | 0 | |
| F | 2 | 1 | 1 | 2 | 0 | 0 | 0 | |
| G | 1 | 1 | 0 | 1 | 1 d | 0 | 0 | |
| SL3 | 11 e | 0 | 3 | 3 | 0 | 3 | 2 | |
| SL3 | 3 | 0 | 1 | 1 | 0 | 1 | 1 | |
| SL3rec | 8 e | 0 | 2 | 2 | 0 | 3 | 1 | |
| PV3-positive Not sequenced | 30 | 2 | 11 | 13 | 6 f | 9 g | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulman, L.M.; Laassri, M.; Handsher, R.; Zagorodnyaya, T.; Sofer, D.; Weil, M.; Mendelson, E.; Chumakov, K. Epidemiology of Type 3 Poliovirus AFP Cases in Israel between 1973 and 1988: Whole Genome Sequencing of RNA Extracted Directly from Archived Stocks to Avoid Re-Culturing Neurovirulent Wild Poliovirus. Vaccines 2022, 10, 2154. https://doi.org/10.3390/vaccines10122154
Shulman LM, Laassri M, Handsher R, Zagorodnyaya T, Sofer D, Weil M, Mendelson E, Chumakov K. Epidemiology of Type 3 Poliovirus AFP Cases in Israel between 1973 and 1988: Whole Genome Sequencing of RNA Extracted Directly from Archived Stocks to Avoid Re-Culturing Neurovirulent Wild Poliovirus. Vaccines. 2022; 10(12):2154. https://doi.org/10.3390/vaccines10122154
Chicago/Turabian StyleShulman, Lester M., Majid Laassri, Rachel Handsher, Tatiana Zagorodnyaya, Danit Sofer, Merav Weil, Ella Mendelson, and Konstantin Chumakov. 2022. "Epidemiology of Type 3 Poliovirus AFP Cases in Israel between 1973 and 1988: Whole Genome Sequencing of RNA Extracted Directly from Archived Stocks to Avoid Re-Culturing Neurovirulent Wild Poliovirus" Vaccines 10, no. 12: 2154. https://doi.org/10.3390/vaccines10122154





