Efficacy of a Novel Multiepitope Vaccine Candidate against Foot-and-Mouth Disease Virus Serotype O and A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Design and Construction of OVM and AVM Genes
2.3. Expression, Purification, and Analysis of Recombinant OVM and AVM Proteins
2.4. Preparation of the Vaccine
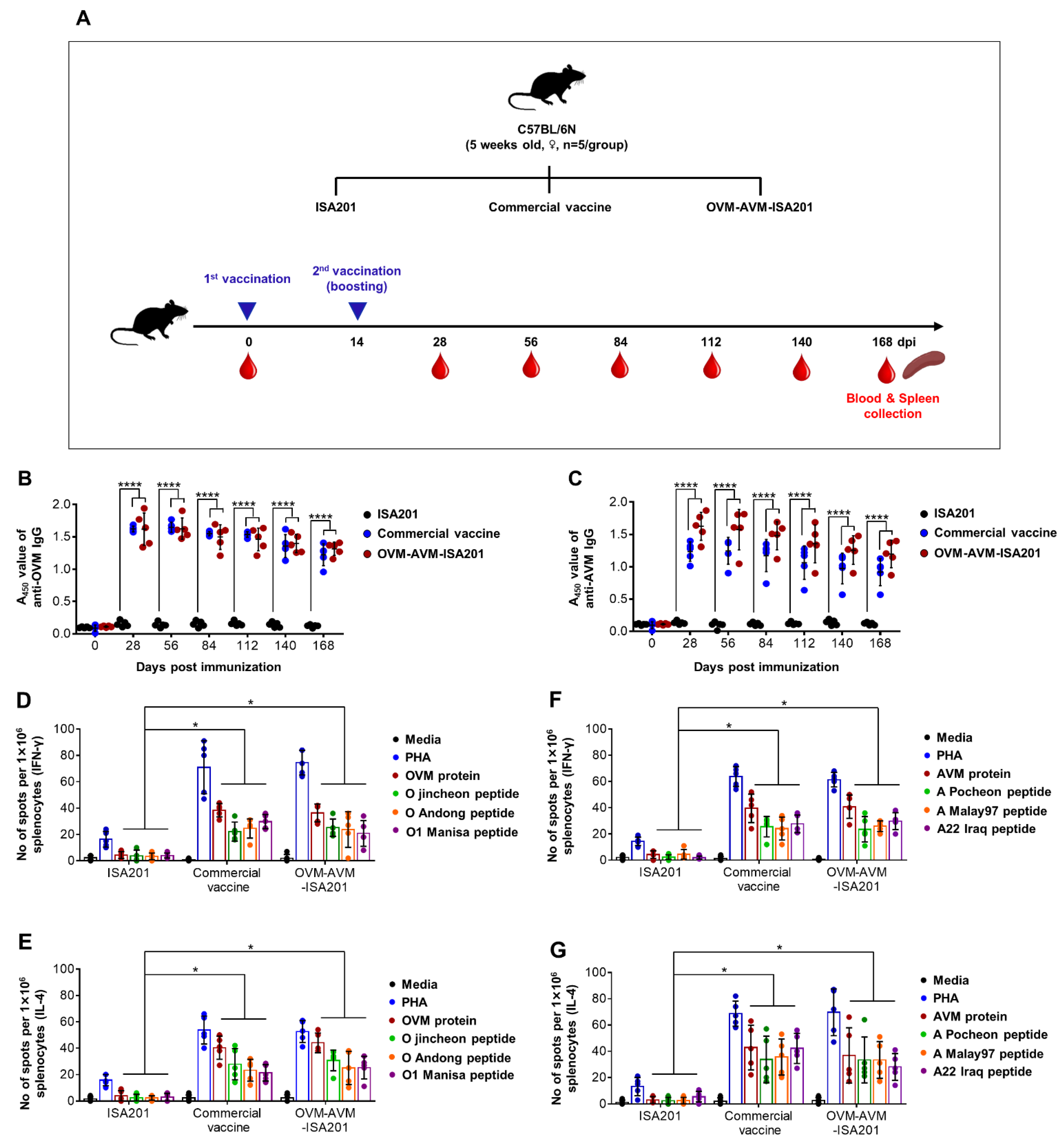
2.5. Evaluation of OVM and AVM Immunogenicity and Protective Potential in Mice
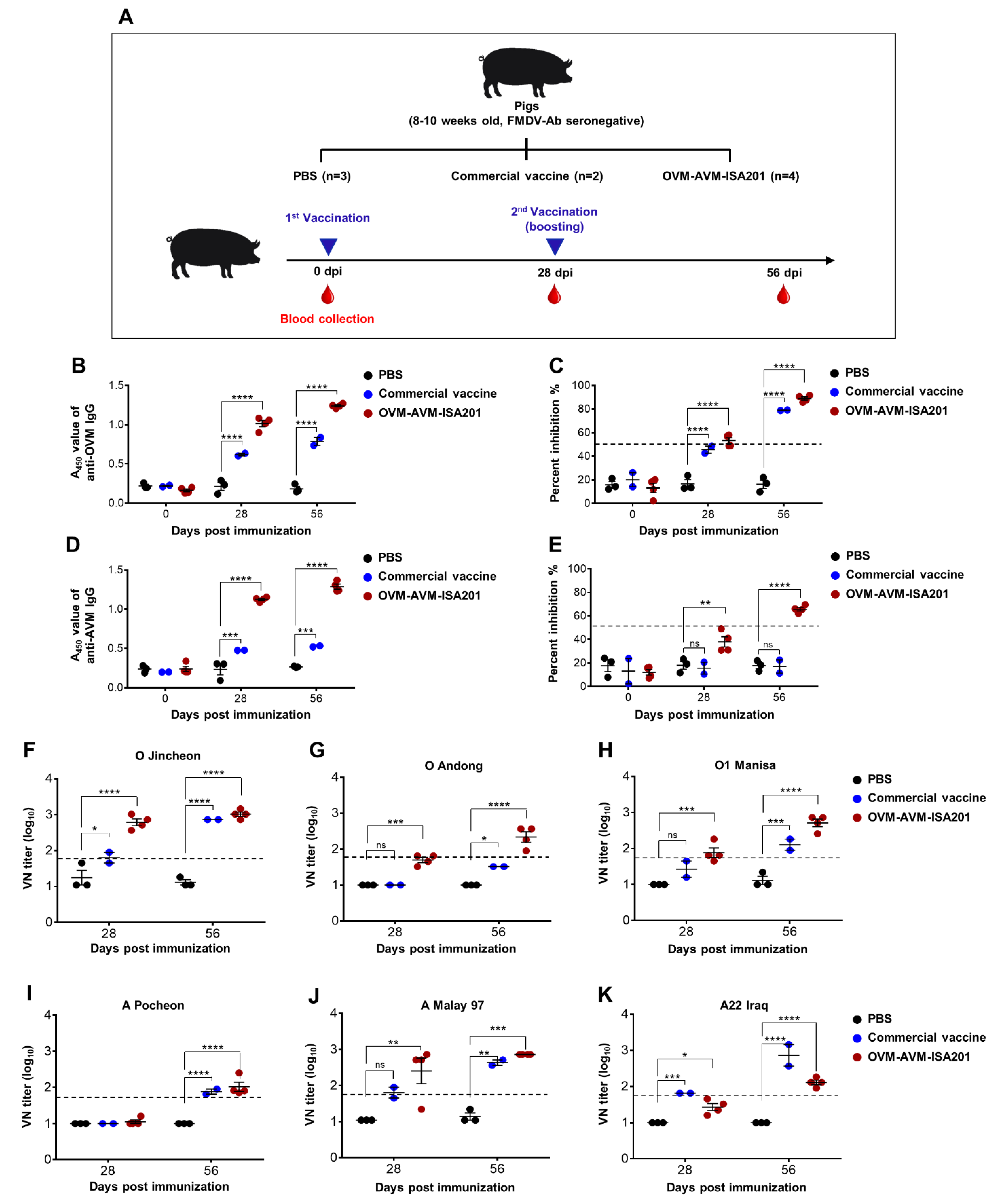
2.6. Confirmation of OVM and AVM Immunogenicity and Protective Potential in Pigs
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. ELISPOT
2.9. Virus Neutralization Test
2.10. Statistical Analysis
3. Results
3.1. Expression and Characterization of Recombinant OVM and AVM Proteins
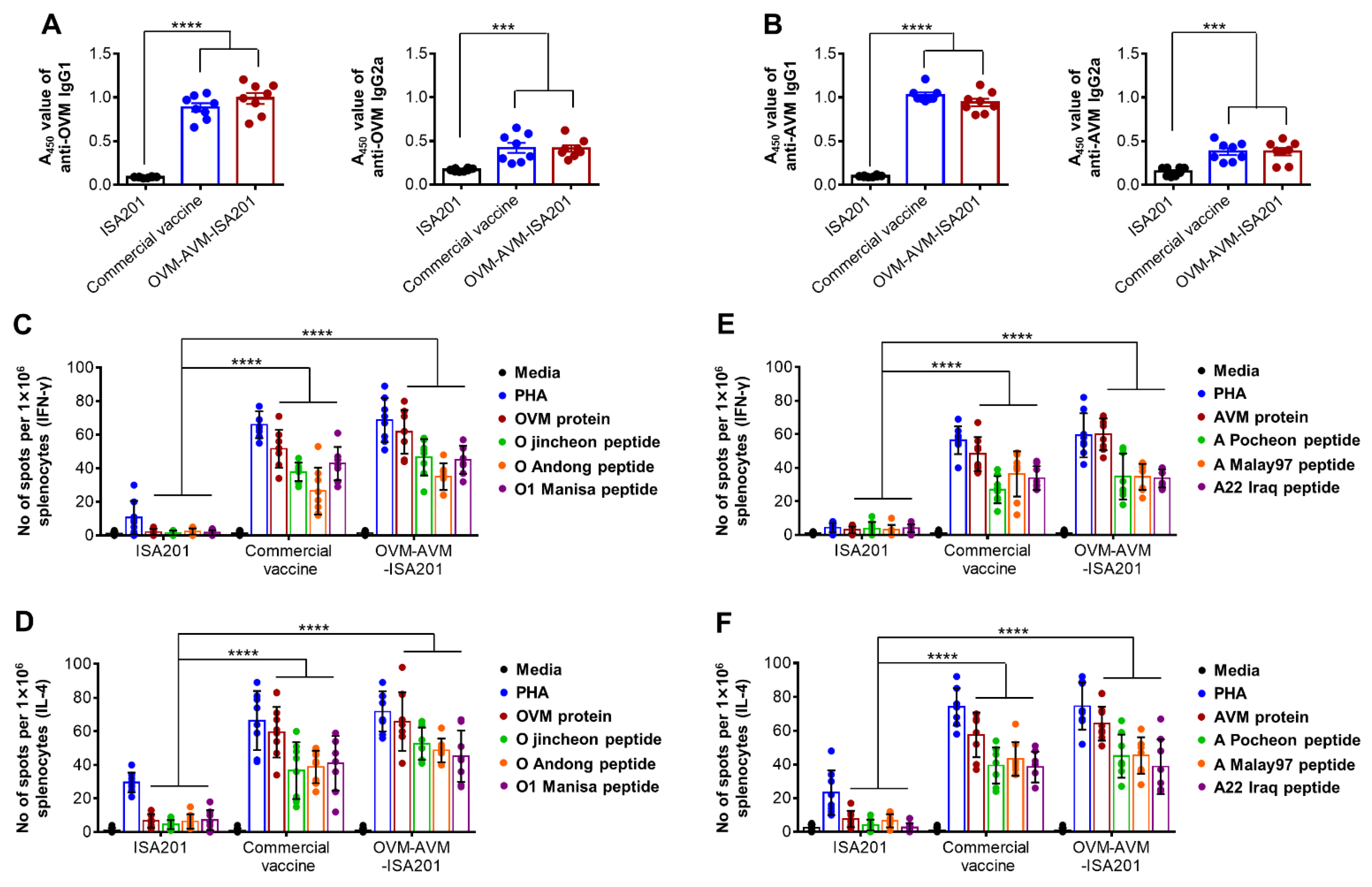
3.2. Recombinant OVM and AVM Emulsified with ISA201 Induce Antigen-Specific Humoral Immune Responses in Mice
3.3. Vaccination with OVM and AVM Emulsified with ISA201 Enhances Antigen-Specific Cell-Mediated Immune Responses in Mice
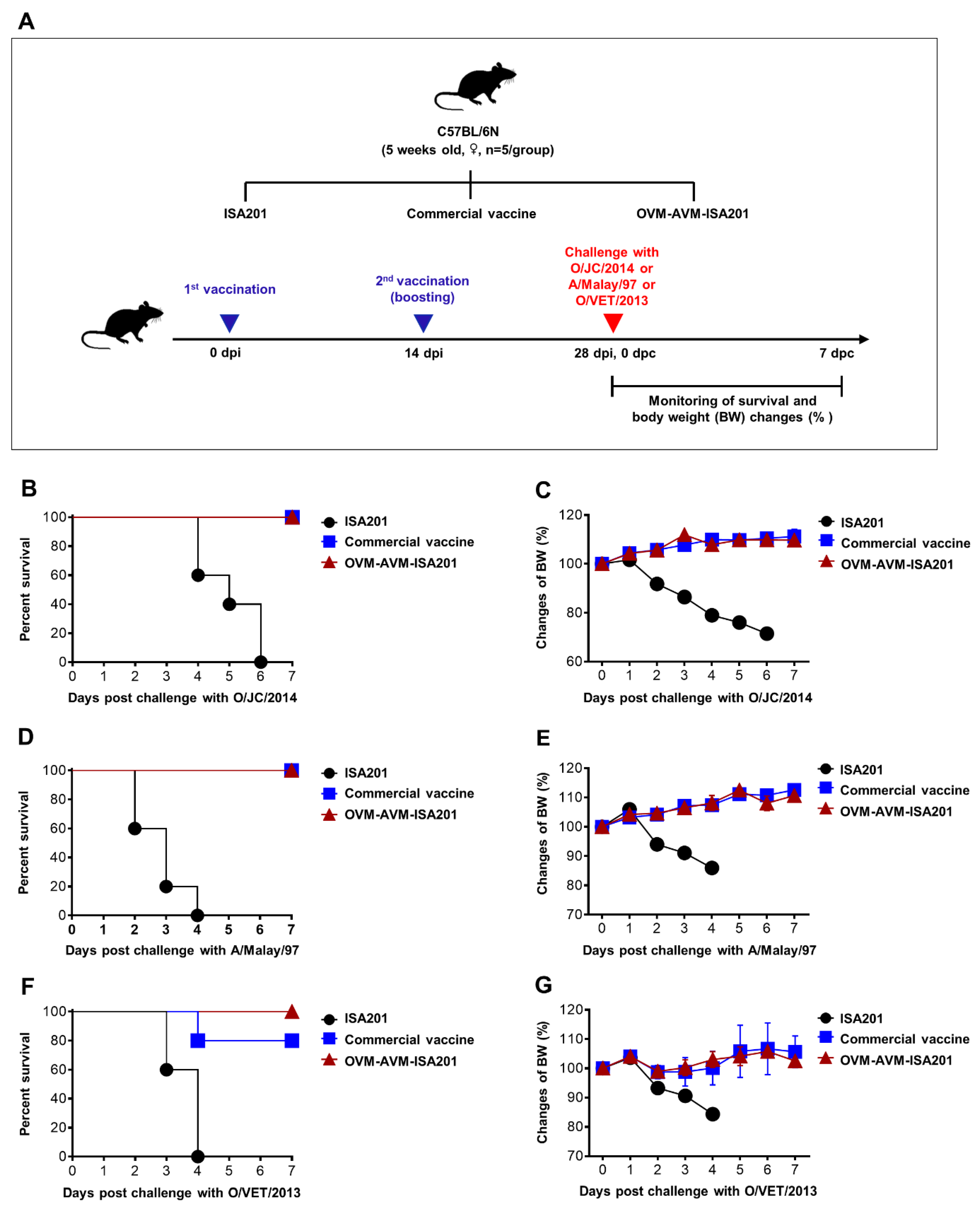
3.4. Vaccination with OVM and AVM Emulsified with ISA201 Protects Mice from Homologous and Heterologous FMDV Challenges
3.5. Vaccination with OVM and AVM Emulsified with ISA201 Induces Long-Lasting Immunity in Mice
3.6. Vaccination with OVM and AVM Emulsified with ISA201 Elicits a Potent Humoral Immune Response in Pigs
3.7. Vaccination with OVM and AVM Emulsified with CAvant®SOE-X Confers Effective Protection against FMDV Serotype O and A Infection in Pigs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamal, S.M.; Belsham, G.J. Foot-and-mouth disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, S.; Tuthill, T.J.; Arias, A.; Killington, R.A.; Rowlands, D.J. Foot-and-mouth disease virus assembly: Processing of recombinant capsid precursor by exogenous protease induces self-assembly of pentamers in vitro in a myristoylation-dependent manner. J. Virol. 2009, 83, 11275–11282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Lu, Z.; Li, D.; Fan, P.; Sun, P.; Bao, H.; Fu, Y.; Li, P.; Bai, X.; Chen, Y. Evaluation of cross-protection against three topotypes of serotype O foot-and-mouth disease virus in pigs vaccinated with multi-epitope protein vaccine incorporated with poly (I: C). Vet. Microbiol. 2014, 168, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Frias-De-Diego, A.; Dellicour, S.; Baele, G.; Machado, G. Global phylogeographical patterns in the spread of foot-and-mouth disease virus. bioRxiv 2019, 590612. [Google Scholar] [CrossRef]
- Jo, H.-E.; Ko, M.-K.; Choi, J.-H.; Shin, S.H.; Jo, H.; You, S.-H.; Lee, M.J.; Kim, S.-M.; Kim, B.; Park, J.-H. New foot-and-mouth disease vaccine, O JC-R, induce complete protection to pigs against SEA topotype viruses occurred in South Korea, 2014–2015. J. Vet. Sci. 2019, 20, e42. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, K.; Ko, Y.; Kim, S.; Lee, H.; Park, J.; Yeh, J.; Kim, M.; Lee, Y.; Sohn, H. Diagnosis and Control Measures of the 2010 Outbreak of Foot-and-Mouth Disease A Type in the Republic of Korea. Transbound. Emerg. Dis. 2013, 60, 188–192. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, S.-Y.; Kim, J.-S.; Kim, A.-Y.; Park, S.-Y.; Lee, J.-H.; Lee, M.; Kim, H.; Lee, S.-I.; Kang, N.-Y. Scale-Up Production of Type O and A Foot-and-Mouth Disease Bivalent Vaccine and Its Protective Efficacy in Pigs. Vaccines 2021, 9, 586. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Chen, H.T.; Zhou, J.H.; Ding, Y.Z.; Liu, Y.S. Research in advance for FMD novel vaccines. Virol. J. 2011, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Chen, H.-Y.; Wang, Y.; Yin, B.; Lv, C.; Mo, X.; Yan, H.; Xuan, Y.; Huang, Y.; Pang, W. Large-scale production of foot-and-mouth disease virus (serotype Asia1) VLP vaccine in Escherichia coli and protection potency evaluation in cattle. BMC Biotechnol. 2016, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-B.; Piao, D.-C.; Lee, J.-Y.; Choi, J.-Y.; Bok, J.-D.; Cho, C.-S.; Kang, S.-K.; Choi, Y.-J. Artificially designed recombinant protein composed of multiple epitopes of foot-and-mouth disease virus as a vaccine candidate. Microb. Cell Factories 2017, 16, 33. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano Terol, G.; Gallego-Jara, J.; Sola Martinez, R.A.; Martinez Vivancos, A.; Canovas Diaz, M.; de Diego Puente, T. Impact of the expression system on recombinant protein production in Escherichia coli BL21. Front. Microbiol. 2021, 12, 682001. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.H.; Huang, C.-J.; Newton, B.S.; Ritter, G.; Old, L.J.; Batt, C.A. Factors affecting endotoxin removal from recombinant therapeutic proteins by anion exchange chromatography. Protein Expr. Purif. 2009, 64, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Fang, Y.; Pan, L.; Lv, J.; Zhang, Z.; Zhou, P.; Ding, Y.; Chen, H.; Shao, J. Evolutionary analysis of structural protein gene VP1 of foot-and-mouth disease virus serotype Asia 1. Sci. World J. 2015, 2015, 734253. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.S.; Amin, R.; Rahman, M.Z.; Hossain, M.A.; Sultana, M. Antigenic heterogeneity of capsid protein VP1 in foot-and-mouth disease virus (FMDV) serotype Asia 1. Adv. Appl. Bioinform. Chem. AABC 2013, 6, 37. [Google Scholar]
- Bolwell, C.; Clarke, B.; Parry, N.; Ouldridge, E.; Brown, F.; Rowlands, D. Epitope mapping of foot-and-mouth disease virus with neutralizing monoclonal antibodies. J. Gen. Virol. 1989, 70, 59–68. [Google Scholar] [CrossRef]
- McCahon, D.; Crowther, J.; Belsham, G.; Kitson, J.; Duchesne, M.; Have, P.; Meloen, R.; Morgan, D.; De Simone, F. Evidence for at least four antigenic sites on type O foot-and-mouth disease virus involved in neutralization; identification by single and multiple site monoclonal antibody-resistant mutants. J. Gen. Virol. 1989, 70, 639–645. [Google Scholar] [CrossRef]
- Kitson, J.D.; McCahon, D.; Belsham, G.J. Sequence analysis of monoclonal antibody resistant mutants of type O foot and mouth disease virus: Evidence for the involvement of the three surface exposed capsid proteins in four antigenic sites. Virology 1990, 179, 26–34. [Google Scholar] [CrossRef]
- Mahapatra, M.; Hamblin, P.; Paton, D. Foot-and-mouth disease virus epitope dominance in the antibody response of vaccinated animals. J. Gen. Virol. 2012, 93, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Crowther, J.; Farias, S.; Carpenter, W.; Samuel, A. Identification of a fifth neutralizable site on type O foot-and-mouth disease virus following characterization of single and quintuple monoclonal antibody escape mutants. J. Gen. Virol. 1993, 74, 1547–1553. [Google Scholar] [CrossRef]
- Bittle, J.L.; Houghten, R.A.; Alexander, H.; Shinnick, T.M.; Sutcliffe, J.G.; Lerner, R.A.; Rowlands, D.J.; Brown, F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature 1982, 298, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Victoria, M.M.; Theron, J.; Maree, F.; Crampton, M. Production of foot-and-mouth disease virus SAT2 VP1 protein. AMB Express 2020, 10, 2. [Google Scholar]
- Jung, J.-G.; Lee, Y.J.; Velmurugan, N.; Ko, Y.-J.; Lee, H.-S.; Jeong, K.J. High-yield production of the VP1 structural protein epitope from serotype O foot-and-mouth disease virus in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2013, 40, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Xin-sheng, L.; Yong-lu, W.; Yong-guang, Z.; Yu-zhen, F.; Li, P.; Jian-liang, L.; Peng, Z.; Zhong-wang, Z.; Cheng, Q.W.; Gang, W.; et al. Cloning, codon-optimized expression and homology modeling of structural protein VP1 from foot and mouth disease virus. Afr. J. Microbiol. Res. 2011, 5, 486–495. [Google Scholar]
- Cao, Y.; Li, D.; Fu, Y.; Bai, Q.; Chen, Y.; Bai, X.; Jing, Z.; Sun, P.; Bao, H.; Li, P. Rational design and efficacy of a multi-epitope recombinant protein vaccine against foot-and-mouth disease virus serotype A in pigs. Antivir. Res. 2017, 140, 133–141. [Google Scholar] [CrossRef]
- Shao, J.-J.; Wong, C.K.; Lin, T.; Lee, S.K.; Cong, G.-Z.; Sin, F.W.Y.; Du, J.-Z.; Gao, S.-D.; Liu, X.-T.; Cai, X.-P. Promising multiple-epitope recombinant vaccine against foot-and-mouth disease virus type O in swine. Clin. Vaccine Immunol. 2011, 18, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Gray, A.R.; Wood, B.A.; Henry, E.; Azhar, M.; King, D.P.; Mioulet, V. Evaluation of cell lines for the isolation of foot-and-mouth disease virus and other viruses causing vesicular disease. Front. Vet. Sci. 2020, 7, 426. [Google Scholar] [CrossRef]
- Moon, H.-J.; Lee, J.-S.; Talactac, M.R.; Chowdhury, M.Y.; Kim, J.-H.; Park, M.-E.; Choi, Y.-K.; Sung, M.-H.; Kim, C.-J. Mucosal immunization with recombinant influenza hemagglutinin protein and poly gamma-glutamate/chitosan nanoparticles induces protection against highly pathogenic influenza A virus. Vet. Microbiol. 2012, 160, 277–289. [Google Scholar] [CrossRef]
- Park, J.-N.; Lee, S.-Y.; Chu, J.-Q.; Lee, Y.-J.; Kim, R.-H.; Lee, K.-N.; Kim, S.-M.; Tark, D.-S.; Kim, B.; Park, J.-H. Protection to homologous and heterologous challenge in pigs immunized with vaccine against foot-and-mouth disease type O caused an epidemic in East Asia during 2010/2011. Vaccine 2014, 32, 1882–1889. [Google Scholar] [CrossRef]
- Alves, M.; Guzylack-Piriou, L.; Juillard, V.; Audonnet, J.-C.; Doel, T.; Dawson, H.; Golde, W.; Gerber, H.; Peduto, N.; McCullough, K. Innate immune defenses induced by CpG do not promote vaccine-induced protection against foot-and-mouth disease virus in pigs. Clin. Vaccine Immunol. 2009, 16, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Oem, J.; Yeh, M.; McKenna, T.; Hayes, J.; Rieder, E.; Giuffre, A.; Robida, J.; Lee, K.; Cho, I.; Fang, X. Pathogenic characteristics of the Korean 2002 isolate of foot-and-mouth disease virus serotype O in pigs and cattle. J. Comp. Pathol. 2008, 138, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, S.M.; Zhao, Z.-S.; Lo, C.-Y.; Misplon, J.A.; Liu, T.; Ye, Z.; Hogan, R.J.; Wu, Z.; Benton, K.A.; Tumpey, T.M. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg. Infect. Dis. 2007, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Golde, W.T.; Pacheco, J.M.; Duque, H.; Doel, T.; Penfold, B.; Ferman, G.S.; Gregg, D.R.; Rodriguez, L.L. Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: Use in emergency outbreak response. Vaccine 2005, 23, 5775–5782. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, C.; McCullough, S.F. Immunology of foot and mouth disease. In Foot and Mouth Disease; Current Perspectives; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- London, C.A.; Abbas, A.K.; Kelso, A. Helper T cell subsets: Heterogeneity, functions and development. Vet. Immunol. Immunopathol. 1998, 63, 37–44. [Google Scholar] [CrossRef]
- Jo, H.; Kim, B.Y.; Park, S.H.; Kim, H.M.; Shin, S.H.; Hwang, S.Y.; Kim, S.-M.; Kim, B.; Park, J.-H.; Lee, M.J. The HSP70-fused foot-and-mouth disease epitope elicits cellular and humoral immunity and drives broad-spectrum protective efficacy. NPJ Vaccines 2021, 6, 42. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Xing, X.; Bao, H.; Huang, N.; Zhu, G.; Bai, X.; Sun, P.; Fu, Y.; Li, P. Selection of Vaccine Candidate for Foot-and-Mouth Disease Virus Serotype O Using a Blocking Enzyme-Linked Immunosorbent Assay. Vaccines 2021, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H. Requirements for improved vaccines against foot-and-mouth disease epidemics. Clin. Exp. Vaccine Res. 2013, 2, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Shao, J.; Ma, F.; Lei, C.; Chang, H.; Zhang, Y. Enhanced efficacy of a multi-epitope vaccine for type A and O foot-and-mouth disease virus by fusing multiple epitopes with Mycobacterium tuberculosis heparin-binding hemagglutinin (HBHA), a novel TLR4 agonist. Mol. Immunol. 2020, 121, 118–126. [Google Scholar] [CrossRef]
- Szczepanek, S.M.; Barrette, R.W.; Rood, D.; Alejo, D.; Silbart, L.K. Xenoepitope substitution avoids deceptive imprinting and broadens the immune response to foot-and-mouth disease virus. Clin. Vaccine Immunol. 2012, 19, 461–467. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Shang, Y.; Zhang, Z.; Liu, X. How foot-and-mouth disease virus receptor mediates foot-and-mouth disease virus infection. Virol. J. 2015, 12, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fry, E.E.; Newman, J.W.; Curry, S.; Najjam, S.; Jackson, T.; Blakemore, W.; Lea, S.M.; Miller, L.; Burman, A.; King, A.M. Structure of Foot-and-mouth disease virus serotype A1061 alone and complexed with oligosaccharide receptor: Receptor conservation in the face of antigenic variation. J. Gen. Virol. 2005, 86, 1909–1920. [Google Scholar] [CrossRef]
- Fry, E.E.; Lea, S.M.; Jackson, T.; Newman, J.W.; Ellard, F.M.; Blakemore, W.E.; Abu-Ghazaleh, R.; Samuel, A.; King, A.M.; Stuart, D.I. The structure and function of a foot-and-mouth disease virus–oligosaccharide receptor complex. EMBO J. 1999, 18, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; McCahon, D.; Crowther, J.; Belsham, G.; McCullough, K. Neutralization of foot-and-mouth disease virus can be mediated through any of at least three separate antigenic sites. J. Gen. Virol. 1987, 68, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, L.; Li, W.; Zhou, G.; Yu, L. Identification of a conformational epitope on the VP1 GH Loop of type Asia1 foot-and-mouth disease virus defined by a protective monoclonal antibody. Vet. Microbiol. 2011, 148, 189–199. [Google Scholar] [CrossRef]
- Asfor, A.S.; Upadhyaya, S.; Knowles, N.J.; King, D.P.; Paton, D.J.; Mahapatra, M. Novel antibody binding determinants on the capsid surface of serotype O foot-and-mouth disease virus. J. Gen. Virol. 2014, 95, 1104. [Google Scholar] [CrossRef]
- Yang, M.; Xu, W.; Goolia, M.; Zhang, Z. Characterization of monoclonal antibodies against foot-and-mouth disease virus serotype O and application in identification of antigenic variation in relation to vaccine strain selection. Virol. J. 2014, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Knowles, N.J.; He, J.; Shang, Y.; Wadsworth, J.; Valdazo-González, B.; Onosato, H.; Fukai, K.; Morioka, K.; Yoshida, K.; Cho, I.-S. Southeast Asian foot-and-mouth disease viruses in Eastern Asia. Emerg. Infect. Dis. 2012, 18, 499. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, K.-N.; Ko, Y.-J.; Kim, S.-M.; Lee, H.-S.; Shin, Y.-K.; Sohn, H.-J.; Park, J.-Y.; Yeh, J.-Y.; Lee, Y.-H. Control of foot-and-mouth disease during 2010–2011 epidemic, South Korea. Emerg. Infect. Dis. 2013, 19, 655. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Tark, D.; Lee, K.-N.; Chun, J.-E.; Lee, H.-S.; Ko, Y.-J.; Kye, S.-J.; Kim, Y.-J.; Oem, J.-K.; Ryoo, S. Control of type O foot-and-mouth disease by vaccination in Korea, 2014–2015. J. Vet. Sci. 2018, 19, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-E.; You, S.-H.; Lee, S.-Y.; Lee, K.-N.; Ko, M.-K.; Choi, J.-H.; Kim, B.; Lee, J.-S.; Park, J.-H. Immune responses in pigs and cattle vaccinated with half-volume foot-and-mouth disease vaccine. J. Vet. Sci. 2017, 18, 323–331. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, M.-E.; Kim, R.-H.; Ko, M.-K.; Lee, K.-N.; Kim, S.-M.; Shim, H.-S.; Kim, B.; Lee, J.-S.; Park, J.-H. Genetic and immunologic relationships between vaccine and field strains for vaccine selection of type A foot-and-mouth disease virus circulating in East Asia. Vaccine 2015, 33, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Taboga, O.; Tami, C.; Carrillo, E.; Nunez, J.; Rodríguez, A.; Saiz, J.C.; Blanco, E.; Valero, M.-L.; Roig, X.; Camarero, J.A. A large-scale evaluation of peptide vaccines against foot-and-mouth disease: Lack of solid protection in cattle and isolation of escape mutants. J. Virol. 1997, 71, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, H.; Tang, T.; Zhao, P.; Du, J.; Guo, S.; Wei, H.; Xu, H.; Wan, M.; Wei, X. Single immunization with a recombinant multiple-epitope protein induced protection against FMDV type Asia 1 in cattle. Int. Immunopharmacol. 2015, 28, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy Chichili, V.P.; Kumar, V.; Sivaraman, J. Linkers in the structural biology of protein–protein interactions. Protein Sci. 2013, 22, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Sabourin, M.; Tuzon, C.T.; Fisher, T.S.; Zakian, V.A. A flexible protein linker improves the function of epitope-tagged proteins in Saccharomyces cerevisiae. Yeast 2007, 24, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Arai, R.; Ueda, H.; Kitayama, A.; Kamiya, N.; Nagamune, T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001, 14, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Schädlich, L.; Senger, T.; Kirschning, C.J.; Müller, M.; Gissmann, L. Refining HPV 16 L1 purification from E. coli: Reducing endotoxin contaminations and their impact on immunogenicity. Vaccine 2009, 27, 1511–1522. [Google Scholar] [CrossRef]
- Nascimento, I.; Leite, L. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- Cloete, M.; Dungu, B.; Van Staden, L.; Ismail-Cassim, N.; Vosloo, W. Evaluation of different adjuvants for foot-and-mouth disease vaccine containing all the SAT serotypes. Onderstepoort J. Vet. Res. 2008, 75, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, A.; Ghosh, S.; Singh, S.; Deshmukh, R. Evaluation of three ‘ready to formulate’ oil adjuvants for foot-and-mouth disease vaccine production. Vaccine 2000, 19, 1097–1105. [Google Scholar] [CrossRef]
- Khorasani, A.; Madadgar, O.; Soleimanjahi, H.; Keyvanfar, H.; Mahravani, H. Evaluation of the efficacy of a new oil-based adjuvant ISA 61 VG FMD vaccine as a potential vaccine for cattle. Iran. J. Vet. Res. 2016, 17, 8. [Google Scholar]
- Park, M.-E.; Lee, S.-Y.; Kim, R.-H.; Ko, M.-K.; Park, J.-N.; Lee, K.-N.; Kim, S.-M.; Choi, J.-H.; You, S.-H.; Kim, B. Altered adjuvant of foot-and-mouth disease vaccine improves immune response and protection from virus challenge. Trials Vaccinol. 2016, 5, 97–104. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; She, D.; Li, P.; Sun, P.; Bai, X.; Chen, Y.; Xie, B.; Liu, Z. The comparison of the efficacy of swine FMD vaccine emulsified with oil adjuvant of ISA 201 VG or ISA 206 VG. J. Biosci. Med. 2013, 1, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.E.-S.; Gamal, W.M.; Hassan, A.I.; Mahdy, S.E.-D.; Hegazy, A.Z.; Abdel-Atty, M.M. Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine. Vet. World 2015, 8, 1189. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.-H.; Chathuranga, W.G.; Shim, Y.-J.; Haluwana, D.; Kim, E.-H.; Yoon, I.-J.; Lim, Y.-T.; Shin, S.H.; Jo, H.; Hwang, S.Y. The Potential Adjuvanticity of CAvant® SOE for Foot-and-Mouth Disease Vaccine. Vaccines 2021, 9, 1091. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Ko, M.-K.; Lee, K.-N.; Choi, J.-H.; You, S.-H.; Pyo, H.-M.; Lee, M.-H.; Kim, B.; Lee, J.-S.; Park, J.-H. Application of mouse model for effective evaluation of foot-and-mouth disease vaccine. Vaccine 2016, 34, 3731–3737. [Google Scholar] [CrossRef]
- Gnazzo, V.; Quattrocchi, V.; Soria, I.; Pereyra, E.; Langellotti, C.; Pedemonte, A.; Lopez, V.; Marangunich, L.; Zamorano, P. Mouse model as an efficacy test for foot-and-mouth disease vaccines. Transbound. Emerg. Dis. 2020, 67, 2507–2520. [Google Scholar] [CrossRef]
- Cao, W.; Chen, Y.; Alkan, S.; Subramaniam, A.; Long, F.; Liu, H.; Diao, R.; Delohery, T.; McCormick, J.; Chen, R. Human T helper (Th) cell lineage commitment is not directly linked to the secretion of IFN-γ or IL-4: Characterization of Th cells isolated by FACS based on IFN-γ and IL-4 secretion. Eur. J. Immunol. 2005, 35, 2709–2717. [Google Scholar] [CrossRef]
- Quan, F.-S.; Vunnava, A.; Compans, R.W.; Kang, S.-M. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS ONE 2010, 5, e9161. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, A.; Schmitz, N.; Storni, T.; Bachmann, M.F. Influenza A vaccine based on the extracellular domain of M2: Weak protection mediated via antibody-dependent NK cell activity. J. Immunol. 2004, 172, 5598–5605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-S.; Chowdhury, M.Y.; Moon, H.-J.; Choi, Y.-K.; Talactac, M.R.; Kim, J.-H.; Park, M.-E.; Son, H.-Y.; Shin, K.-S.; Kim, C.-J. The highly conserved HA2 protein of the influenza A virus induces a cross protective immune response. J. Virol. Methods 2013, 194, 280–288. [Google Scholar] [CrossRef]
- Zamorano, P.; Wigdorovitz, A.; Chaher, M.; Fernandez, F.; Carrillo, C.; Marcovecchio, F.E.; Sadir, A.M.; Borca, M. Recognition of B and T cell epitopes by cattle immunized with a synthetic peptide containing the major immunogenic site of VP1 FMDV 01 Campos. Virology 1994, 201, 383–387. [Google Scholar] [CrossRef]
- Zamorano, P.; Wigdorovitz, A.; Perez-Filgueira, M.; Carrillo, C.; Escribano, J.; Sadir, A.; Borca, M. A 10-amino-acid linear sequence of VP1 of foot and mouth disease virus containing B-and T-cell epitopes induces protection in mice. Virology 1995, 212, 614–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, D.; Reeve, R.; Capozzo, A.V.; Ludi, A. Estimating the protection afforded by foot-and-mouth disease vaccines in the laboratory. Vaccine 2019, 37, 5515–5524. [Google Scholar] [CrossRef]


| Antigen | Strain | Epitope Sequences | |
|---|---|---|---|
| VP1-1 (aa 132–162) | VP1-2 (aa 192–212) | ||
| OVM | O/Jincheon/SKR/2014 | GKCKYTGGSLPNVRGDLQVLAPRAARPLPTS | LAVHPSAARHKQKIVAPVKQS |
| O/Andong/SKR/2010 | GNCKYAGGSLPNVRGDLQVLAQKAARPLPTS | LAVHPSAARHKQKIVAPVKQS | |
| O1 Manisa/Turkey/69 | GNSKYGDGTVANVRGDLQVLAQKAARALPTS | LAIHPDQARHKQKIVAPVKQL | |
| AVM | A/Pocheon/KOR/2010 | GTSRYSAPATRRGDLGSLAARLAAQLPASFN | VEVTSQDRHKQKIIAPAKQLL |
| A/Malay/97 | GTSKYSTPGARRGDLGSLAARDAAQLPASFN | VEVLSQDRHKQRIIAPAKQLL | |
| A22/Iraq/24/64 | GTSKYSAGGTGRRGDLGPLAARVAAQLPASF | AVEVSSQDRHKQKIIAPAKQL | |
| Antigen | Strain | aa Position | aa Sequence |
|---|---|---|---|
| OVM | O/Jincheon/SKR/2014 | VP1140~159 | SLPNVRGDLQVLAPRAARPL |
| O/Andong/SKR/2010 | VP1140~160 | SLPNVRGDLQVLAQKAARPLP | |
| O1 Manisa/Turkey/69 | VP1141~159 | VANVRGDLQVLAQKAARAL | |
| AVM | A/Pocheon/KOR/2010 | VP1139~157 | PATRRGDLGSLAARLAAQL |
| A/Malay/97 | VP1138~157 | TPGARRGDLGSLAARDAAQL | |
| A22/Iraq/24/64 | VP1139~158 | GGTGRRGDLGPLAARVAAQL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chathuranga, W.A.G.; Hewawaduge, C.; Nethmini, N.A.N.; Kim, T.-H.; Kim, J.H.; Ahn, Y.-H.; Yoon, I.-J.; Yoo, S.-S.; Park, J.-H.; Lee, J.-S. Efficacy of a Novel Multiepitope Vaccine Candidate against Foot-and-Mouth Disease Virus Serotype O and A. Vaccines 2022, 10, 2181. https://doi.org/10.3390/vaccines10122181
Chathuranga WAG, Hewawaduge C, Nethmini NAN, Kim T-H, Kim JH, Ahn Y-H, Yoon I-J, Yoo S-S, Park J-H, Lee J-S. Efficacy of a Novel Multiepitope Vaccine Candidate against Foot-and-Mouth Disease Virus Serotype O and A. Vaccines. 2022; 10(12):2181. https://doi.org/10.3390/vaccines10122181
Chicago/Turabian StyleChathuranga, W. A. Gayan, Chamith Hewawaduge, N. A. Nadeeka Nethmini, Tae-Hwan Kim, Ju Hun Kim, Young-Hoon Ahn, In-Joong Yoon, Sung-Sik Yoo, Jong-Hyeon Park, and Jong-Soo Lee. 2022. "Efficacy of a Novel Multiepitope Vaccine Candidate against Foot-and-Mouth Disease Virus Serotype O and A" Vaccines 10, no. 12: 2181. https://doi.org/10.3390/vaccines10122181
APA StyleChathuranga, W. A. G., Hewawaduge, C., Nethmini, N. A. N., Kim, T. -H., Kim, J. H., Ahn, Y. -H., Yoon, I. -J., Yoo, S. -S., Park, J. -H., & Lee, J. -S. (2022). Efficacy of a Novel Multiepitope Vaccine Candidate against Foot-and-Mouth Disease Virus Serotype O and A. Vaccines, 10(12), 2181. https://doi.org/10.3390/vaccines10122181






