The Population-Wide Risk-Benefit Profile of Extending the Primary COVID-19 Vaccine Course Compared with an mRNA Booster Dose Program
Abstract
:1. Introduction
2. Methods
2.1. Disease Transmission Model Overview
2.2. Investigating the Impact of COVID-19 Vaccine Waning on Disease Burden
2.3. Quantifying the Risk–Benefit Profile of COVID-19 Vaccination at the Population Level
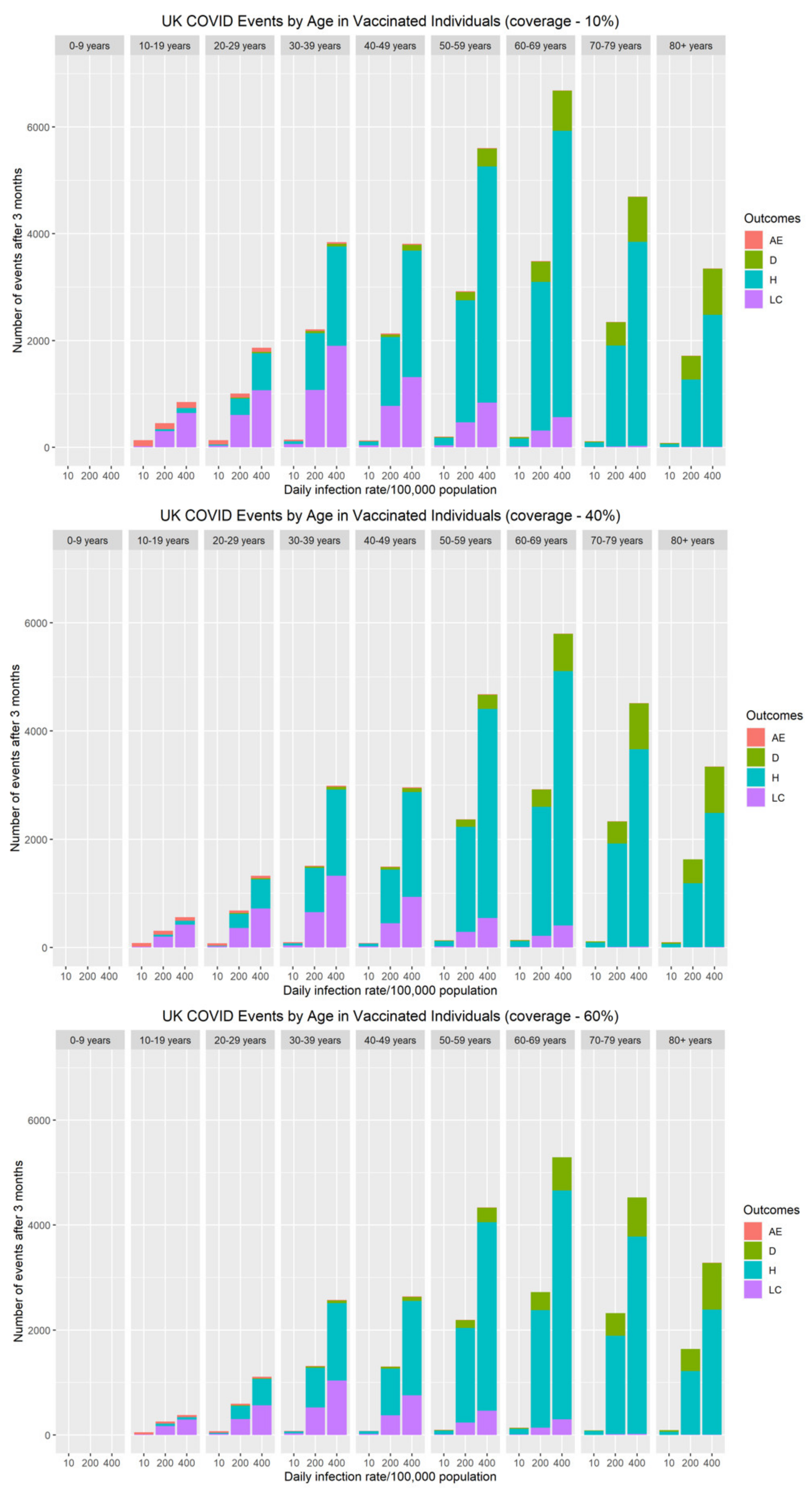
3. Results
3.1. Impact of Vaccine Waning on COVID-19 Hospitalisations
3.2. Balancing the Benefit of Targeting Primary Series or Booster Vaccine Program
3.3. Population-Wide Risk–Benefit Profile of COVID-19 Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, L.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Shrotri, M.; Krutikov, M.; Palmer, T.; Giddings, R.; Azmi, B.; Subbarao, S.; Fuller, C.; Irwin-Singer, A.; Davies, D.; Tut, G.; et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Infect. Dis. 2021, 21, 1529–1538. [Google Scholar] [CrossRef]
- Moustsen-Helms, I.R.; Emborg, H.-D.; Nielsen, J.; Nielsen, K.F.; Krause, T.G.; Mølbak, K.; Møller, K.L.; Berthelsen, A.-S.N.; Valentiner-Branth, P. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 vaccine in long-term care facility residents and healthcare workers—A Danish cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Tov, A.B.; Cohen, D.; Muhsen, K. Assessment of effectiveness of 1 dose of BNT162b2 vaccine for SARS-CoV-2 infection 13 to 24 days after immunization. JAMA Netw. Open 2021, 4, e2115985. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lee, J.; Ta, C.; Soroush, A.; Rogers, J.R.; Kim, J.H.; Natarajan, K.; Zucker, J.; Weng, C. A retrospective analysis of COVID-19 mRNA vaccine breakthrough infections—Risk factors and vaccine effectiveness. medRxiv 2021. [Google Scholar] [CrossRef]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. Morb. Mortal. Wkl. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England. Technical Briefing 28. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1033101/Technical_Briefing_28_12_Nov_2021.pdf (accessed on 12 November 2021).
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Wu, J.; Wan, J.; Huang, Y.; Song, J.; Fu, Z.; Wang, H.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microb. Infect. 2021, 22, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, P.P.; Al Kanaani, Z.; et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2021, 385, e38. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Elmer, A.; Kingston, N.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Iacobucci, G. Covid-19: Protection from two doses of vaccine wanes within six months, data suggest. BMJ 2021, 374, n2113. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, E.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Lee, G.S.; Tian, Y.; Florea, A.; Aragones, M.; Tubert, J.E.; et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: Test negative case-control study. BMJ 2021, 375, e068848. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Expands Eligibility for COVID-19 Vaccine Boosters. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters (accessed on 20 November 2021).
- Centers for Disease Control and Prevention. Overview of COVID-19 Vaccine Recommendations. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#overview-covid19-vax-recommendations (accessed on 20 November 2021).
- Wise, J. Covid-19: Booster doses to be offered to 30 million people in UK. BMJ 2021, 374, n2261. [Google Scholar] [CrossRef]
- GOV.UK. Press Release: JCVI Issues Updated Advice on COVID-19 Booster Vaccination. Available online: https://www.gov.uk/government/news/jcvi-issues-updated-advice-on-covid-19-booster-vaccination (accessed on 20 November 2021).
- GOV.UK. Press Release: JCVI Issues Advice on COVID-19 Booster Vaccines for Those Aged 40 to 49 and Second Doses for 16 to 17 Year Olds. Available online: https://www.gov.uk/government/news/jcvi-issues-advice-on-covid-19-booster-vaccines-for-those-aged-40-to-49-and-second-doses-for-16-to-17-year-olds (accessed on 20 November 2021).
- University Hospital Southampton NHS Foundation Trust. COV-Boost Vaccine Trial. Available online: https://www.covboost.org.uk/home (accessed on 20 November 2021).
- National Institute for Health Research. Data from NIHR-Supported Studies Inform UK COVID-19 Booster Programme. Available online: https://www.nihr.ac.uk/news/data-from-nihr-supported-studies-inform-uk-covid-19-booster-programme/28663 (accessed on 20 November 2021).
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Gee, J.; Marquez, P.; Myers, T.R.; Shimabukuro, T.T.; Shay, D.K. Safety monitoring of an additional dose of COVID-19 vaccine—United States, August 12–September 19, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: Update from the Advisory committee on immunization practices—United States, June 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- World Health Organization. Statement of the WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID-19 Subcommittee on Reports of Guillain-Barré Syndrome (GBS) Following Adenovirus Vector COVID-19 Vaccines. Available online: https://www.who.int/news/item/26-07-2021-statement-of-the-who-gacvs-covid-19-subcommittee-on-gbs (accessed on 20 November 2021).
- Lai, C.-C.; Ko, W.-C.; Chen, C.-J.; Chen, P.-Y.; Huang, Y.-C.; Lee, P.-I.; Hsueh, P.-R. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Rev. Vaccines 2021, 20, 1027–1035. [Google Scholar] [CrossRef]
- Shiri, T.; Evans, M.; Talarico, C.A.; Morgan, A.R.; Mussad, M.; Buck, P.O.; McEwan, P.; Strain, W.D. Vaccinating adolescents and children significantly reduces COVID-19 morbidity and mortality across all ages: A population-based modeling study using the UK as an example. Vaccines 2021, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. Vaccinations in United Kingdom 2021. Available online: https://coronavirus.data.gov.uk/details/vaccinations (accessed on 20 November 2021).
- GOV.UK. Coronavirus (COVID-19) in the UK 2021. Available online: https://coronavirus.data.gov.uk/details/cases (accessed on 20 November 2021).
- Our World in Data. Daily New Confirmed COVID-19 Cases. Available online: https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&time=2020-06-09..&country=GBR~DEU~FRA~ITA~BEL~AUT~FIN~HUN~IRL~SWE~ESP~SVK~SVN~ROU~PRT~POL~NOR~NLD~LUX~LTU~MLT~LVA~LIE~ISL~GRC~DNK~CYP~HRV®ion=Europe&pickerMetric=location&pickerSort=asc&tab=map&yScale=log&Interval=New+per+day&Align+outbreaks=true&Relative+to+Population=false&Metric=Confirmed+cases (accessed on 20 November 2021).
- GOV.UK. Coronavirus in the Vaccinated in the UK 2022. Available online: https://www.gov.uk/government/publications/coronavirus-press-conferences-quality-and-methodology-information-report (accessed on 4 January 2022).
- Strain, W.D.; Mansi, J.; Boikos, C.; Boivin, M.; Fisher, W.A. Achieving influenza vaccine uptake target in Canada via a pharmacy-led telephone discussion during the 2019–2020 season. Vaccines 2021, 9, 312. [Google Scholar] [CrossRef]
- Singer, M.E.; Taub, I.B.; Kaelber, D.C. Risk of myocarditis from COVID-19 infection in people under age 20: A population-based analysis. medRxiv 2021. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiri, T.; Evans, M.; Talarico, C.A.; Morgan, A.R.; Mussad, M.; Buck, P.O.; McEwan, P.; Strain, W.D. The Population-Wide Risk-Benefit Profile of Extending the Primary COVID-19 Vaccine Course Compared with an mRNA Booster Dose Program. Vaccines 2022, 10, 140. https://doi.org/10.3390/vaccines10020140
Shiri T, Evans M, Talarico CA, Morgan AR, Mussad M, Buck PO, McEwan P, Strain WD. The Population-Wide Risk-Benefit Profile of Extending the Primary COVID-19 Vaccine Course Compared with an mRNA Booster Dose Program. Vaccines. 2022; 10(2):140. https://doi.org/10.3390/vaccines10020140
Chicago/Turabian StyleShiri, Tinevimbo, Marc Evans, Carla A. Talarico, Angharad R. Morgan, Maaz Mussad, Philip O. Buck, Phil McEwan, and William David Strain. 2022. "The Population-Wide Risk-Benefit Profile of Extending the Primary COVID-19 Vaccine Course Compared with an mRNA Booster Dose Program" Vaccines 10, no. 2: 140. https://doi.org/10.3390/vaccines10020140
APA StyleShiri, T., Evans, M., Talarico, C. A., Morgan, A. R., Mussad, M., Buck, P. O., McEwan, P., & Strain, W. D. (2022). The Population-Wide Risk-Benefit Profile of Extending the Primary COVID-19 Vaccine Course Compared with an mRNA Booster Dose Program. Vaccines, 10(2), 140. https://doi.org/10.3390/vaccines10020140





