Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anti-SARS-CoV-2 Spike IgG Antibodies
2.3. Microneutralization Assay
2.4. Peripheral Blood Mononuclear Cells Isolation and Stimulation
2.5. Multiparameter Flow Cytometry Analysis
2.6. Interferon-γ Quantification
2.7. Statistical Analysis
2.8. Limitations
3. Results
3.1. Monitoring of Humoral Response after Vaccination

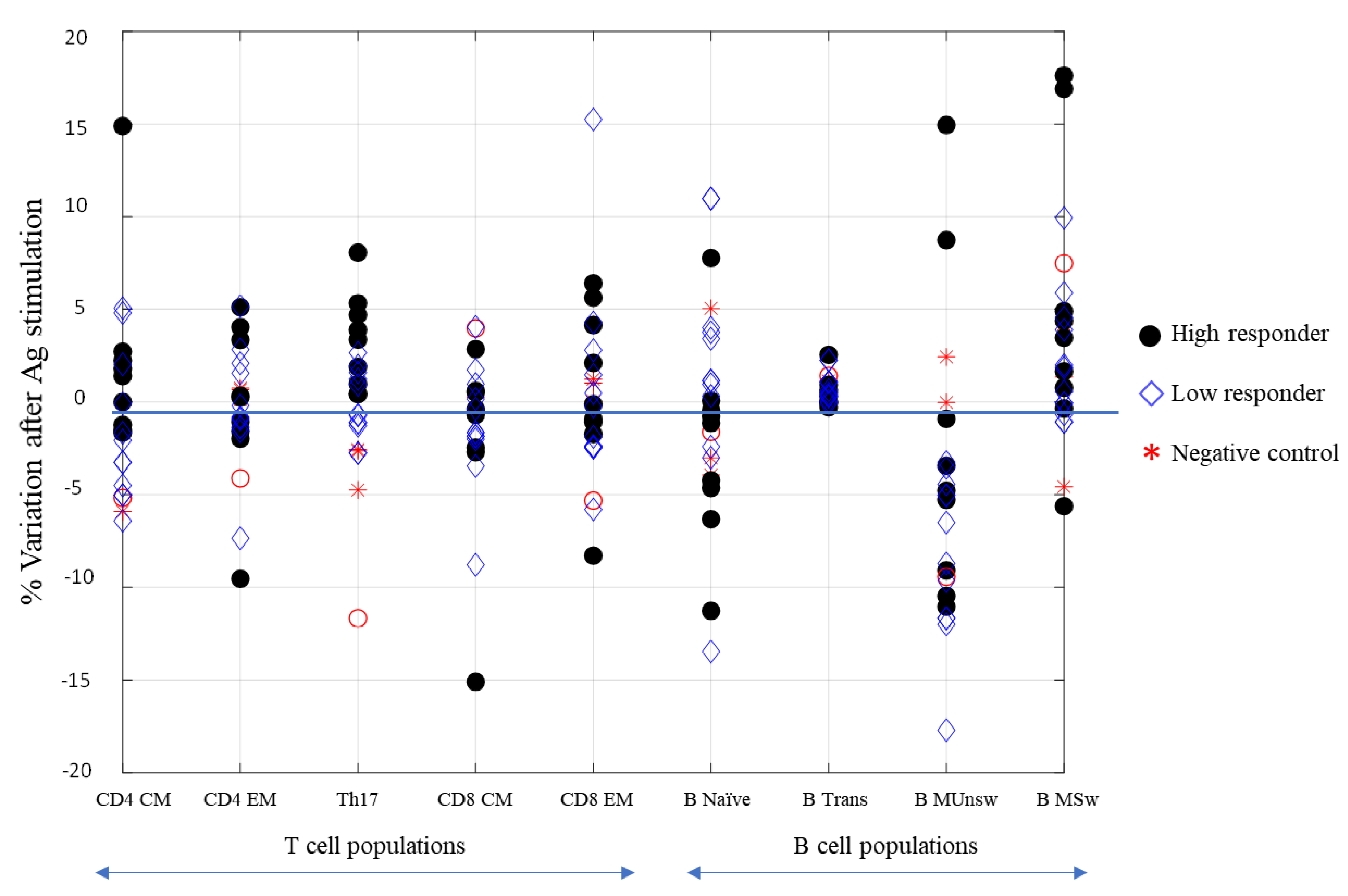
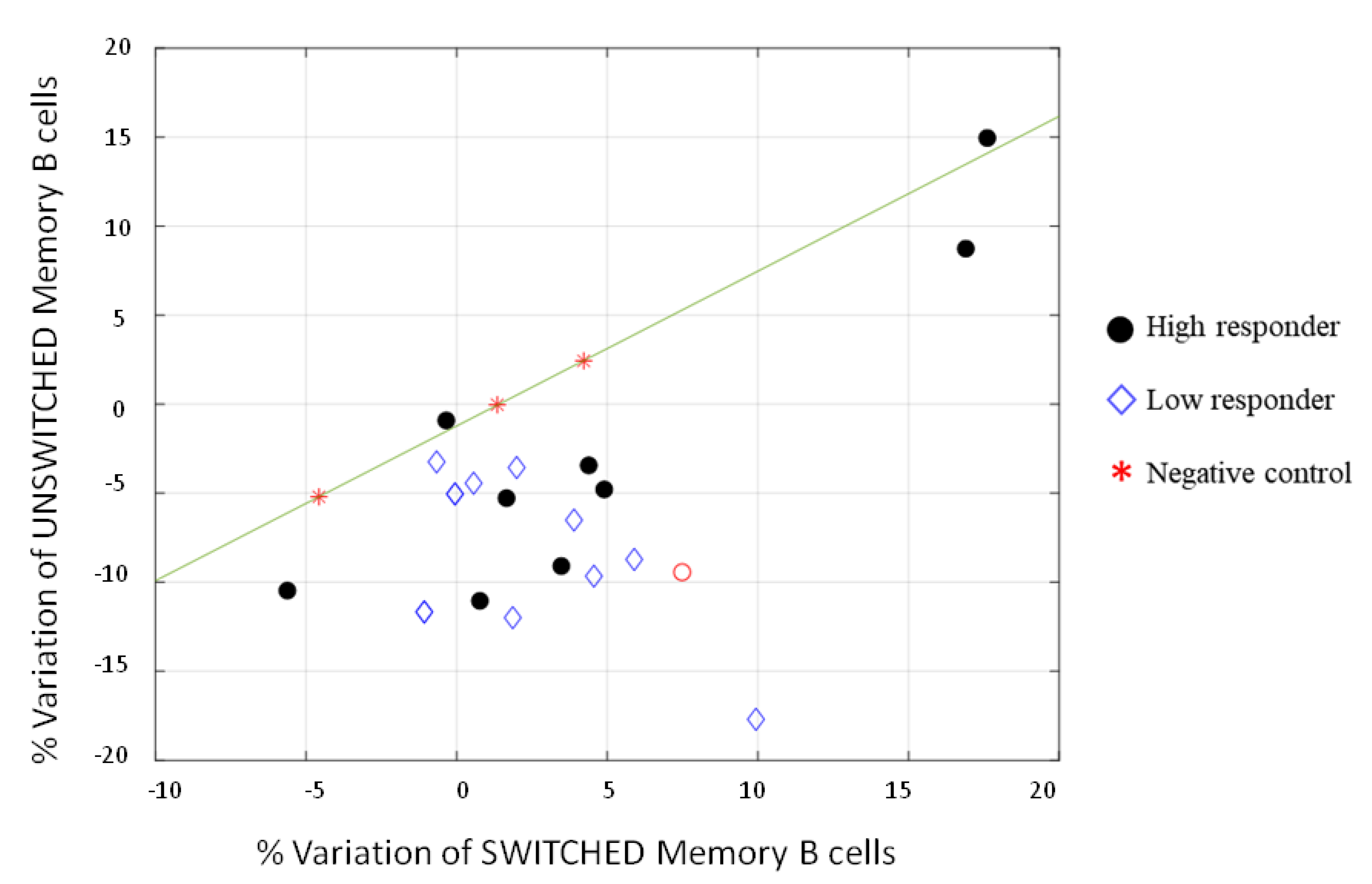
3.2. Cell-Mediated Immune Response to SARS-CoV-2 mRNA Vaccine
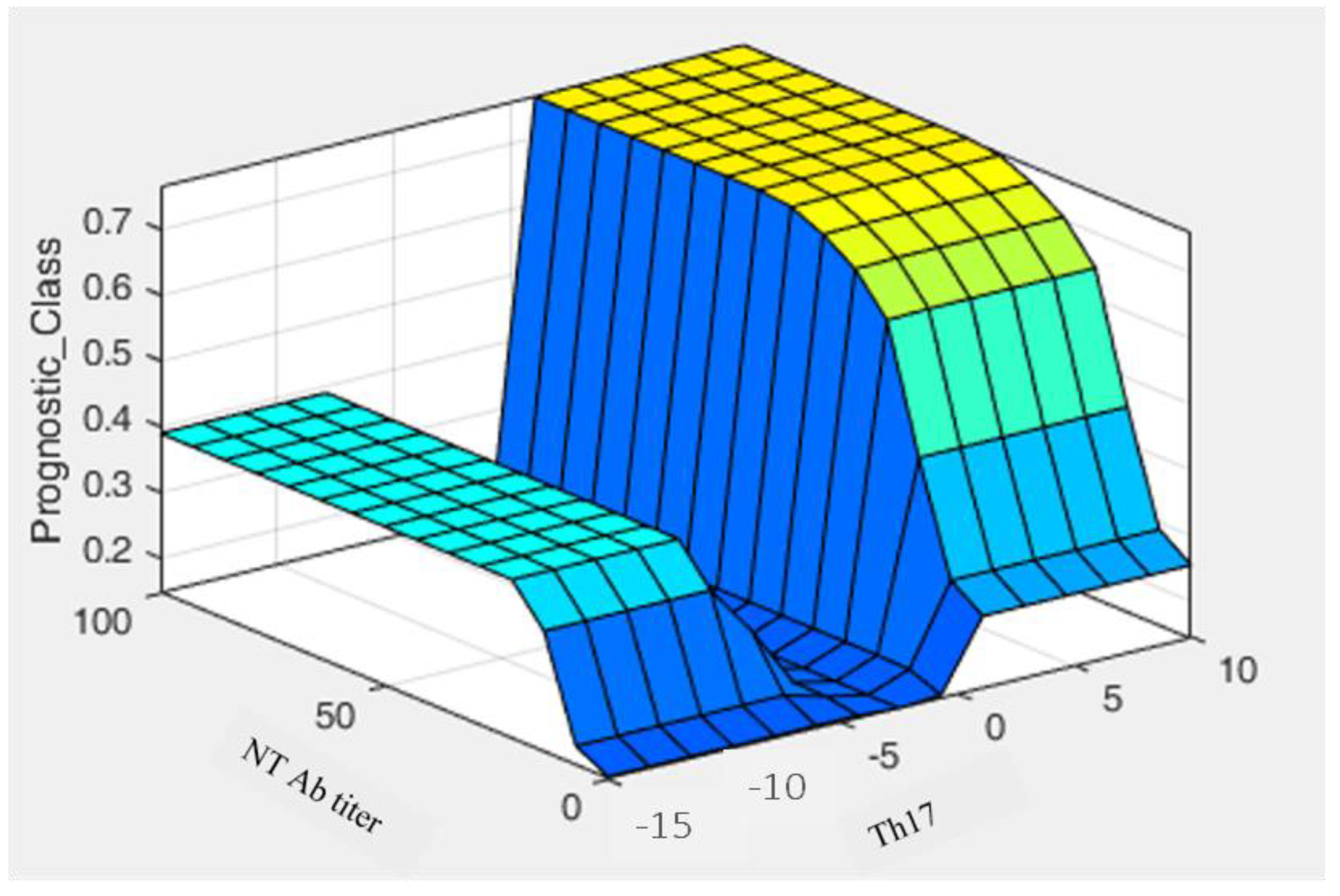
3.3. Fuzzy System
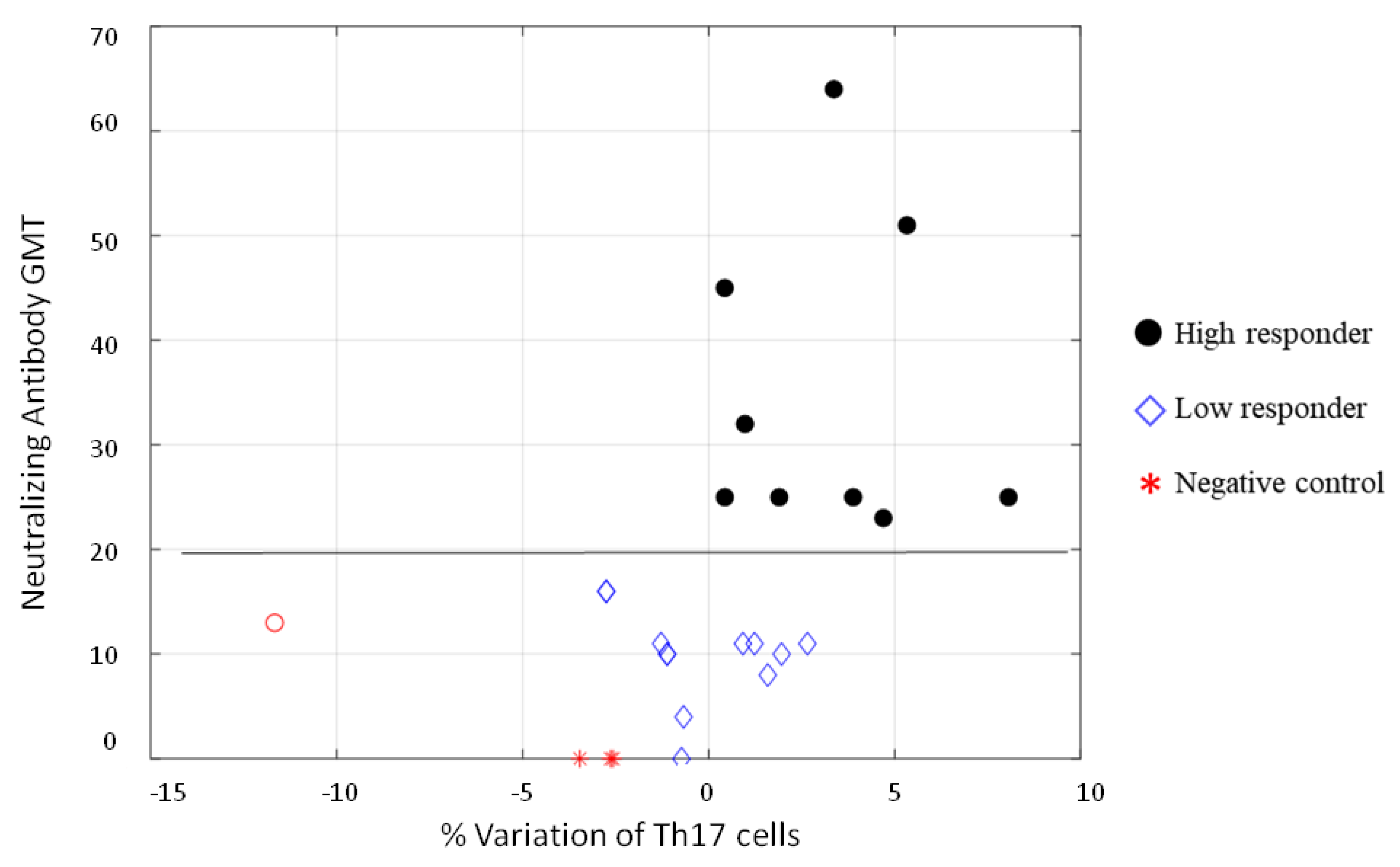
3.4. Interferon-γ Production by SARS-CoV-2 Specific T Cells in the Whole Blood
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 December 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Savellini, G.G.; Fabrizi, S.; Miceli, G.B.; Cusi, M.G. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021, 385, 90–92. [Google Scholar] [CrossRef]
- Brewer, R.C.; Ramadoss, N.S.; Lahey, L.J.; Jahanbani, S.; Robinson, W.H.; Lanz, T.V. BNT162b2 vaccine induces divergent B cell responses to SARS-CoV-2 S1 and S2. Nat. Immunol. 2021, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health-Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Almendro-Vázquez, P.; Laguna-Goya, R.; Ruiz-Ruigomez, M.; Utrero-Rico, A.; Lalueza, A.; de la Calle, G.M.; Delgado, P.; Perez-Ordoño, L.; Muro, E.; Vila, J.; et al. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLOS Pathog. 2021, 17, e1010211. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Maddur, M.S.; Miossec, P.; Kaveri, S.V.; Bayry, J. Th17 Cells: Biology, Pathogenesis of Autoimmune and Inflammatory Diseases, and Therapeutic Strategies. Am. J. Pathol. 2012, 181, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Liu, B.; Ji, H.; Gao, D. Prognostic of electronic equipment based on adaptive neural network ensemble. Jiefangjun Ligong Daxue Xuebao J. PLA Univ. Sci. Technol. Nat. Sci. Ed. 2013, 14, 565–568. [Google Scholar]
- Liang, D.; Wang, M.; Xu, Z. A novel approach of three-way decisions with information interaction strategy for intelligent decision making under uncertainty. Inf. Sci. 2021, 581, 106–135. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Carsetti, R.; Zaffina, S.; Mortari, E.P.; Terreri, S.; Corrente, F.; Capponi, C.; Palomba, P.; Mirabella, M.; Cascioli, S.; Palange, P.; et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front. Immunol. 2020, 11, 3365. [Google Scholar] [CrossRef] [PubMed]
- Anichini, G.; Gandolfo, C.; Terrosi, C.; Fabrizi, S.; Miceli, G.B.; Savellini, G.G.; Prathyumnan, S.; Franchi, F.; Cusi, M.G. Antibody response to SARS-CoV-2 in infected patients with different clinical outcome. J. Med. Virol. 2021, 93, 2548–2552. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. Correlates of protection from SARS-CoV-2 infection. Lancet 2021, 397, 1421–1423. [Google Scholar] [CrossRef]
- Mortari, E.P.; Russo, C.; Vinci, M.R.; Terreri, S.; Salinas, A.F.; Piccioni, L.; Alteri, C.; Colagrossi, L.; Coltella, L.; Ranno, S.; et al. Highly Specific Memory B Cells Generation after the 2nd Dose of BNT162b2 Vaccine Compensate for the Decline of Serum Antibodies and Absence of Mucosal IgA. Cells 2021, 10, 2541. [Google Scholar] [CrossRef]
- Simon, Q.; Pers, J.-O.; Cornec, D.; Le Pottier, L.; Mageed, R.A.; Hillion, S. In-depth characterization of CD24 high CD38 high transitional human B cells reveals different regulatory profiles. J. Allergy Clin. Immunol. 2016, 137, 1577–1584.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patakas, A.; Benson, R.A.; Withers, D.R.; Conigliaro, P.; McInnes, I.B.; Brewer, J.M.; Garside, P. Th17 Effector Cells Support B Cell Responses Outside of Germinal Centres. PLoS ONE 2012, 7, e49715. [Google Scholar] [CrossRef]
- Mitsdoerffer, M.; Lee, Y.; Jäger, A.; Kim, H.-J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef] [Green Version]


| Neutralizing Antibody GMT > 128 at 10 Days Post-Vaccination | Neutralizing Antibody GMT < 128 at 10 Days Post-Vaccination | |||||
|---|---|---|---|---|---|---|
| 1 Month | 3 Months | 6 Months | 1 Month | 3 Months | 6 Months | |
| Ab level increase average | 2 (6.66%) + 21.7% | 0 | 0 | 17 (56.66%) + 22.0% | 0 | 0 |
| Ab level stability | 2 (6.66%) | 0 | 0 | 6 (20.0%) | 0 | 0 |
| Ab level decrease average | 26 (86.66%) − 28.6% | 30 (100%) − 70.7% | 30 (100%) − 86.5% | 7 (23.33%) − 6.8% | 30 (100%) − 58.7% | 30 (100%) − 76.9% |
| N | CoviFERON FIA Results (IU/mL) | ||||
|---|---|---|---|---|---|
| Nil | Original SP Antigen | Mitogen | Results Interpretation | ||
| 1 | <0.145 | 1.42 | >10.00 | 1.28 | Reactive |
| 2 | <0.145 | 4.55 | >10.00 | 4.41 | Reactive |
| 3 | <0.145 | 1.12 | >10.00 | 0.98 | Reactive |
| 4 | <0.145 | 0.17 | >10.00 | 0.03 | Non-Reactive |
| 5 | <0.145 | 1.76 | >10.00 | 1.62 | Reactive |
| 6 | <0.145 | 0.93 | >10.00 | 0.79 | Reactive |
| 7 | <0.145 | 0.24 | >10.00 | 0.10 | Non-Reactive |
| 8 | <0.145 | <0.145 | >10.00 | 0.00 | Non-Reactive |
| 9 | 2.38 | 2.23 | >10.00 | −0.15 | Non-Reactive |
| 10 | <0.145 | 0.56 | >10.00 | 0.42 | Reactive |
| 11 | <0.145 | 1.34 | >10.00 | 1.20 | Reactive |
| 12 | <0.145 | <0.145 | >10.00 | 0.00 | Non-Reactive |
| 13 | <0.145 | 1.08 | >10.00 | 0.94 | Reactive |
| 14 | <0.145 | 2.74 | >10.00 | 2.60 | Reactive |
| 15 | <0.145 | 1.17 | >10.00 | 1.03 | Reactive |
| 16 | 0.63 | 0.89 | >10.00 | 0.26 | Non-Reactive |
| 17 | 0.27 | 7.00 | >10.00 | 6.73 | Reactive |
| 18 | <0.145 | 0.22 | >10.00 | 0.08 | Non-Reactive |
| 19 | <0.145 | <0.145 | >10.00 | 0.00 | Non-Reactive |
| 20 | <0.145 | 0.18 | >10.00 | 0.04 | Non-Reactive |
| CTRL | <0.145 | <0.145 | >10.00 | 0.00 | Non-Reactive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandolfo, C.; Anichini, G.; Mugnaini, M.; Bocchia, M.; Terrosi, C.; Sicuranza, A.; Gori Savellini, G.; Gozzetti, A.; Franchi, F.; Cusi, M.G. Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 171. https://doi.org/10.3390/vaccines10020171
Gandolfo C, Anichini G, Mugnaini M, Bocchia M, Terrosi C, Sicuranza A, Gori Savellini G, Gozzetti A, Franchi F, Cusi MG. Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine. Vaccines. 2022; 10(2):171. https://doi.org/10.3390/vaccines10020171
Chicago/Turabian StyleGandolfo, Claudia, Gabriele Anichini, Marco Mugnaini, Monica Bocchia, Chiara Terrosi, Anna Sicuranza, Gianni Gori Savellini, Alessandro Gozzetti, Federico Franchi, and Maria Grazia Cusi. 2022. "Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine" Vaccines 10, no. 2: 171. https://doi.org/10.3390/vaccines10020171








