Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Approval
2.2. Cell, Tissue Explant, and Virus Culture Conditions
2.3. IgG/A Semi-Quantitative ELISA
2.4. Neutralization
2.5. Infectivity Assay
2.6. Multiplex Cytokine Analysis
2.7. Statistical Analysis
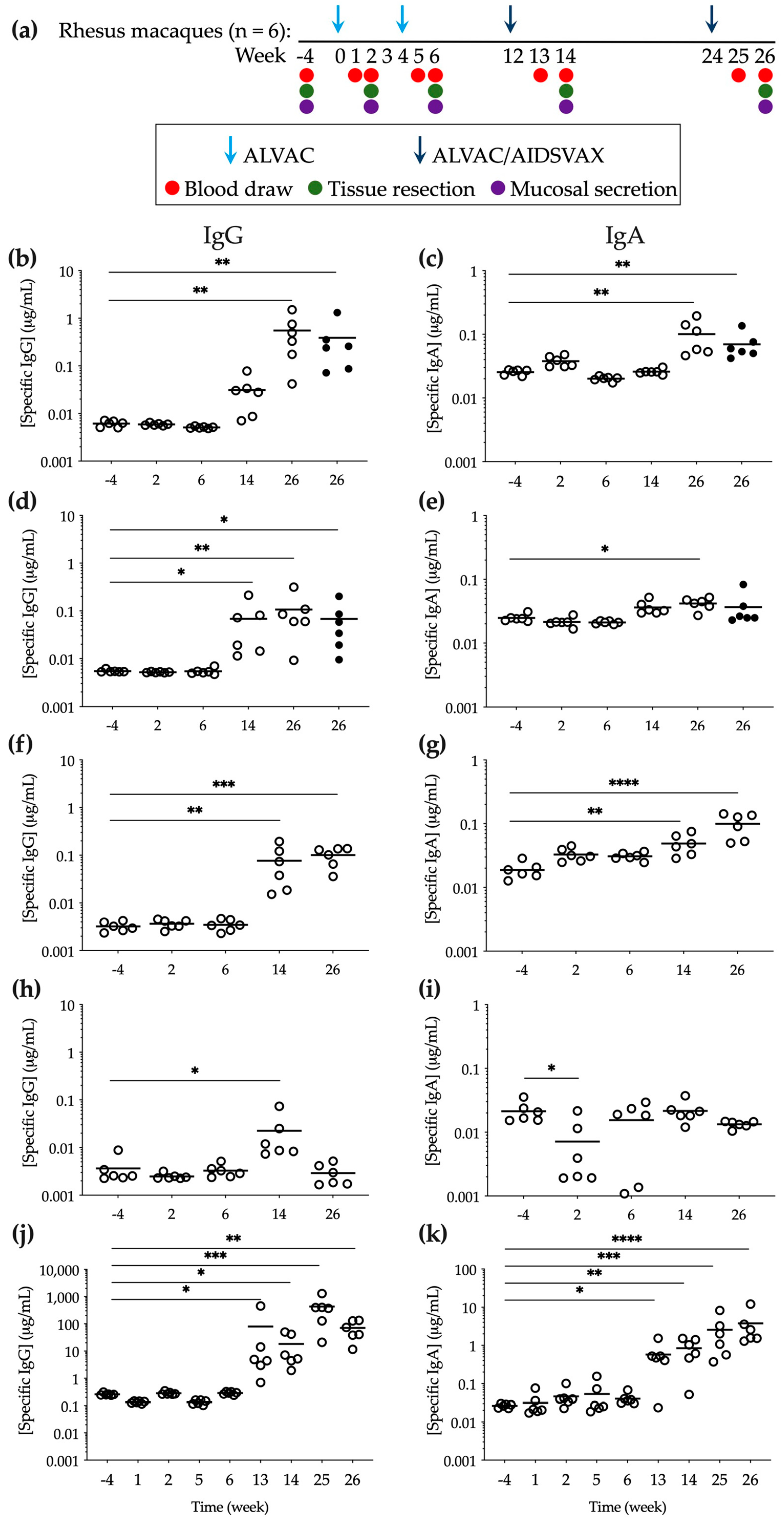
3. Results
3.1. Specific Anti-gp120 HIV-1CM244 IgG and IgA Were Detected in Mucosal Compartments following ALVAC/AIDSVAX Vaccination
3.2. Ex Vivo Efficacy of Abs Elicited by Prime-Boosting Vaccination with ALVAC/AIDSVAX
3.3. Proteomic Analysis of Responses to ALVAC and AIDSVAX
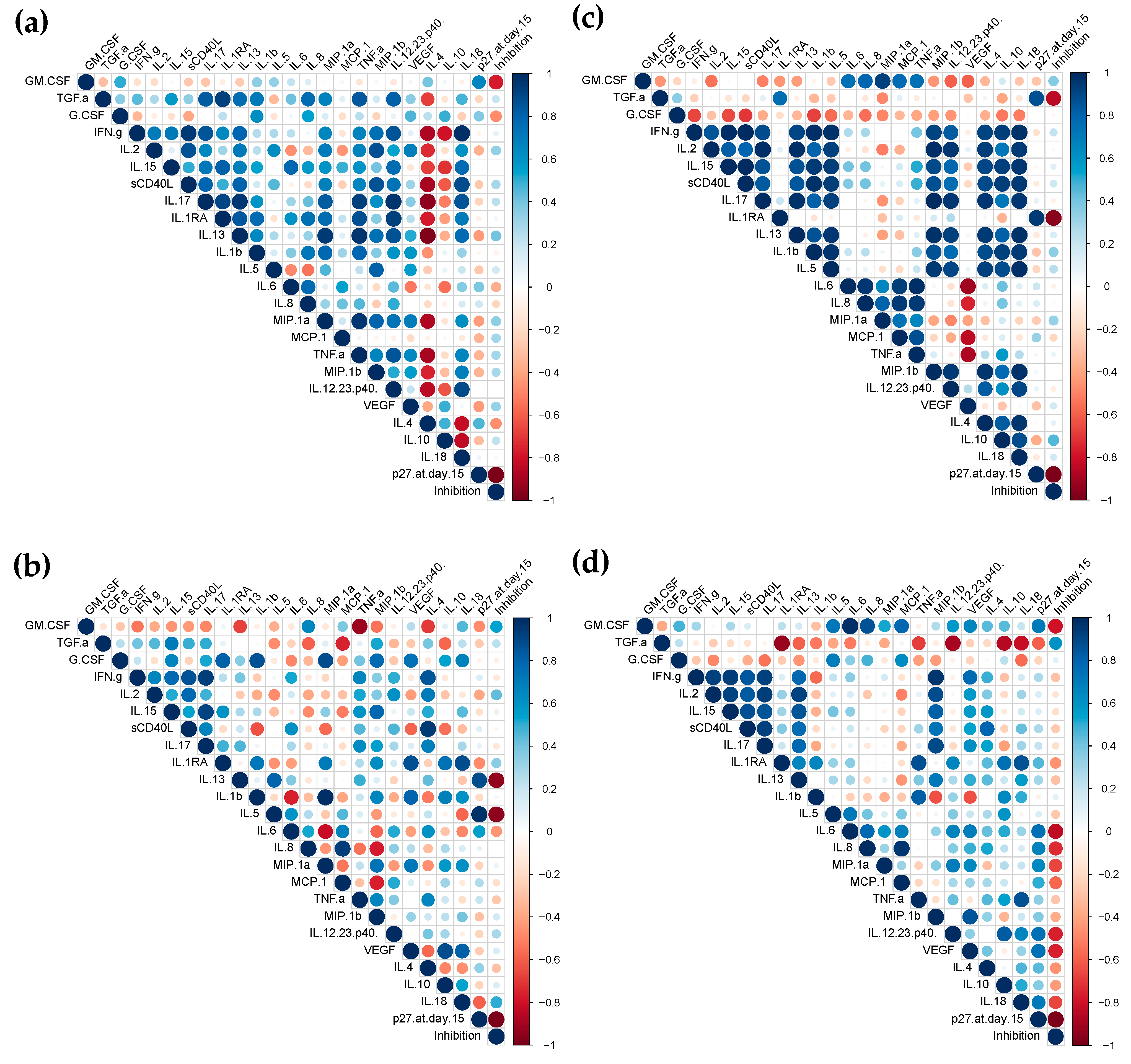
3.4. Correlation Analysis of Responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.E.; Bekker, L.G.; Laher, F.; Malahleha, M.; Allen, M.; Moodie, Z.; Grunenberg, N.; Huang, Y.; Grove, D.; Prigmore, B.; et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C gp120-MF59 in Adults. N. Engl. J. Med. 2021, 384, 1089–1100. [Google Scholar] [CrossRef]
- Burton, D.R. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, T.; Schmidt, S.; Duan, H.; Cheng, C.; Chuang, G.-Y.; Gu, Y.; Louder, M.; Lin, B.; Shen, C.-H.; et al. Vaccination induces maturation of diverse unmutated VRC01-class precursors to HIV-1 broadly neutralizing antibodies in an Ig-humanized mouse model. In Proceedings of the 4th HIV Research for Prevention conference (HIVR4P//Virtual), 27–28 January–3–4 February 2021. J. Int. AIDS Soc. 2021, 24, e25659. [Google Scholar] [CrossRef]
- Haynes, B.F.; Kelsoe, G.; Harrison, S.C.; Kepler, T.B. B-cell–lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 2012, 30, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, K.M.; Carnathan, D.G.; Nogal, B.; Martin, J.T.; Rodriguez, O.L.; Upadhyay, A.A.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2019, 177, 1153–1171.e1128. [Google Scholar] [CrossRef]
- D’Souza, M.P.; Rele, S.; Haynes, B.F.; Hu, D.J.; Kaplan, D.L.; Mamaghani, S.; Rampulla, D. Engineering immunity for next generation HIV vaccines: The intersection of bioengineering and immunology. Vaccine 2020, 38, 187–193. [Google Scholar] [CrossRef]
- Boopathy, A.V.; Mandal, A.; Kulp, D.W.; Menis, S.; Bennett, N.R.; Watkins, H.C.; Wang, W.; Martin, J.T.; Thai, N.T.; He, Y.; et al. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc. Natl. Acad. Sci. USA 2019, 116, 16473–16478. [Google Scholar] [CrossRef]
- Pattani, A.; McKay, P.F.; Garland, M.J.; Curran, R.M.; Migalska, K.; Cassidy, C.M.; Malcolm, R.K.; Shattock, R.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J. Control Release 2012, 162, 529–537. [Google Scholar] [CrossRef]
- Hansen, S.G.; Ford, J.C.; Lewis, M.S.; Ventura, A.B.; Hughes, C.M.; Coyne-Johnson, L.; Whizin, N.; Oswald, K.; Shoemaker, R.; Swanson, T.; et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011, 473, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Marshall, E.E.; Malouli, D.; Ventura, A.B.; Hughes, C.M.; Ainslie, E.; Ford, J.C.; Morrow, D.; Gilbride, R.M.; Bae, J.Y.; et al. A live-attenuated RhCMV/SIV vaccine shows long-term efficacy against heterologous SIV challenge. Sci. Transl. Med. 2019, 11, eaaw2607. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Klasse, P.J.; Dufour, J.; Veazey, R.S.; Moore, J.P. Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission. Proc. Natl. Acad. Sci. USA 2012, 109, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Mayer, C.; Huang, Y.; Gettie, A.; Tsai, L.; Ren, W.; Shakirzyanova, M.; Sina, S.T.; Trunova, N.; Blanchard, J.; Jenkins, L.M.; et al. Delay of simian human immunodeficiency virus infection and control of viral replication in vaccinated macaques challenged in the presence of a topical microbicide. Aids 2011, 25, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, R.; Dereuddre-Bosquet, N.; Dispinseri, S.; Gosse, L.; Desjardins, D.; Shen, X.; Tolazzi, M.; Ochsenbauer, C.; Saidi, H.; Tomaras, G.; et al. Superior Efficacy of a Human Immunodeficiency Virus Vaccine Combined with Antiretroviral Prevention in Simian-Human Immunodeficiency Virus-Challenged Nonhuman Primates. J. Virol. 2016, 90, 5315–5328. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Tomaka, F.L.; Wegmann, F.; Stieh, D.J.; Alter, G.; Robb, M.L.; Michael, N.L.; Peter, L.; Nkolola, J.P.; Borducchi, E.N.; et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018, 392, 232–243. [Google Scholar] [CrossRef]
- Pegu, A.; Yang, Z.Y.; Boyington, J.C.; Wu, L.; Ko, S.Y.; Schmidt, S.D.; McKee, K.; Kong, W.P.; Shi, W.; Chen, X.; et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 2014, 6, 243ra288. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Gilbert, P.B.; Juraska, M.; Montefiori, D.C.; Morris, L.; Karuna, S.T.; Edupuganti, S.; Mgodi, N.M.; deCamp, A.C.; Rudnicki, E.; et al. Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N. Engl. J. Med. 2021, 384, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Mgodi, N.M.; Takuva, S.; Edupuganti, S.; Karuna, S.; Andrew, P.; Lazarus, E.; Garnett, P.; Shava, E.; Mukwekwerere, P.G.; Kochar, N.; et al. A Phase 2b Study to Evaluate the Safety and Efficacy of VRC01 Broadly Neutralizing Monoclonal Antibody in Reducing Acquisition of HIV-1 Infection in Women in Sub-Saharan Africa: Baseline Findings. J. Acquir. Immune Defic. Syndr. 2021, 87, 680–687. [Google Scholar] [CrossRef]
- Sui, Y.; Gordon, S.; Franchini, G.; Berzofsky, J.A. Nonhuman primate models for HIV/AIDS vaccine development. Curr. Protoc. Immunol. 2013, 102, 12.14.11–12.14.30. [Google Scholar] [CrossRef]
- Bett, A.J.; Dubey, S.A.; Mehrotra, D.V.; Guan, L.; Long, R.; Anderson, K.; Collins, K.; Gaunt, C.; Fernandez, R.; Cole, S.; et al. Comparison of T cell immune responses induced by vectored HIV vaccines in non-human primates and humans. Vaccine 2010, 28, 7881–7889. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- McElrath, M.J.; Haynes, B.F. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 2010, 33, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C.; Mascola, J.R. Neutralizing antibodies against HIV-1: Can we elicit them with vaccines and how much do we need? Curr. Opin. HIV AIDS 2009, 4, 347–351. [Google Scholar] [CrossRef]
- Alam, S.M.; Scearce, R.M.; Parks, R.J.; Plonk, K.; Plonk, S.G.; Sutherland, L.L.; Gorny, M.K.; Zolla-Pazner, S.; Vanleeuwen, S.; Moody, M.A.; et al. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: Antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 2008, 82, 115–125. [Google Scholar] [CrossRef]
- Muro-Cacho, C.A.; Pantaleo, G.; Fauci, A.S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 1995, 154, 5555–5566. [Google Scholar]
- Kuhrt, D.; Faith, S.A.; Leone, A.; Rohankedkar, M.; Sodora, D.L.; Picker, L.J.; Cole, K.S. Evidence of early B-cell dysregulation in simian immunodeficiency virus infection: Rapid depletion of naive and memory B-cell subsets with delayed reconstitution of the naive B-cell population. J. Virol. 2010, 84, 2466–2476. [Google Scholar] [CrossRef]
- Peruchon, S.; Chaoul, N.; Burelout, C.; Delache, B.; Brochard, P.; Laurent, P.; Cognasse, F.; Prevot, S.; Garraud, O.; Le Grand, R.; et al. Tissue-specific B-cell dysfunction and generalized memory B-cell loss during acute SIV infection. PLoS ONE 2009, 4, e5966. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.Q.; Casimiro, D.R.; Schleif, W.A.; Chen, M.; Citron, M.; Davies, M.E.; Burns, J.; Liang, X.; Fu, T.M.; Handt, L.; et al. Early depletion of proliferating B cells of germinal center in rapidly progressive simian immunodeficiency virus infection. Virology 2007, 361, 455–464. [Google Scholar] [CrossRef]
- Malcolm, R.K.; Veazey, R.S.; Geer, L.; Lowry, D.; Fetherston, S.M.; Murphy, D.J.; Boyd, P.; Major, I.; Shattock, R.J.; Klasse, P.J.; et al. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob. Agents Chemother. 2012, 56, 2251–2258. [Google Scholar] [CrossRef]
- Derdeyn, C.A.; Decker, J.M.; Sfakianos, J.N.; Wu, X.; O’Brien, W.A.; Ratner, L.; Kappes, J.C.; Shaw, G.M.; Hunter, E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 2000, 74, 8358–8367. [Google Scholar] [CrossRef] [PubMed]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Cranage, M.; McGowan, I.; Anton, P.; Shattock, R.J. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob. Agents Chemother. 2009, 53, 1797–1807. [Google Scholar] [CrossRef]
- Hu, Q.; Frank, I.; Williams, V.; Santos, J.J.; Watts, P.; Griffin, G.E.; Moore, J.P.; Pope, M.; Shattock, R.J. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 2004, 199, 1065–1075. [Google Scholar] [CrossRef]
- Brown, B.K.; Darden, J.M.; Tovanabutra, S.; Oblander, T.; Frost, J.; Sanders-Buell, E.; de Souza, M.S.; Birx, D.L.; McCutchan, F.E.; Polonis, V.R. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J. Virol. 2005, 79, 6089–6101. [Google Scholar] [CrossRef]
- Pal, R.; Taylor, B.; Foulke, J.S.; Woodward, R.; Merges, M.; Praschunus, R.; Gibson, A.; Reitz, M. Characterization of a simian human immunodeficiency virus encoding the envelope gene from the CCR5-tropic HIV-1 Ba-L. J. Acquir. Immune Defic. Syndr. 2003, 33, 300–307. [Google Scholar] [CrossRef]
- Santra, S.; Tomaras, G.D.; Warrier, R.; Nicely, N.I.; Liao, H.X.; Pollara, J.; Liu, P.; Alam, S.M.; Zhang, R.; Cocklin, S.L.; et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS Pathog. 2015, 11, e1005042. [Google Scholar] [CrossRef]
- Gordon, C.J.; Muesing, M.A.; Proudfoot, A.E.; Power, C.A.; Moore, J.P.; Trkola, A. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 1999, 73, 684–694. [Google Scholar] [CrossRef]
- Aldon, Y.; McKay, P.F.; Moreno Herrero, J.; Vogel, A.B.; Levai, R.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Haas, H.; Fabian, K.; Le Grand, R.; et al. Immunogenicity of stabilized HIV-1 Env trimers delivered by self-amplifying mRNA. Mol. Ther. Nucleic Acids 2021, 25, 483–493. [Google Scholar] [CrossRef]
- Joseph, S.; Quinn, K.; Greenwood, A.; Cope, A.V.; McKay, P.F.; Hayes, P.J.; Kopycinski, J.T.; Gilmour, J.; Miller, A.N.; Geldmacher, C.; et al. A Comparative Phase I Study of Combination, Homologous Subtype-C DNA, MVA, and Env gp140 Protein/Adjuvant HIV Vaccines in Two Immunization Regimes. Front. Immunol. 2017, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, J.P.; Belec, L.; Pires, R.; Pillot, J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect. Immun. 1994, 62, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Lycke, N.Y. Immunology of the human genital tract. Curr. Opin. Infect. Dis. 2003, 16, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.X. Predominate HIV1-specific IgG activity in various mucosal compartments of HIV1-infected individuals. Clin. Immunol. 2000, 97, 59–68. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef]
- Montefiori, D.C.; Karnasuta, C.; Huang, Y.; Ahmed, H.; Gilbert, P.; de Souza, M.S.; McLinden, R.; Tovanabutra, S.; Laurence-Chenine, A.; Sanders-Buell, E.; et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 2012, 206, 431–441. [Google Scholar] [CrossRef]
- Prescott, M.J.; Lidster, K. Improving quality of science through better animal welfare: The NC3Rs strategy. Lab Anim. 2017, 46, 152–156. [Google Scholar] [CrossRef]
- Luo, K.; Liao, H.X.; Zhang, R.; Easterhoff, D.; Wiehe, K.; Gurley, T.C.; Armand, L.C.; Allen, A.A.; Von Holle, T.A.; Marshall, D.J.; et al. Tissue memory B cell repertoire analysis after ALVAC/AIDSVAX B/E gp120 immunization of rhesus macaques. JCI Insight 2016, 1, e88522. [Google Scholar] [CrossRef]
- Vaccari, M.; Gordon, S.N.; Fourati, S.; Schifanella, L.; Liyanage, N.P.; Cameron, M.; Keele, B.F.; Shen, X.; Tomaras, G.D.; Billings, E.; et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat. Med. 2016, 22, 762–770. [Google Scholar] [CrossRef]
- Cheeseman, H.M.; Olejniczak, N.J.; Rogers, P.M.; Evans, A.B.; King, D.F.L.; Ziprin, P.; Liao, H.X.; Haynes, B.F.; Shattock, R.J. Broadly Neutralizing Antibodies Display Potential for Prevention of HIV-1 Infection of Mucosal Tissue Superior to that of Nonneutralizing Antibodies. J. Virol. 2017, 91, e01762-16. [Google Scholar] [CrossRef]
- Mall, A.S.; Habte, H.; Mthembu, Y.; Peacocke, J.; de Beer, C. Mucus and Mucins: Do they have a role in the inhibition of the human immunodeficiency virus? Virol. J. 2017, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Shukair, S.A.; Allen, S.A.; Cianci, G.C.; Stieh, D.J.; Anderson, M.R.; Baig, S.M.; Gioia, C.J.; Spongberg, E.J.; Kauffman, S.M.; McRaven, M.D.; et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013, 6, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Mhlekude, B.; Lenman, A.; Sidoyi, P.; Joseph, J.; Kruppa, J.; Businge, C.B.; Mdaka, M.L.; Konietschke, F.; Pich, A.; Gerold, G.; et al. The barrier functions of crude cervical mucus plugs against HIV-1 infection in the context of cell-free and cell-to-cell transmission. AIDS 2021, 35, 2105–2117. [Google Scholar] [CrossRef]
- Stax, M.J.; Mouser, E.E.; van Montfort, T.; Sanders, R.W.; de Vries, H.J.; Dekker, H.L.; Herrera, C.; Speijer, D.; Pollakis, G.; Paxton, W.A. Colorectal mucus binds DC-SIGN and inhibits HIV-1 trans-infection of CD4+ T-lymphocytes. PLoS ONE 2015, 10, e0122020. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, K.M.; Malykhina, O.; Stieh, D.J.; Hope, T.J. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS ONE 2013, 8, e76176. [Google Scholar] [CrossRef] [PubMed]
- Bonsignori, M.; Pollara, J.; Moody, M.A.; Alpert, M.D.; Chen, X.; Hwang, K.K.; Gilbert, P.B.; Huang, Y.; Gurley, T.C.; Kozink, D.M.; et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 2012, 86, 11521–11532. [Google Scholar] [CrossRef]
- Su, B.; Dispinseri, S.; Iannone, V.; Zhang, T.; Wu, H.; Carapito, R.; Bahram, S.; Scarlatti, G.; Moog, C. Update on Fc-Mediated Antibody Functions against HIV-1 beyond Neutralization. Front. Immunol. 2019, 10, 2968. [Google Scholar] [CrossRef]
- Perez, L.G.; Martinez, D.R.; deCamp, A.C.; Pinter, A.; Berman, P.W.; Francis, D.; Sinangil, F.; Lee, C.; Greene, K.; Gao, H.; et al. V1V2-specific complement activating serum IgG as a correlate of reduced HIV-1 infection risk in RV144. PLoS ONE 2017, 12, e0180720. [Google Scholar] [CrossRef]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J.; et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 2014, 6, 228ra238. [Google Scholar] [CrossRef]
- Liao, H.X.; Bonsignori, M.; Alam, S.M.; McLellan, J.S.; Tomaras, G.D.; Moody, M.A.; Kozink, D.M.; Hwang, K.K.; Chen, X.; Tsao, C.Y.; et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 2013, 38, 176–186. [Google Scholar] [CrossRef]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Liu, P.; Alam, S.M.; Hwang, K.K.; Gurley, T.C.; Kozink, D.M.; Armand, L.C.; Marshall, D.J.; et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J. Virol. 2014, 88, 7715–7726. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, G.D.; Ferrari, G.; Shen, X.; Alam, S.M.; Liao, H.X.; Pollara, J.; Bonsignori, M.; Moody, M.A.; Fong, Y.; Chen, X.; et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. USA 2013, 110, 9019–9024. [Google Scholar] [CrossRef] [PubMed]
- Harenberg, A.; Guillaume, F.; Ryan, E.J.; Burdin, N.; Spada, F. Gene profiling analysis of ALVAC infected human monocyte derived dendritic cells. Vaccine 2008, 26, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.J.; Harenberg, A.; Burdin, N. The Canarypox-virus vaccine vector ALVAC triggers the release of IFN-gamma by Natural Killer (NK) cells enhancing Th1 polarization. Vaccine 2007, 25, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jones, B.; Hu, N.; Chang, H.; Ahmad, S.; Liu, J.; Parrington, M.; Ostrowski, M. Comparative analysis of tropism between canarypox (ALVAC) and vaccinia viruses reveals a more restricted and preferential tropism of ALVAC for human cells of the monocytic lineage. Vaccine 2006, 24, 6376–6391. [Google Scholar] [CrossRef] [PubMed]
- Andersen-Nissen, E.; Fiore-Gartland, A.; Ballweber Fleming, L.; Carpp, L.N.; Naidoo, A.F.; Harper, M.S.; Voillet, V.; Grunenberg, N.; Laher, F.; Innes, C.; et al. Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk. PLoS Pathog. 2021, 17, e1009363. [Google Scholar] [CrossRef]
- Teigler, J.E.; Phogat, S.; Franchini, G.; Hirsch, V.M.; Michael, N.L.; Barouch, D.H. The canarypox virus vector ALVAC induces distinct cytokine responses compared to the vaccinia virus-based vectors MVA and NYVAC in rhesus monkeys. J. Virol. 2014, 88, 1809–1814. [Google Scholar] [CrossRef]
- Sabbaj, S.; Mulligan, M.J.; Hsieh, R.H.; Belshe, R.B.; McGhee, J.R. Cytokine profiles in seronegative volunteers immunized with a recombinant canarypox and gp120 prime-boost HIV-1 vaccine. NIAID AIDS Vaccine Evaluation Group. AIDS 2000, 14, 1365–1374. [Google Scholar] [CrossRef]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef]
- Herrera, C.; Shattock, R.J. Candidate microbicides and their mechanisms of action. Curr. Top. Microbiol. Immunol. 2014, 383, 1–25. [Google Scholar]
- Anton, P.A.; Cranston, R.D.; Kashuba, A.; Hendrix, C.W.; Bumpus, N.N.; Richardson-Harman, N.; Elliott, J.; Janocko, L.; Khanukhova, E.; Dennis, R.; et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res. Hum. Retrovir. 2012, 28, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Tiraboschi, J.M.; Herrera, C.; Else, L.; Egan, D.; Dickinson, L.; Jackson, A.; Olejniczak, N.; Back, D.; Khoo, S.; et al. Brief Report: Pharmacokinetic/Pharmacodynamic Investigation of Single-Dose Oral Maraviroc in the Context of HIV-1 Pre-exposure Prophylaxis. J. Acquir. Immune Defic. Syndr. 2016, 73, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Lwanga, J.; Lee, M.; Mantori, S.; Amara, A.; Else, L.; Penchala, S.D.; Egan, D.; Challenger, E.; Dickinson, L.; et al. Pharmacokinetic/pharmacodynamic investigation of raltegravir with or without lamivudine in the context of HIV-1 pre-exposure prophylaxis (PrEP). J. Antimicrob. Chemother. 2021, 76, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.; Cranston, R.D.; Duffill, K.; Siegel, A.; Engstrom, J.C.; Nikiforov, A.; Jacobson, C.; Rehman, K.K.; Elliott, J.; Khanukhova, E.; et al. A Phase 1 Randomized, Open Label, Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Three Formulations of Tenofovir 1% Gel (the CHARM-01 Study). PLoS ONE 2015, 10, e0125363. [Google Scholar] [CrossRef]
- Richardson-Harman, N.; Hendrix, C.W.; Bumpus, N.N.; Mauck, C.; Cranston, R.D.; Yang, K.; Elliott, J.; Tanner, K.; McGowan, I.; Kashuba, A.; et al. Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PLoS ONE 2014, 9, e111507. [Google Scholar] [CrossRef]
- Richardson-Harman, N.; Mauck, C.; McGowan, I.; Anton, P. Dose-response relationship between tissue concentrations of UC781 and explant infectibility with HIV type 1 in the RMP-01 rectal safety study. AIDS Res. Hum. Retrovir. 2012, 28, 1422–1433. [Google Scholar] [CrossRef]
- Richardson-Harman, N.; Lackman-Smith, C.; Fletcher, P.S.; Anton, P.A.; Bremer, J.W.; Dezzutti, C.S.; Elliott, J.; Grivel, J.C.; Guenthner, P.; Gupta, P.; et al. Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J. Clin. Microbiol. 2009, 47, 3530–3539. [Google Scholar] [CrossRef]
- Anton, P.A.; Elliott, J.; Poles, M.A.; McGowan, I.M.; Matud, J.; Hultin, L.E.; Grovit-Ferbas, K.; Mackay, C.R.; Chen, I.S.Y.; Giorgi, J.V. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. Aids 2000, 14, 1761–1765. [Google Scholar] [CrossRef]
- Lapenta, C.; Boirivant, M.; Marini, M.; Santini, S.M.; Logozzi, M.; Viora, M.; Belardelli, F.; Fais, S. Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur. J. Immunol. 1999, 29, 1202–1208. [Google Scholar] [CrossRef]
- Poles, M.A.; Elliott, J.; Taing, P.; Anton, P.A.; Chen, I.S. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 2001, 75, 8390–8399. [Google Scholar] [CrossRef]
- Herrera, C.; McRaven, M.D.; Laing, K.G.; Dennis, J.; Hope, T.J.; Shattock, R.J. Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs. Vaccines 2021, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Shacklett, B.L.; Greenblatt, R.M. Immune Responses to HIV in the Female Reproductive Tract, Immunologic Parallels with the Gastrointestinal Tract, and Research Implications. Am. J. Reprod. Immunol. 2011, 65, 230–241. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.S.; Ratto-Kim, S.; Chuenarom, W.; Schuetz, A.; Chantakulkij, S.; Nuntapinit, B.; Valencia-Micolta, A.; Thelian, D.; Nitayaphan, S.; Pitisuttithum, P.; et al. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J. Immunol. 2012, 188, 5166–5176. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, C.; Veazey, R.; Lemke, M.M.; Arnold, K.; Kim, J.H.; Shattock, R.J. Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates. Vaccines 2022, 10, 187. https://doi.org/10.3390/vaccines10020187
Herrera C, Veazey R, Lemke MM, Arnold K, Kim JH, Shattock RJ. Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates. Vaccines. 2022; 10(2):187. https://doi.org/10.3390/vaccines10020187
Chicago/Turabian StyleHerrera, Carolina, Ronald Veazey, Melissa M. Lemke, Kelly Arnold, Jerome H. Kim, and Robin J. Shattock. 2022. "Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates" Vaccines 10, no. 2: 187. https://doi.org/10.3390/vaccines10020187
APA StyleHerrera, C., Veazey, R., Lemke, M. M., Arnold, K., Kim, J. H., & Shattock, R. J. (2022). Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates. Vaccines, 10(2), 187. https://doi.org/10.3390/vaccines10020187





