The Ongoing Journey of a Shigella Bioconjugate Vaccine
Abstract
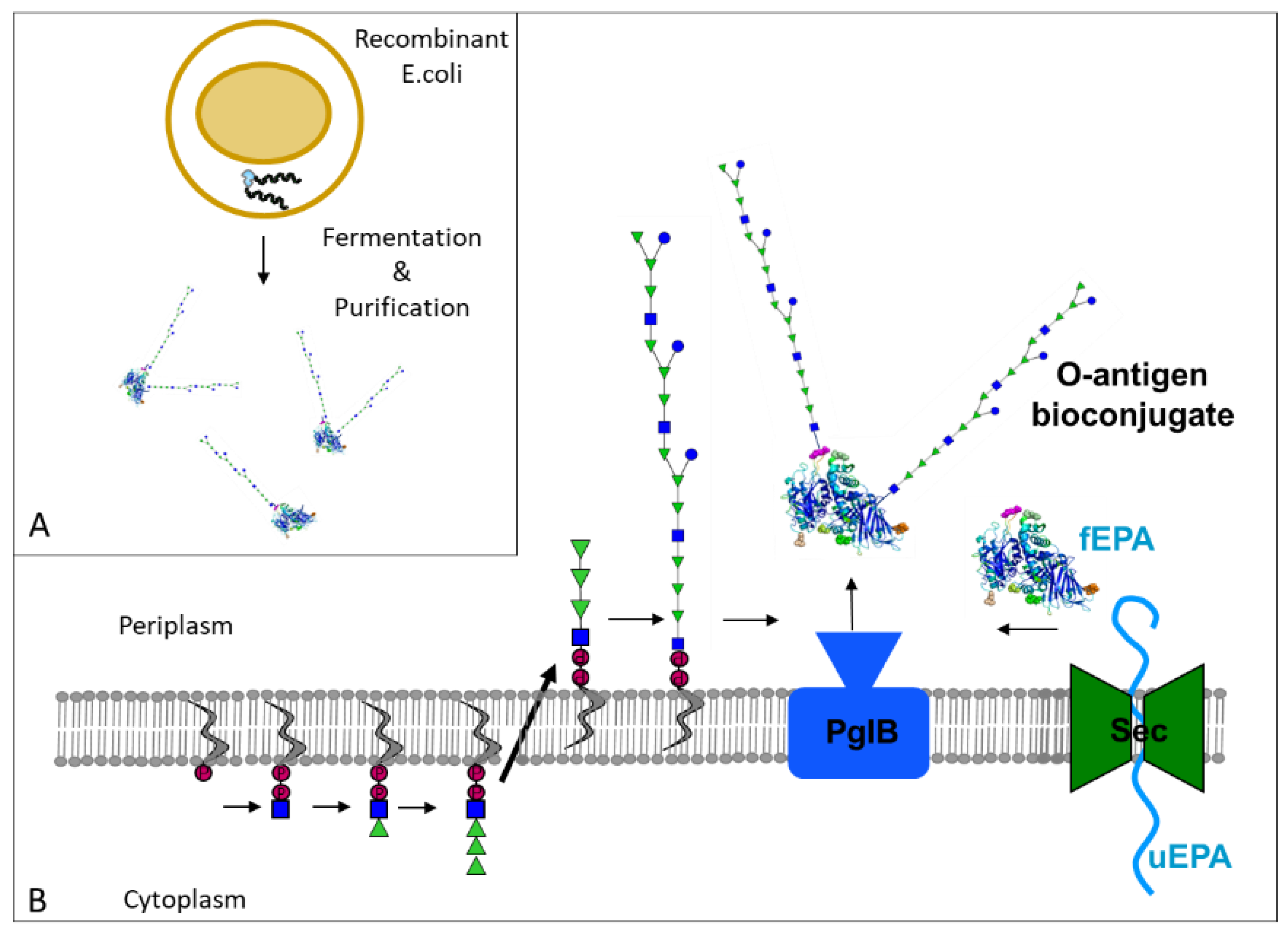
:1. Introduction
2. O-Antigen-Based Shigella Vaccines: From Chemical to Bioconjugates
2.1. Shigella Dysenteriae Bioconjugate Vaccine
2.2. Shigella Flexneri Bioconjugate Vaccine
2.3. Quadrivalent Shigella Bioconjugate Vaccine
3. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotloff, K.L.; Platts-Mills, J.A.; Nasrin, D.; Roose, A.; Blackwelder, W.C.; Levine, M.M. Global burden of diarrheal diseases among children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017, 35, 6783–6789. [Google Scholar] [CrossRef] [PubMed]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Giersing, B.; Khalil, I. DRAFT WHO Preferred Product Characteristics for Vaccines against Shigella. Available online: https://www.who.int/immunization/research/ppc-tpp/PPC_Shigella_draft_for_review_april2020.pdf (accessed on 16 December 2021).
- Baker, S.; The, H.C. Recent insights into Shigella. Curr. Opin. Infect. Dis. 2018, 31, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Chompook, P. Shigellosis. Encycl. Environ. Health 2019, 626–632. [Google Scholar] [CrossRef]
- Worley, J.N.; Javkar, K.; Hoffmann, M.; Hysell, K.; Garcia-Williams, A.; Tagg, K.; Kanjilal, S.; Strain, E.; Pop, M.; Allard, M.; et al. Genomic Drivers of Multidrug-Resistant Shigella Affecting Vulnerable Patient Populations in the United States and Abroad. Mbio 2021, 12, e03188-20. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States—2019. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 21 October 2021).
- Kahsay, A.G.; Muthupandian, S. A review on Sero diversity and antimicrobial resistance patterns of Shigella species in Africa, Asia and South America, 2001–2014. BMC Res. Notes 2016, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Ashkenazi, S.; Cohen, D. An update on vaccines against Shigella. Ther. Adv. Vaccines 2013, 1, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimanovich, A.A.; Buskirk, A.D.; Heine, S.J.; Blackwelder, W.C.; Wahid, R.; Kotloff, K.L.; Pasetti, M.F. Functional and Antigen-Specific Serum Antibody Levels as Correlates of Protection Against Shigellosis in a Controlled Human Challenge Study. Clin. Vaccine Immunol. 2016, 24, e00412-16. [Google Scholar] [CrossRef] [Green Version]
- Raqib, R.; Sarker, P.; Zaman, K.; Alam, N.H.; Wierzba, T.F.; Maier, N.; Talukder, K.; Baqui, A.H.; Suvarnapunya, A.E.; Qadri, F.; et al. A phase I trial of WRSS1, a Shigella sonnei live oral vaccine in Bangladeshi adults and children. Hum. Vaccin Immunother. 2019, 15, 1326–1337. [Google Scholar] [CrossRef]
- Frenck, R.W.; Baqar, S.; Alexander, W.; Dickey, M.; McNeal, M.; El-Khorazaty, J.; Baughman, H.; Hoeper, A.; Barnoy, S.; Suvarnapunya, A.E.; et al. A Phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine 2018, 36, 4880–4889. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Harro, C.; DeNearing, B.; Bream, J.; Bauers, N.; Dally, L.; Flores, J.; Van de Verg, L.; Sack, D.A.; Walker, R. Evaluation of the Safety, Tolerability, and Immunogenicity of an Oral, Inactivated Whole-Cell Shigella flexneri 2a Vaccine in Healthy Adult Subjects. Clin. Vaccine Immunol. 2016, 23, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M.-L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2021, 21, 546–558. [Google Scholar] [CrossRef]
- Riddle, M.S.; Kaminski, R.W.; Di Paolo, C.; Porter, C.K.; Gutierrez, R.L.; Clarkson, K.A.; Weerts, H.E.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: A Single-Blind, Randomized Phase I Study. Clin. Vaccine Immunol. 2016, 23, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Launay, O.; Ndiaye, A.G.W.; Conti, V.; Loulergue, P.; Sciré, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schütte, L.D.; Auerbach, J.; et al. Booster Vaccination With GVGH Shigella sonnei 1790GAHB GMMA Vaccine Compared to Single Vaccination in Unvaccinated Healthy European Adults: Results From a Phase 1 Clinical Trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef] [Green Version]
- Mo, Y.; Fang, W.; Li, H.; Chen, J.; Hu, X.; Wang, B.; Feng, Z.; Shi, H.; He, Y.; Huang, D.; et al. Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China. Vaccines 2022, 10, 33. [Google Scholar] [CrossRef]
- Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.M.; Kaminski, R.W.; Porter, C.K.; Chakraborty, S.; Clarkson, K.A.; Brubaker, J.; et al. Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection. EBioMedicine 2021, 66, 103310. [Google Scholar] [CrossRef]
- Frenck, R.W.; Conti, V.; Ferruzzi, P.; Ndiaye, A.G.W.; Parker, S.; McNeal, M.M.; Dickey, M.; Granada, J.P.; Cilio, G.L.; de Ryck, I.; et al. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: Results from a phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine 2021, 39, 101076. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Losonsky, G.A.; Nataro, J.P.; Wasserman, S.S.; Hale, T.L.; Taylor, D.N.; Newland, J.W.; Sadoff, J.C.; Formal, S.B.; Levine, M.M. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine 1995, 13, 495–502. [Google Scholar] [CrossRef]
- Passwell, J.H.; Ashkenzi, S.; Banet-Levi, Y.; Ramon-Saraf, R.; Farzam, N.; Lerner-Geva, L.; Even-Nir, H.; Yerushalmi, B.; Chu, C.; Shiloach, J.; et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine 2009, 28, 2231–2235. [Google Scholar] [CrossRef]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Obiero, C.W.; Ndiaye, A.G.W.; Sciré, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schütte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017, 8, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, K.A.; Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.; Porter, C.K.; Chakraborty, S.; Brubaker, J.; Elwood, D.; et al. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. EBioMedicine 2021, 66, 103308. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D. Conjugate vaccines. Clin. Exp. Immunol. 2000, 119, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.; Casey, J.; Blatter, M.; Rothstein, E.; Ryall, R.; Bybel, M.; Gilmet, G.; Papa, T. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatric Infect. Dis. J. 2005, 24, 57–62. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Halperin, S.A.; Law, B.J.; Forrest, B.; Danzig, L.E.; Granoff, D.M. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: A randomized controlled trial. JAMA 1998, 280, 1685–1689. [Google Scholar] [CrossRef]
- Englund, J.A.; Glezen, W.P.; Turner, C.; Harvey, J.; Thompson, C.; Siber, G.R. Transplacental antibody transfer following maternal immunization with polysaccharide and conjugate Haemophilus influenzae type b vaccines. J. Infect. Dis. 1995, 171, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Chang, S.-Y.; Chuang, Y.-C.; Liu, W.-C.; Su, C.-T.; Su, Y.-C.; Chang, S.-F.; Hung, C.-C. Revaccination with 7-valent pneumococcal conjugate vaccine elicits better serologic response than 23-valent pneumococcal polysaccharide vaccine in HIV-infected adult patients who have undergone primary vaccination with 23-valent pneumococcal polysaccharide vaccine in the era of combination antiretroviral therapy. Vaccine 2014, 32, 1031–1035. [Google Scholar] [CrossRef]
- Sorensen, R.U.; Leiva, L.E.; Giangrosso, P.A.; Butler, B.; Javier, F.C.; Sacerdote, D.M.; Bradford, N.; Moore, C. Response to a heptavalent conjugate Streptococcus pneumoniae vaccine in children with recurrent infections who are unresponsive to the polysaccharide vaccine. Pediatric Infect. Dis. J. 1998, 17, 685–691. [Google Scholar] [CrossRef]
- Pichichero, M.E. Meningococcal conjugate vaccine in adolescents and children. Clin. Pediatr. 2005, 44, 479–489. [Google Scholar] [CrossRef]
- Watson, W. Pneumococcal conjugate vaccines. Pediatric Infect. Dis. J. 2000, 19, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Slack, M.; Esposito, S.; Haas, H.; Mihalyi, A.; Nissen, M.; Mukherjee, P.; Harrington, L. Haemophilus influenzae type b disease in the era of conjugate vaccines: Critical factors for successful eradication. Expert Rev. Vaccines 2020, 19, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.F.; Wacker, M.; Hernandez, M.; Hitchen, P.G.; Marolda, C.L.; Kowarik, M.; Morris, H.R.; Dell, A.; Valvano, M.A.; Aebi, M. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. USA 2005, 102, 3016–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, M.; Linton, D.; Hitchen, P.G.; Nita-Lazar, M.; Haslam, S.M.; North, S.J.; Panico, M.; Morris, H.R.; Dell, A.; Wren, B.W.; et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 2002, 298, 1790–1793. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Li, J.; Shiloach, Y.; Robbins, J.B.; Szu, S.C. Safety and immunogenicity of Escherichia coli O157 O-specific polysaccharide conjugate vaccine in 2–5-year-old children. J. Infect. Dis. 2006, 193, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.N.; Trofa, A.C.; Sadoff, J.; Chu, C.; Bryla, D.; Shiloach, J.; Cohen, D.; Ashkenazi, S.; Lerman, Y.; Egan, W.; et al. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect. Immun. 1993, 61, 3678–3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passwell, J.H.; Harlev, E.; Ashkenazi, S.; Chu, C.; Miron, D.; Ramon, R.; Farzan, N.; Shiloach, J.; Bryla, D.A.; Majadly, F.; et al. Safety and immunogenicity of improved Shigella O-specific polysaccharide-protein conjugate vaccines in adults in Israel. Infect. Immun. 2001, 69, 1351–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passwell, J.H.; Ashkenazi, S.; Harlev, E.; Miron, D.; Ramon, R.; Farzam, N.; Lerner-Geva, L.; Levi, Y.; Chu, C.; Shiloach, J.; et al. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPAsucc conjugate vaccines in one- to four-year-old children. Pediatric Infect. Dis. J. 2003, 22, 701–706. [Google Scholar] [CrossRef]
- Robbins, J.B.; Kubler-Kielb, J.; Vinogradov, E.; Mocca, C.; Pozsgay, V.; Shiloach, J.; Schneerson, R. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc. Natl. Acad. Sci. USA 2009, 106, 7974–7978. [Google Scholar] [CrossRef] [Green Version]
- Ravenscroft, N.; Haeuptle, M.A.; Kowarik, M.; Fernandez, F.S.; Carranza, P.; Brunner, A.; Steffen, M.; Wetter, M.; Keller, S.; Ruch, C.; et al. Purification and characterization of a Shigella conjugate vaccine, produced by glycoengineering Escherichia coli. Glycobiology 2016, 26, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatz, C.; Bally, B.; Rohrer, S.; Steffen, R.; Kramme, S.; Siegrist, C.-A.; Wacker, M.; Alaimo, C.; Fonck, V.G. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: A single blind, partially randomized Phase I study. Vaccine 2015, 33, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Wang, L.; Kowarik, M.; Dowd, M.; Lipowsky, G.; Faridmoayer, A.; Shields, K.; Park, S.; Alaimo, C.; Kelley, K.A.; et al. Prevention of Staphylococcus aureus Infections by Glycoprotein Vaccines Synthesized in Escherichia coli. J. Infect. Dis. 2014, 209, 1551–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttner, A.; Hatz, C.; van den Dobbelsteen, G.; Abbanat, D.; Hornacek, A.; Frölich, R.; Dreyer, A.M.; Martin, P.; Davies, T.; Fae, K.; et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: A randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 2017, 17, 528–537. [Google Scholar] [CrossRef]
- Frenck, R.W.; Ervin, J.; Chu, L.; Abbanat, D.; Spiessens, B.; Go, O.; Haazen, W.; van den Dobbelsteen, G.; Poolman, J.; Thoelen, S.; et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): A phase 2 randomised controlled trial. Lancet Infect. Dis. 2019, 19, 631–640. [Google Scholar] [CrossRef]
- Clarkson, K.A.; Porter, C.K.; Talaat, K.R.; Frenck, R.W.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Kaminski, R.W. Shigella-Specific Immune Profiles Induced after Parenteral Immunization or Oral Challenge with Either Shigella flexneri 2a or Shigella sonnei. mSphere 2021, 6, e0012221. [Google Scholar] [CrossRef]
- Noriega, F.R.; Liao, F.M.; Maneval, D.R.; Ren, S.; Formal, S.B.; Levine, M.M. Strategy for cross-protection among Shigella flexneri serotypes. Infect. Immun. 1999, 67, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Chen, W.H.; Cohen, M.B.; Kirkpatrick, B.D.; Brady, R.C.; Galloway, D.; Gurwith, M.; Hall, R.H.; Kessler, R.A.; Lock, M.; Haney, D.; et al. Single-dose Live Oral Cholera Vaccine CVD 103-HgR Protects Against Human Experimental Infection With Vibrio cholerae O1 El Tor. Clin. Infect. Dis. 2016, 62, 1329–1335. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Gibani, M.M.; Moore, M.; Juel, H.B.; Jones, E.; Meiring, J.; Harris, V.; Gardner, J.; Nebykova, A.; Kerridge, S.A.; et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: A randomised controlled, phase 2b trial. Lancet 2017, 390, 2472–2480. [Google Scholar] [CrossRef] [Green Version]
- Giersing, B.K.; Porter, C.K.; Kotloff, K.; Neels, P.; Cravioto, A.; MacLennan, C.A. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine 2019, 37, 4778–4783. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Bioconjugate | Doses * & schedule | Number (n) & Age | Results |
|---|---|---|---|---|
| NCT01069471 Phase I, Switzerland, in 2010 | Vaccine against S.dysenteriae (GVXN SD133-EPA) | 2 µg PS 2 µg PS + AlOH3 10 µg PS 10 µg PS + AlOH3 2 injections: Day 0 and 2 months | n = 40 18–50 years |
|
| NCT02388009 Phase I, US, in 2015 | Vaccine against S. flexneri-2a (Flexyn2a-EPA) | 10 µg PS 10 µg PS + AlOH3 Placebo 2 injections: Day 0 and 1 month | n = 30 18–50 years |
|
| NCT02646371 Phase IIb, US, in 2016 | 10 µg PS Placebo 2 injections: Day 0 and 1 month | n = 67 18–50 years | ||
| NCT04056117 Phase I/II, Kenya, Started in 2019, ongoing | Vaccine against S. flexneri 2a, 3a, 6 and sonnei (S4V-EPA) |
| n = 16 18–50 years n = 48 2–5 years n = 528 9 months | Ongoing, enrollment completed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, P.; Alaimo, C. The Ongoing Journey of a Shigella Bioconjugate Vaccine. Vaccines 2022, 10, 212. https://doi.org/10.3390/vaccines10020212
Martin P, Alaimo C. The Ongoing Journey of a Shigella Bioconjugate Vaccine. Vaccines. 2022; 10(2):212. https://doi.org/10.3390/vaccines10020212
Chicago/Turabian StyleMartin, Patricia, and Cristina Alaimo. 2022. "The Ongoing Journey of a Shigella Bioconjugate Vaccine" Vaccines 10, no. 2: 212. https://doi.org/10.3390/vaccines10020212





