Human Milk Antibodies after BNT162b2 Vaccination Exhibit Reduced Binding against SARS-CoV-2 Variants of Concern
Abstract
:1. Introduction
2. Materials and Methods
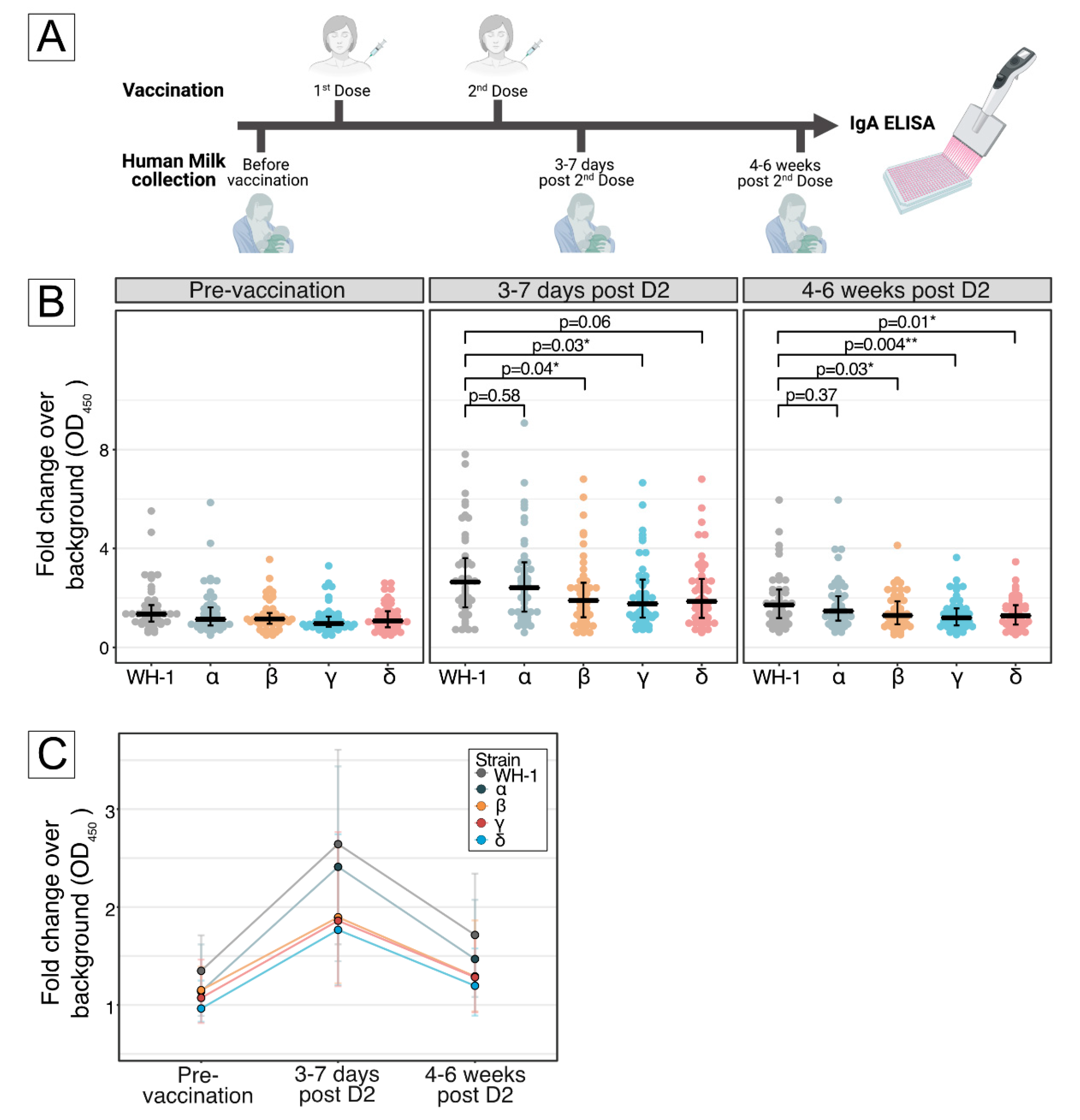
2.1. Subjects and Milk Sample Collection
2.2. Biochemical Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Low, J.M.; Gu, Y.; Ng, M.S.F.; Amin, Z.; Lee, L.Y.; Ng, Y.P.M.; Shunmuganathan, B.D.O.; Niu, Y.; Gupta, R.; Tambyah, P.A.; et al. Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. NPJ Vaccines 2021, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Lechosa-Muñiz, C.; Paz-Zulueta, M.; Mendez-Legaza, J.M.; Irure-Ventura, J.; Cuesta González, R.; Calvo Montes, J.; López-Hoyos, M.; Llorca, J.; Cabero-Pérez, M.J. Induction of SARS-CoV-2-Specific IgG and IgA in Serum and Milk with Different SARS-CoV-2 Vaccines in Breastfeeding Women: A Cross-Sectional Study in Northern Spain. Int. J. Environ. Res. Public Health 2021, 18, 8831. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: How many variants are there, and what do we know about them? BMJ 2021, 374, n1971. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Bixler, D.; Miller, A.D.; Mattison, C.P.; Taylor, B.; Komatsu, K.; Peterson Pompa, X.; Moon, S.; Karmarkar, E.; Liu, C.Y.; Openshaw, J.J.; et al. SARS-CoV-2–Associated Deaths Among Persons Aged < 21 Years—United States, February 12–July 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- The Straits Times. More Unvaccinated Pregnant Women with Covid-19 Hospitalised in Singapore. Available online: https://www.straitstimes.com/singapore/health/more-unvaccinated-pregnant-women-with-covid-19-hospitalised-in-singapore (accessed on 21 January 2022).
- Ministry of Health Singapore. Update on Local COVID-19 Situation and Vaccination Progress (30 August 2021). Available online: https://www.moh.gov.sg/news-highlights/details/update-on-local-covid-19-situation-and-vaccination-progress-(30-august-2021) (accessed on 21 January 2022).
- Pace Ryan, M.; Williams Janet, E.; Järvinen Kirsi, M.; Belfort Mandy, B.; Pace Christina, D.W.; Lackey Kimberly, A.; Gogel Alexandra, C.; Nguyen-Contant, P.; Kanagaiah, P.; Fitzgerald, T.; et al. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio 2021, 12, e03192-20. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef] [PubMed]
| Number of participants | No. (%) |
| Study participants, No. | 46 |
| Maternal characteristics | |
| Maternal Age, mean (standard deviation, SD), years | 31.5 (0.9) |
| Ethnicity Singaporean Chinese Singaporean Malay | 41 (89.1) 5 (10.9) |
| Chronic disease Asthma Polycystic Ovarian Syndrome Thalassemia Minor | 1 (2.2) 1 (2.2) 1 (2.2) |
| Antenatal conditions | 0 |
| Smoker | 0 |
| Postpartum (standard deviation, SD), months | 13.5 (SD 2.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, J.M.; Gu, Y.; Ng, M.S.F.; Wang, L.W.; Amin, Z.; Zhong, Y.; MacAry, P.A. Human Milk Antibodies after BNT162b2 Vaccination Exhibit Reduced Binding against SARS-CoV-2 Variants of Concern. Vaccines 2022, 10, 225. https://doi.org/10.3390/vaccines10020225
Low JM, Gu Y, Ng MSF, Wang LW, Amin Z, Zhong Y, MacAry PA. Human Milk Antibodies after BNT162b2 Vaccination Exhibit Reduced Binding against SARS-CoV-2 Variants of Concern. Vaccines. 2022; 10(2):225. https://doi.org/10.3390/vaccines10020225
Chicago/Turabian StyleLow, Jia Ming, Yue Gu, Melissa Shu Feng Ng, Liang Wei Wang, Zubair Amin, Youjia Zhong, and Paul A. MacAry. 2022. "Human Milk Antibodies after BNT162b2 Vaccination Exhibit Reduced Binding against SARS-CoV-2 Variants of Concern" Vaccines 10, no. 2: 225. https://doi.org/10.3390/vaccines10020225
APA StyleLow, J. M., Gu, Y., Ng, M. S. F., Wang, L. W., Amin, Z., Zhong, Y., & MacAry, P. A. (2022). Human Milk Antibodies after BNT162b2 Vaccination Exhibit Reduced Binding against SARS-CoV-2 Variants of Concern. Vaccines, 10(2), 225. https://doi.org/10.3390/vaccines10020225






