Recombinant HBsAg of the Wild-Type and the G145R Escape Mutant, included in the New Multivalent Vaccine against Hepatitis B Virus, Dramatically Differ in their Effects on Leukocytes from Healthy Donors In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Sera
2.2. rHBsAg
2.3. Immunophenotyping of PBMC
2.4. Stimulation of PBMC with rHBsAg
2.5. Evaluation of the Expression of Activation and Depletion Markers on PBMC after Antigenic Stimulation
2.6. Statistical Analysis
3. Results
3.1. Immunophenotyping of PBMC from Healthy Donors
3.2. Evaluation of Activation Markers on PBMC from Healthy Donors after In Vitro Stimulation with rHBsAg
3.3. Cytokine Secretion by PBMC from Healthy Donors after Stimulation with rHBsAg In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahamat, G.; Kenmoe, S.; Akazong, E.W.; Ebogo-Belobo, J.T.; Mbaga, D.S.; Bowo-Ngandji, A.; Foe-Essomba, J.R.; Amougou-Atsama, M.; Monamele, C.G.; Mikangue, C.A.M.; et al. Global prevalence of hepatitis B virus serological markers among healthcare workers: A systematic review and meta-analysis. World J. Hepatol. 2021, 13, 1190–1202. [Google Scholar] [CrossRef]
- Cox, A.L.; El-Sayed, M.H.; Kao, J.-H.; Lazarus, J.V.; Lemoine, M.; Lok, A.S.; Zoulim, F. Progress towards elimination goals for viral hepatitis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 533–542. [Google Scholar] [CrossRef]
- Ho, J.K.-T.; Jeevan-Raj, B.; Netter, H.-J. Hepatitis B Virus (HBV) Subviral Particles as Protective Vaccines and Vaccine Platforms. Viruses 2020, 12, 126. [Google Scholar] [CrossRef]
- Maksvitis, R.Y.; Ivanov, R.V.; Amelin, A.V.; Goncharova, A.V.; Andreeva, A.S.; Grinyov, I.A. New Russian Trivalent Hepatitis B vaccine (Bubo®-Unigep): Phase I Clinical Study Results and Perspectives of Further Investigations Aiming Registration in Russia. Epidemiol. Vaccinal Prev. 2020, 18, 45–52. [Google Scholar] [CrossRef][Green Version]
- Han, Q.; Zhang, C.; Zhang, J.; Tian, Z. The role of innate immunity in HBV infection. Semin. Immunopathol. 2012, 35, 23–38. [Google Scholar] [CrossRef]
- François, G.; Kew, M.; Van Damme, P.; Mphahlele, J.; Meheus, A. Mutant hepatitis B viruses: A matter of academic interest only or a problem with far-reaching implications? Vaccine 2001, 19, 3799–3815. [Google Scholar] [CrossRef]
- Rosenberg, W. Mechanisms of immune escape in viral hepatitis. Gut 1999, 44, 759–764. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Konopleva, M.V.; Semenenko, T.A.; Akimkin, V.G.; Tutelyan, A.V.; Suslov, A.P. The mechanisms of immune escape by hepatitis B virus. Ann. Russ. Acad. Med. Sci. 2017, 72, 408–419. [Google Scholar] [CrossRef]
- Wieland, S.F.; Chisari, F.V. Stealth and Cunning: Hepatitis B and Hepatitis C Viruses. J. Virol. 2005, 79, 9369–9380. [Google Scholar] [CrossRef]
- Wieland, S.; Thimme, R.; Purcell, R.H.; Chisari, F.V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 2004, 101, 6669–6674. [Google Scholar] [CrossRef]
- Weinberger, K.M.; Bauer, T.; Böhm, S.; Jilg, W. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. Microbiology 2000, 81, 1165–1174. [Google Scholar] [CrossRef]
- Datta, S.; Panigrahi, R.; Biswas, A.; Chandra, P.K.; Banerjee, A.; Mahapatra, P.K.; Panda, C.K.; Chakrabarti, S.; Bhattacharya, S.K.; Biswas, K.; et al. Genetic Characterization of Hepatitis B Virus in Peripheral Blood Leukocytes: Evidence for Selection and Compartmentalization of Viral Variants with the Immune Escape G145R Mutation. J. Virol. 2009, 83, 9983–9992. [Google Scholar] [CrossRef]
- Coffin, C.S.; Osiowy, C.; Gao, S.; Nishikawa, S.; van der Meer, F.; Van Marle, G. Hepatitis B virus (HBV) variants fluctuate in paired plasma and peripheral blood mononuclear cells among patient cohorts during different chronic hepatitis B (CHB) disease phases. J. Viral Hepat. 2014, 22, 416–426. [Google Scholar] [CrossRef]
- Vanlandschoot, P.; Leroux-Roels, G. Viral apoptotic mimicry: An immune evasion strategy developed by the hepatitis B virus? Trends Immunol. 2003, 24, 144–147. [Google Scholar] [CrossRef]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef]
- Woltman, A.M.; Brouw, M.L.O.D.; Biesta, P.J.; Shi, C.C.; Janssen, H.L.A. Hepatitis B Virus Lacks Immune Activating Capacity, but Actively Inhibits Plasmacytoid Dendritic Cell Function. PLoS ONE 2011, 6, e15324. [Google Scholar] [CrossRef]
- Waters, J.A.; Kennedy, M.; Voet, P.; Hauser, P.; Petre, J.; Carman, W.; Thomas, H.C. Loss of the common “A” determinant of hepatitis B surface antigen by a vaccine-induced escape mutant. J. Clin. Investig. 1992, 90, 2543–2547. [Google Scholar] [CrossRef]
- Cooreman, M.P.; Van Roosmalen, M.H.; Morsche, R.T.; Sünnen, C.M.G.; De Ven, E.M.E.S.-V.; Jansen, J.B.M.J.; Tytgat, G.N.J.; De Wit, P.L.M.; Paulij, W.P. Characterization of the reactivity pattern of murine monoclonal antibodies against wild-type hepatitis B surface antigen to g145r and other naturally occurring “a” loop escape mutations. Hepatology 1999, 30, 1287–1292. [Google Scholar] [CrossRef]
- Limeres, M.J.; Gomez, E.R.; Noseda, D.G.; Cerrudo, C.S.; Ghiringhelli, P.D.; Nusblat, A.D.; Cuestas, M.L. Impact of hepatitis B virus genotype F on in vitro diagnosis: Detection efficiency of HBsAg from Amerindian subgenotypes F1b and F4. Arch. Virol. 2019, 164, 2297–2307. [Google Scholar] [CrossRef]
- Hossain, G.; Ueda, K. Investigation of a Novel Hepatitis B Virus Surface Antigen (HBsAg) Escape Mutant Affecting Immunogenicity. PLoS ONE 2017, 12, e0167871. [Google Scholar] [CrossRef]
- Schilling, R.; Ijaz, S.; Davidoff, M.; Lee, J.-Y.; Locarnini, S.; Williams, R.; Naoumov, N.V. Endocytosis of Hepatitis B Immune Globulin into Hepatocytes Inhibits the Secretion of Hepatitis B Virus Surface Antigen and Virions. J. Virol. 2003, 77, 8882–8892. [Google Scholar] [CrossRef]
- Araujo, N.M.; Teles, S.A.; Spitz, N. Comprehensive Analysis of Clinically Significant Hepatitis B Virus Mutations in Relation to Genotype, Subgenotype and Geographic Region. Front. Microbiol. 2020, 11, 616023. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Chang, M.-H.; Ni, Y.-H.; Chen, H.-L. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut 2004, 53, 1499–1503. [Google Scholar] [CrossRef]
- Komatsu, H.; Inui, A.; Umetsu, S.; Tsunoda, T.; Sogo, T.; Konishi, Y.; Fujisawa, T. Evaluation of the G145R Mutant of the Hepatitis B Virus as a Minor Strain in Mother-to-Child Transmission. PLoS ONE 2016, 11, e0165674. [Google Scholar] [CrossRef]
- Konopleva, M.; Belenikin, M.; Shanko, A.; Bazhenov, A.; Kiryanov, S.; Tupoleva, T.; Sokolova, M.; Pronin, A.; Semenenko, T.; Suslov, A. Detection of S-HBsAg Mutations in Patients with Hematologic Malignancies. Diagnostics 2021, 11, 969. [Google Scholar] [CrossRef]
- Ogata, N.; Cote, P.J.; Zanetti, A.R.; Miller, R.H.; Shapiro, M.; Gerin, J.; Purcell, R.H. Licensed recombinant hepatitis B vaccines protect chimpanzees against infection with the prototype surface gene mutant of hepatitis B virus. Hepatology 1999, 30, 779–786. [Google Scholar] [CrossRef]
- Kamili, S. Infectivity and vaccination efficacy studies in animal models of HBV S and pol gene mutants. Antivir. Ther. 2010, 15, 477–485. [Google Scholar] [CrossRef][Green Version]
- Bazhenov, A.I.; Elgort, D.A.; Feldsherova, A.A.; Budnitskaya, P.Z.; Nikitina, G.I.; Hats, Y.S.; Konopleva, M.V.; Godkov, M.A.; Borisova, V.N.; Yarosh, L.V.; et al. Detection of antibodies to HBsAg mutant forms in individuals immunized of different subtypes hepatitis B vaccines. Epidemiol. Vaccinal Prev. 2011, 5, 49–53. [Google Scholar]
- Wilson, J.N.; Nokes, D.J.; Carman, W.F. Current status of HBV vaccine escape variants—A mathematical model of their epidemiology. J. Viral Hepat. 1998, 5, 25–30. [Google Scholar] [CrossRef]
- Asatryan, M.N.; Salman, E.R.; Kilikovsky, V.V.; Kiselev, K.V.; Sipacheva, N.B.; Semenenko, T.A. Investigation of the spread of a vaccine-induced escape mutant of hepatitis B virus, by using a computer-based epidemiological model. Epidemiol.-Fectious Dis. Curr. Items 2013, 6, 34–38. [Google Scholar]
- Kniskern, P.J.; Hagopian, A.I. HBsAg Escape Mutant Vaccine. EU Patent 0511,855,A1, 4 November 1992. [Google Scholar]
- Thomas, H.C.; Carman, W.F. Hepatitis B Vaccine. U.S. Patent 5,639,637, 17 June 1997. [Google Scholar]
- Primi, D.; Fiordalisi, G.; Palla, M. Escape Mutant of the Surface Antigen of Hepatitis B Virus. U.S. Patent 6,172,193,B1, 9 January 2001. [Google Scholar]
- Zheng, X.; Weinberger, K.M.; Gehrke, R.; Isogawa, M.; Hilken, G.; Kemper, T.; Xu, Y.; Yang, D.; Jilg, W.; Roggendorf, M.; et al. Mutant hepatitis B virus surface antigens (HBsAg) are immunogenic but may have a changed specificity. Virology 2004, 329, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, A.I. Improvement of Immunodetection Methods of HBsAg Mutants of the Hepatitis B Virus. Ph.D. Thesis, Federal State Budget Institution “National Research Center for Epidemiology and Microbiology Named after Honorary Academician, N.F. Gamaleya” of the Ministry of Health of the Russian Federation, Moscow, Russia, 2009; 148p. (In Russian). Available online: https://www.dissercat.com/content/sovershenstvovanie-metodov-immunodetektsii-nvsag-mutantov-virusa-gepatita-v (accessed on 18 October 2021).
- Rezaee, R.; Poorebrahim, M.; Najafi, S.; Sadeghi, S.; Pourdast, A.; Alavian, S.M.; Poortahmasebi, V.; Alavian, S.E. Impacts of the G145R Mutation on the Structure and Immunogenic Activity of the Hepatitis B Surface Antigen: A Computational Analysis. Zahedan J. Res. Med. Sci. 2016, 16, e39097. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, T.; Iwanski, A.; Will, H.; Sterneck, M. Deficiency in virion secretion and decreased stability of the hepatitis B virus immune escape mutant G145R. Hepatology 2003, 38, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.V.; Sokolova, M.V.; Shevlyagina, N.V.; Bazhenov, A.I.; Fel’dsherova, A.A.; Krymskij, M.A.; Borisova, V.N.; Se-menenko, T.A.; Nesterenko, V.G.; Suslov, A.P. Morphological analysis of hepatitis B virus with escape mutations in S-gene G145R and S143L. Vopr. Virusol. 2017, 62, 119–128. [Google Scholar] [CrossRef]
- Konopleva, M.; Borisova, V.N.; Sokolova, M.V.; Feldsherova, A.A.; Krymskij, M.A.; Semenenko, T.A.; Suslov, A.P. A comparative characteristic of antigenic properties of recombinant and native hbs-antigens with G145R mutation and evaluation of their immunogenicity. Vopr. Virusol. 2017, 62, 179–186. [Google Scholar] [CrossRef]
- Bellecave, P.; Gouttenoire, J.; Gajer, M.; Brass, V.; Koutsoudakis, G.; Blum, H.E.; Bartenschlager, R.; Nassal, M.; Moradpour, D. Hepatitis B and C virus coinfection: A novel model system reveals the absence of direct viral interference. Hepatology 2009, 50, 46–55. [Google Scholar] [CrossRef]
- Krymskij, M.A.; Borisov, I.A.; Yakovlev, M.S.; Agafonov, M.O.; Ter-Avanesyan, M.D.; Suslov, A.P.; Semenenko, T.A. Recombinant Hansenula Polymorpha Yeast Strain-Producer of Mutant Hepatitis B Virus Surface Antigen (Versions). RU Patent 2586513,C1, 10 June 2016. [Google Scholar]
- Krymskij, M.A.; Borisov, I.A.; Yakovlev, M.S.; Melnikov, V.A.; Suslov, A.P.; Semenenko, T.A.; Konopleva, M.V.; Sokolova, M.V.; Feldsherova, A.A.; Elgort, D.A. Method for Assessing Antibody Level Specific to Different Variants of Hepatitis B Virus HBsAg. RU Patent 2,616,236,C1, 13 April 2017. [Google Scholar]
- Khaidukov, S.V.; Zurochka, A.V.; Totolian, A.A.; Chereshnev, V.A. Major and lymphocyte populations of human peripheral blood lymphocytes and their reference values, as assayed by multi-colour cytometry. Med. Immunol. 2009, 11, 227–238. [Google Scholar] [CrossRef]
- Hünig, T. Method for Preclinical Testing of Immunomodulatory Agents. WO Patent 2011/036308, 31 March 2011. [Google Scholar]
- Lobaina, Y.; Hardtke, S.; Wedemeyer, H.; Aguilar, J.C.; Schlaphoff, V. In vitro stimulation with HBV therapeutic vaccine candidate Nasvac activates B and T cells from chronic hepatitis B patients and healthy donors. Mol. Immunol. 2015, 63, 320–327. [Google Scholar] [CrossRef]
- Shi, M.; Qian, S.; Chen, W.-W.; Zhang, H.; Zhang, B.; Tang, Z.-R.; Zhang, Z.; Wang, F.-S. Hepatitis B virus (HBV) antigen-pulsed monocyte-derived dendritic cells from HBV-associated hepatocellular carcinoma patients significantly enhance specific T cell responses in vitro. Clin. Exp. Immunol. 2006, 147, 277–286. [Google Scholar] [CrossRef]
- Fenner, J.E.; Starr, R.; Cornish, A.L.; Zhang, J.-G.; Metcalf, D.; Schreiber, R.D.; Sheehan, K.; Hilton, D.J.; Alexander, W.S.; Hertzog, P.J. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat. Immunol. 2006, 7, 33–39. [Google Scholar] [CrossRef]
- Lanzavecchia, A.; Bove, S. Specific B lymphocytes efficiently pick up, process and present antigen to T cells. Behring. Inst. Mitt. 1985, 77, 82–87. [Google Scholar]
- Lanzavecchia, A. Antigen-specific interaction between T and B cells. Nature 1985, 314, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, E.V. The kingdom of B-lymphocytes. Med. Immunol. 2004, 6, 176–185. [Google Scholar]
- Tharinger, H.; Rebbapragada, I.; Samuel, D.; Novikov, N.; Nguyen, M.H.; Jordan, R.; Frey, C.R.; Pflanz, S. Antibody-dependent and antibody-independent uptake of HBsAg across human leucocyte subsets is similar between individuals with chronic hepatitis B virus infection and healthy donors. J. Viral Hepat. 2017, 9, 164–513. [Google Scholar] [CrossRef] [PubMed]
- Couillin, I.; Tiollais, P.; Pol, S.; Mancini, M.; Driss, F.; Bréchot, C.; Michel, M.L. Specific Vaccine Therapy in Chronic Hepatitis B: Induction of T Cell Proliferative Responses Specific for Envelope Antigens. J. Infect. Dis. 1999, 180, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Krymsky, M.A.; Krymsky, R.M.; Budanov, M.V.; Borisova, V.N. Compliance of hepatitis B vaccines to the subtype of hepatitis B virus prevalent in Russian Federation. Russ. J. Biopharm. 2010, 2, 8–15. [Google Scholar]
- Pritulina, Y.G. Differentiated Immunotherapy of Persistent HBV Infection with Recombinant HBV Vaccine. Doctorate Thesis, Voronezh State Medical University named after N.N. Burdenko, Voronezh, Russia, 2007; 32p. (In Russian). Available online: https://search.rsl.ru/ru/record/01003059796 (accessed on 18 October 2021).
- Borisova, V.N.; Melnikov, V.A.; Pritulina, Y.G.; Rychnev, B.E.; Zemskov, A.M.; Solomakchin, G.G.; Blinova, O.G.; Zaporozhko, L.V. The use of the viral hepatitis B vaccine “Combiotech”for a complex therapy in patients with chronic hepatitis B and car-riers of hepatitis B virus. Public Health Life Environ. 1999, 6, 8–11. [Google Scholar]
- Petchakup, C.; Hutchinson, P.E.; Tay, H.M.; Leong, S.Y.; Li, K.H.H.; Hou, H.W. Label-free quantitative lymphocyte activation profiling using microfluidic impedance cytometry. Sens. Actuators B Chem. 2021, 339, 129864. [Google Scholar] [CrossRef]
- Lomakova, Y.D.; Londregan, J.; Maslanka, J.; Goldman, N.; Somerville, J.; Riggs, J.E. PHA eludes macrophage suppression to activate CD8+ T cells. Immunobiology 2018, 224, 94–101. [Google Scholar] [CrossRef]
- Lugovaya, A.V.; Kalinina, N.M.; Mitreikin, V.P.; Emanuel, Y.V.; Kovalchuk, Y.P.; Artyomova, A.V. Spontaneous and activation-induced apoptosis of peripheral blood mononuclear cells in the pathogenesis of type 1 diabetes mellitus. Med. Immunol. (Russia) 2020, 22, 123–134. [Google Scholar] [CrossRef]
- Tchórzewski, H.; Głowacka, E.; Banasik, M.; Lewkowicz, P.; Szałapska-Zawodniak, M. Activated T lymphocytes from patients with high risk of type I diabetes mellitus have different ability to produce interferon-γ, interleukin-6 and interleukin-10 and undergo anti-CD95 induced apoptosis after insulin stimulation. Immunol. Lett. 2001, 75, 225–234. [Google Scholar] [CrossRef]
- Schlaak, J.F.; Tully, G.; Löhr, H.F.; Gerken, G.; Büschenfelde, K.-H.M.Z. HBV-specific immune defect in chronic hepatitis B (CHB) is correlated with a dysregulation of pro- and anti-inflammatory cytokines. Clin. Exp. Immunol. 1999, 115, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Meng, H.; Fan, Y.; Song, Y.; Wang, S.; Zhu, F.; Guo, C.; Zhang, L.; Shi, Y. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol. Immunother. 2012, 61, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.F.; Jeor, S.C.S. Hantavirus Immunology. Viral Immunol. 2002, 15, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, F.G.; Sayegh, M.H. Memory T Cells: A Hurdle to Immunologic Tolerance. J. Am. Soc. Nephrol. 2003, 14, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.R.; Dubey, C.; Swain, S.L. Qualitative Changes Accompany Memory T Cell Generation: Faster, More Effective Responses at Lower Doses of Antigen. J. Immunol. 2000, 164, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Paul, W.E. Acquisition of Lymphokine-Producing Phenotype by CD4+ T Cells. Annu. Rev. Immunol. 1994, 12, 635–673. [Google Scholar] [CrossRef]
- Fischer, H.; Dohlsten, M.; Andersson, U.; Hedlund, G.; Ericsson, P.; Hansson, J.; Sjögren, H.O. Production of TNF-alpha and TNF-beta by staphylococcal enterotoxin A activated human T cells. J. Immunol. 1990, 144, 4663–4669. [Google Scholar] [PubMed]
- Salmon, M.; Kitas, G.D.; Bacon, P.A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from peripheral blood and by human CD4+ T cell clones. J. Immunol. 1989, 143, 907–912. [Google Scholar] [PubMed]
- Sewell, W.A.; Valentine, J.E.; Cooley, M.A. Expression of Interleukin 5 by the CD4+CD45R0+ Subset of Human T Cells. Growth Factors 1992, 6, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Dohlsten, M.; Hedlund, G.; Sjögren, H.-O.; Carlsson, R. Two subsets of human CD4+T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-γ can be defined by the Leu-18 and UCHLl monoclonal antibodies. Eur. J. Immunol. 1988, 18, 1173–1178. [Google Scholar] [CrossRef]
- Ehlers, S.; Smith, K.A. Differentiation of T cell lymphokine gene expression: The in vitro acquisition of T cell memory. J. Exp. Med. 1991, 173, 25–36. [Google Scholar] [CrossRef]
- Keating, S.M.; Heitman, J.D.; Wu, S.; Deng, X.; Stramer, S.L.; Kuhns, M.C.; Mullen, C.; Norris, P.J.; Busch, M.P. Cytokine and Chemokine Responses in the Acute Phase of Hepatitis B Virus Replication in Naive and Previously Vaccinated Blood and Plasma Donors. J. Infect. Dis. 2013, 209, 845–854. [Google Scholar] [CrossRef][Green Version]
- Werner, J.M.; Abdalla, A.; Gara, N.; Ghany, M.G.; Rehermann, B. The Hepatitis B Vaccine Protects Re-Exposed Health Care Workers, But Does Not Provide Sterilizing Immunity. Gastroenterology 2013, 145, 1026–1034. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Ni, Y.-H.; Chiang, B.-L.; Chen, P.-J.; Chang, M.H.; Chang, L.-Y.; Su, I.; Kuo, H.; Huang, L.; Chen, D.-S.; et al. Humoral and Cellular Immune Responses to a Hepatitis B Vaccine Booster 15–18 Years after Neonatal Immunization. J. Infect. Dis. 2008, 197, 1419–1426. [Google Scholar] [CrossRef]
- Simons, B.C.; Spradling, P.R.; Bruden, D.J.T.; Zanis, C.; Case, S.; Choromanski, T.L.; Apodaca, M.; Brogdon, H.D.; Dwyer, G.; Snowball, M.; et al. A Longitudinal Hepatitis B Vaccine Cohort Demonstrates Long-lasting Hepatitis B Virus (HBV) Cellular Immunity Despite Loss of Antibody Against HBV Surface Antigen. J. Infect. Dis. 2016, 214, 273–280. [Google Scholar] [CrossRef]
- Carollo, M.; Palazzo, R.; Bianco, M.; Pandolfi, E.; Chionne, P.; Fedele, G.; Tozzi, A.E.; Carsetti, R.; Romanò, L.; Ausiello, C.M. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine 2013, 31, 506–513. [Google Scholar] [CrossRef]
- Wang, X.; Mosmann, T. In Vivo Priming of Cd4 T Cells That Produce Interleukin (Il)-2 but Not IL-4 or Interferon (Ifn)-γ, and Can Subsequently Differentiate into IL-4–Or IFN-γ–Secreting Cells. J. Exp. Med. 2001, 194, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Bucy, R.P.; Karr, L.; Huang, G.Q.; Li, J.; Carter, D.; Honjo, K.; Lemons, J.A.; Murphy, K.M.; Weaver, C. Single cell analysis of cytokine gene coexpression during CD4+ T-cell phenotype development. Proc. Natl. Acad. Sci. USA 1995, 92, 7565–7569. [Google Scholar] [CrossRef]
- Assenmacher, M.; Löhning, M.; Scheffold, A.; Manz, R.A.; Schmitz, J.; Radbruch, A. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur. J. Immunol. 1998, 28, 1534–1543. [Google Scholar] [CrossRef]
- Olsen, I.; Sollid, L.M. Pitfalls in determining the cytokine profile of human T cells. J. Immunol. Methods 2013, 390, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Kowalczyk, D.; Ozóg, M.; Zembala, M. Three-Color Flow Cytometry Detection of Intracellular Cytokines in Peripheral Blood Mononuclear Cells: Comparative Analysis of Phorbol Myristate Acetate-Ionomycin and Phytohemagglutinin Stimulation. Clin. Diagn. Lab. Immunol. 2001, 8, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Sunder-Plassmann, R.; Reinherz, E.L. A p56 -independent Pathway of CD2 Signaling Involves Jun Kinase. J. Biol. Chem. 1998, 273, 24249–24257. [Google Scholar] [CrossRef]
- Unitt, J.; Hornigold, D. Plant lectins are novel Toll-like receptor agonists. Biochem. Pharmacol. 2011, 81, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Poloskov, V.V.; Sokolova, Z.A.; Burova, O.S.; Shuvalov, A.N.; Sokolova, T.M. The effect of mitogens on the differentiation of THP-1 cells and the expression of TLR/RLR genes. Cytokines Inflamm. 2016, 15, 161–165. [Google Scholar]
- Kaewthawee, N.; Brimson, S. The effects of ursolic acid on cytokine production via the MAPK pathways in leukemic T-cells. Excli J. 2013, 12, 102–114. [Google Scholar]
- Feau, S.; Facchinetti, V.; Granucci, F.; Citterio, S.; Jarrossay, D.; Seresini, S.; Protti, M.P.; Lanzavecchia, A.; Ricciardi-Castagnoli, P. Dendritic cell-derived IL-2 production is regulated by IL-15 in humans and in mice. Blood 2005, 105, 697–702. [Google Scholar] [CrossRef]
- Ryu, K.A.; McGonnigal, B.; Moore, T.; Kargupta, T.; Mancini, R.J.; Esser-Kahn, A.P. Light Guided In-vivo Activation of Innate Immune Cells with Photocaged TLR 2/6 Agonist. Sci. Rep. 2017, 7, 8074. [Google Scholar] [CrossRef] [PubMed]
- Granucci, F.; Feau, S.; Angeli, V.; Trottein, F.; Ricciardi-Castagnoli, P. Early IL-2 Production by Mouse Dendritic Cells Is the Result of Microbial-Induced Priming. J. Immunol. 2003, 170, 5075–5081. [Google Scholar] [CrossRef] [PubMed]
- Granucci, F.; Feau, S.; Zanoni, I.; Pavelka, N.; Vizzardelli, C.; Raimondi, G.; Ricciardi-Castagnoli, P. The Immune Response Is Initiated by Dendritic Cells via Interaction with Microorganisms and Interleukin-2 Production. J. Infect. Dis. 2003, 187, S346–S350. [Google Scholar] [CrossRef] [PubMed]
- Rosadini, C.V.; Kagan, J.C. Microbial strategies for antagonizing Toll-like-receptor signal transduction. Curr. Opin. Immunol. 2015, 32, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Z.; Hu, C.; Qian, F.; Cheng, Y.; Wu, M.; Shi, B.; Chen, J.; Hu, Y.; Yuan, Z. Hepatitis B Virus Surface Antigen Selectively Inhibits TLR2 Ligand–Induced IL-12 Production in Monocytes/Macrophages by Interfering with JNK Activation. J. Immunol. 2013, 190, 5142–5151. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, A.I.; Elgort, D.A.; Feldsherova, A.A.; Budnitskaya, P.Z.; Nikitina, G.I.; Hats, Y.S.; Konopleva, M.V.; Godkov, M.A.; Borisova, V.N.; Semenenko, T.A.; et al. The comparative estimation of anti-HBs activity against native and recombinant type HBsAg. Epidemiol. Vaccinal Prev. 2012, 2, 76–81. [Google Scholar]



| Cell Population | Relative Amount, % | Reference Values for Blood, % |
|---|---|---|
| T cells | 71.56 | 61–85 |
| B cells | 9.39 | 7–17 |
| CD8+ T cells (CTL) | 30.44 | 19–35 |
| CD4+ T cells (Th) | 65.29 | 35–65 |
| NK cells | 8.56 | 8–18 |
| NKT cells | 4.03 | 0.5–6 |
| Neutrophils | 1.73 | 37–63 |
| Eosinophils | 0.44 | 0–3 |
| Monocytes | 8.59 | 2–9 |
| Immunoregulatory index CD4+/CD8+ | 2.30 | 1.5–2.6 |
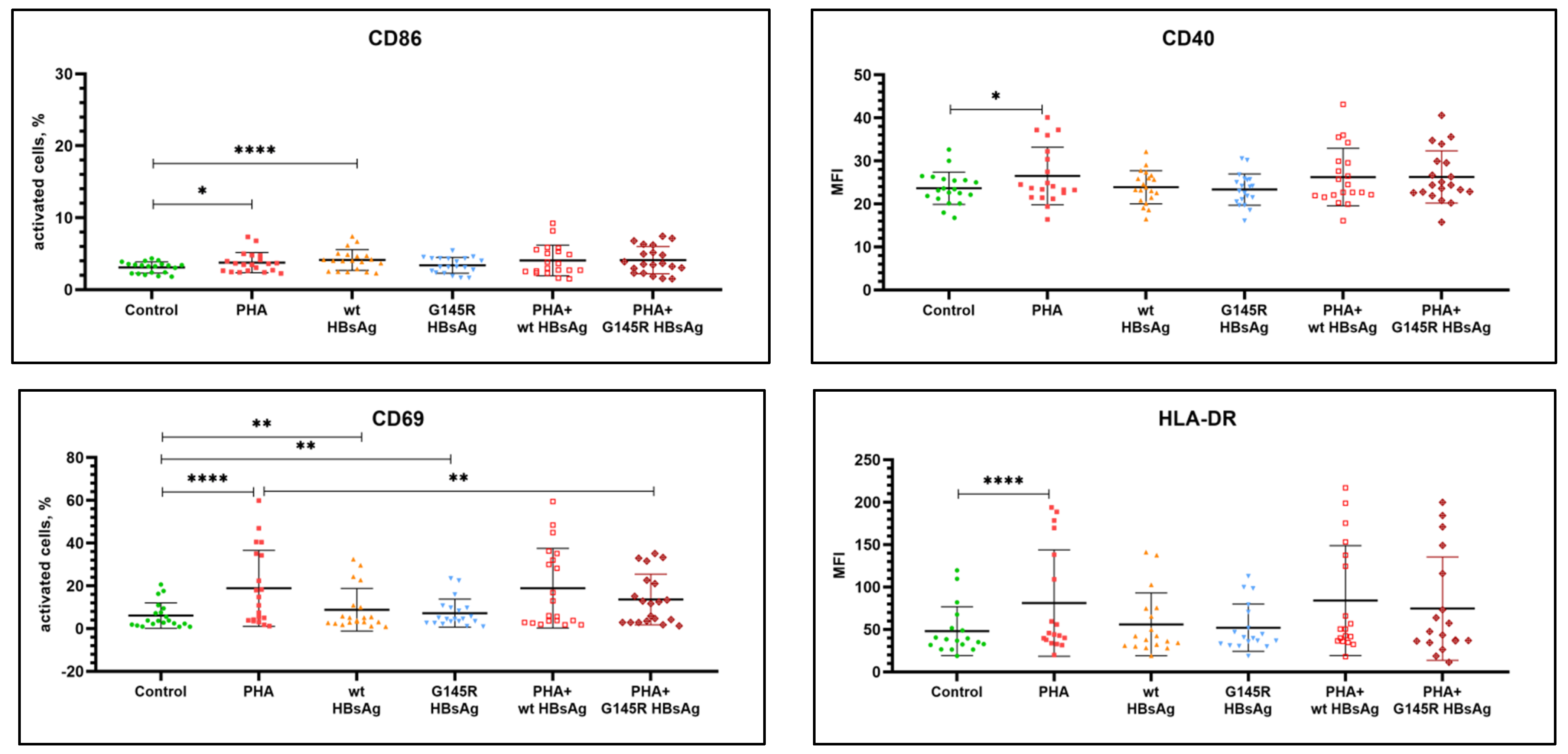
| Activation Markers | Mean Value ± SEM | p-Value, Group vs. Negative Control | p-Value, Group vs. PHA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative Control | PHA | wt HBsAg | G145R HBsAg | PHA+ wt HBsAg | PHA+ G145R HBsAg | PHA | wt HBsAg | G145R HBsAg | PHA+ wt HBsAg | PHA+ G145R HBsAg | |
| B-cell activation; % cells for CD69 and CD86, MFI for CD40 and HLA-DR | |||||||||||
| CD69 | 6.11 ± 1.34 | 18.84 ± 3.98 | 8.83 ± 2.23 | 7.24 ± 1.46 | 18.91 ± 4.17 | 13.62 ± 2.65 | <0.0001 (*) | 0.0073 (*) | 0.0086 (*) | 0.5459 (ns) | 0.0010 (*) |
| CD86 | 3.08 ± 0.17 | 3.76 ± 0.31 | 4.13 ± 0.32 | 3.38 ± 0.25 | 4.07 ± 0.47 | 4.11 ± 0.42 | 0.0133(*) | 0.0001 (*) | 0.0570 (ns) | 0.2413 (ns) | 0.4749 (ns) |
| CD40 | 23.64 ± 0.84 | 26.51 ± 1.50 | 23.88 ± 0.86 | 23.34 ± 0.82 | 26.25 ± 1.50 | 26.26 ± 1.36 | 0.0139 (*) | 0.5706 (ns) | 0.1117 (ns) | 0.3488 (ns) | 0.2815 (ns) |
| HLA-DR | 48.05 ± 6.77 | 81.20 ± 14.79 | 56.01 ± 8.72 | 52.08 ± 6.56 | 84.00 ± 15.26 | 74.66 ± 14.37 | <0.0001 (*) | 0.1084 (ns) | 0.0898 (ns) | 0.6397 (ns) | 0.4951 (ns) |
| NK-cell activation; % cells | |||||||||||
| CD69 | 25.11 ± 4.37 | 39.61 ± 5.08 | 31.21 ± 6.03 | 26.86 ± 4.75 | 39.23 ± 5.64 | 34.11 ± 4.47 | <0.0001 (*) | 0.0010 (*) | 0.0385 (*) | 0.9323 (ns) | <0.0001 (*) |
| T-cell activation; % cells | |||||||||||
| HLA-DR | 5.90 ± 0.99 | 7.87 ± 1.12 | 5.82 ± 0.99 | 6.30 ± 1.27 | — | — | 0.0045 (*) | 0.5509 (ns) | 0.7578 (ns) | — | — |
| CD4+ T-cell activation; % cells | |||||||||||
| CD69 | 1.70 ± 0.44 | 21.72 ± 5.00 | 2.74 ± 0.83 | 2.08 ± 0.50 | 23.64 ± 5.14 | 18.92 ± 4.10 | <0.0001 (*) | 0.0210 (*) | 0.0104 (*) | 0.0637 (ns) | 0.1231 (ns) |
| CD279 (PD-1) | 3.62 ± 0.46 | 3.58 ± 0.48 | 3.72 ± 0.52 | 3.51 ± 0.46 | 3.56 ± 0.48 | 3.33 ± 0.47 | 0.5039 (ns) | 0.4123 (ns) | 0.6410 (ns) | 0.6215 (ns) | 0.0896 (ns) |
| CD8+ T-cell activation; % cells | |||||||||||
| CD69 | 4.80 ± 1.21 | 21.94 ± 4.66 | 7.28 ± 1.65 | 5.81 ± 1.15 | 22.29 ± 5.02 | 17.36 ± 3.95 | <0.0001 (*) | 0.0012 (*) | 0.0391 (*) | 0.8408 (ns) | 0.0001 (*) |
| CD279 (PD-1) | 4.07 ± 0.69 | 3.77 ± 0.68 | 4.18 ± 0.69 | 4.32 ± 0.72 | 3.88 ± 0.66 | 3.55 ± 0.67 | 0.8052 (ns) | 0.5706 (ns) | 0.5217 (ns) | 0.9273 (ns) | 0.2492 (ns) |
| Cytokine | N of Tested Donors | Antigen for PBMC Stimulation | p-Value (Responders), wt HBsAg vs. G145R HBsAg | |||||
|---|---|---|---|---|---|---|---|---|
| wt HBsAg | G145R HBsAg | |||||||
| N of Responders | % of Total Donors | Cytokine Range, pg/mL | N of Responders | % of Total Donors | Cytokine Range, pg/mL | |||
| IFN-γ | 20 | 13 | 65 | 0–1390 | 14 | 70 | 0–820 | >0.9999 (ns) |
| IFN-α | 20 | 1 | 5 | 0–2 | 0 | 0 | 0–0 | >0.9999 (ns) |
| TNF-α | 18 | 15 | 83 | 5–890 | 16 | 89 | 5–174 | >0.9999 (ns) |
| IL-2 | 20 | 4 | 20 | 0–17 | 18 | 90 | 0–69 | <0.0001 (*) |
| IL-10 | 20 | 15 | 75 | 8–870 | 17 | 85 | 7–260 | 0.6948 (ns) |
| Cytokine | Mean Value of Cytokine Concentration (pg/mL) ± SEM | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group vs. Negative Control | Group Vs. PHA | PHA+ wt HBsAg vs. wt HBsAg | PHA+ G145R HBsAg vs. G145R HBsAg | ||||||||||
| Negative Control | PHA | wt HBsAg | G145R HBsAg | PHA+ wt HBsAg | PHA+ G145R HBsAg | PHA | wt HBsAg | G145R HBsAg | PHA+ wt HBsAg | PHA+ G145R HBsAg | |||
| IFN-γ | 65.75 ± 29.63 | 186.60 ± 86.37 | 232.05 ± 91.08 | 111.95 ± 50.28 | 408.60 ± 160.43 | 204.10 ± 84.03 | 0.0020 (*) | 0.0005 (*) | 0.0001 (*) | 0.0222 (*) | 0.2466 (ns) | 0.0154 (*) | 0.0337 (*) |
| IFN-α | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.10 ± 0.10 | 0.00 ± 0.00 | 035 ± 0.35 | 0.00 ± 0.00 | — | >0.9999 (ns) | — | >0.9999 (ns) | — | >0.9999 (ns) | — |
| TNF-α | 24.78 ± 6.66 | 45.22 ± 10.04 | 218.39 ± 69.95 | 38.50 ± 10.83 | 220.83 ± 67.57 | 50.44 ± 12.18 | 0.0038 (*) | 0.0002 (*) | 0.0009 (*) | 0.0038 (*) | 0.2477 (ns) | 0.9278 (ns) | 0.1710 (ns) |
| IL-2 | 4.65 ± 1.19 | 20.50 ± 5.13 | 4.30 ± 1.17 | 21.85 ± 3.63 | 13.55 ± 3.60 | 28.00 ± 5.11 | 0.0002 (*) | 0.6289 (ns) | <0.0001 (*) | 0.0002 (*) | 0.0153 (*) | 0.0035 (*) | 0.4004 (ns) |
| IL-10 | 59.25 ± 10.43 | 127.10 ± 19.12 | 274.15 ± 65.12 | 79.50 ± 15.20 | 340.40 ± 65.52 | 129.80 ± 19.35 | <0.0001 (*) | 0.0002 (*) | 0.0004 (*) | 0.0007 (*) | 0.8517 (ns) | 0.0137 (*) | <0.0001 (*) |
| Cytokine | Group 1 (n = 7), Anti-HBsAg < 10 mIU/mL | Group 2 (n = 9), Anti-HBsAg > 10 mIU/mL | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Responders | Cytokine Concentration, pg/mL | Responders | Cytokine Concentration, pg/mL | Responders | Cytokine Concentration | |||||
| Number | % of Total | M ± m | Range | Number | % of Total | M ± m | Range | |||
| IFN-γ | 5 | 71 | 212.86 ± 136.10 | 0–990 | 6 | 67 | 324.22 ± 172.74 | 0–1390 | >0.9999 (ns) | 0.7535 (ns) |
| IFN-α | 0 | 0 | 0.00 ± 0.00 | 0–0 | 1 | 11 | 0.22 ± 0.22 | 0–2 | >0.9999 (ns) | — |
| TNF-α | 5 | 71 | 194.86 ± 94.94 | 6–689 | 8 | 89 | 274.56 ± 119.33 | 5–890 | 0.5500 (ns) | 0.7577 (ns) |
| IL-2 | 2 | 29 | 6.43 ± 2.17 | 0–17 | 2 | 22 | 4.56 ± 1.75 | 0–13 | >0.9999 (ns) | 0.4751 (ns) |
| IL-10 | 5 | 71 | 319.14 ± 121.71 | 19–780 | 8 | 89 | 335.00 ± 101.75 | 41–870 | 0.5500 (ns) | 0.5360 (ns) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopleva, M.V.; Borisova, V.N.; Sokolova, M.V.; Semenenko, T.A.; Suslov, A.P. Recombinant HBsAg of the Wild-Type and the G145R Escape Mutant, included in the New Multivalent Vaccine against Hepatitis B Virus, Dramatically Differ in their Effects on Leukocytes from Healthy Donors In Vitro. Vaccines 2022, 10, 235. https://doi.org/10.3390/vaccines10020235
Konopleva MV, Borisova VN, Sokolova MV, Semenenko TA, Suslov AP. Recombinant HBsAg of the Wild-Type and the G145R Escape Mutant, included in the New Multivalent Vaccine against Hepatitis B Virus, Dramatically Differ in their Effects on Leukocytes from Healthy Donors In Vitro. Vaccines. 2022; 10(2):235. https://doi.org/10.3390/vaccines10020235
Chicago/Turabian StyleKonopleva, Maria V., Vera N. Borisova, Maria V. Sokolova, Tatyana A. Semenenko, and Anatoly P. Suslov. 2022. "Recombinant HBsAg of the Wild-Type and the G145R Escape Mutant, included in the New Multivalent Vaccine against Hepatitis B Virus, Dramatically Differ in their Effects on Leukocytes from Healthy Donors In Vitro" Vaccines 10, no. 2: 235. https://doi.org/10.3390/vaccines10020235
APA StyleKonopleva, M. V., Borisova, V. N., Sokolova, M. V., Semenenko, T. A., & Suslov, A. P. (2022). Recombinant HBsAg of the Wild-Type and the G145R Escape Mutant, included in the New Multivalent Vaccine against Hepatitis B Virus, Dramatically Differ in their Effects on Leukocytes from Healthy Donors In Vitro. Vaccines, 10(2), 235. https://doi.org/10.3390/vaccines10020235






