Metrics from Wearable Devices as Candidate Predictors of Antibody Response Following Vaccination against COVID-19: Data from the Second TemPredict Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measures
2.3. Analytic Plan
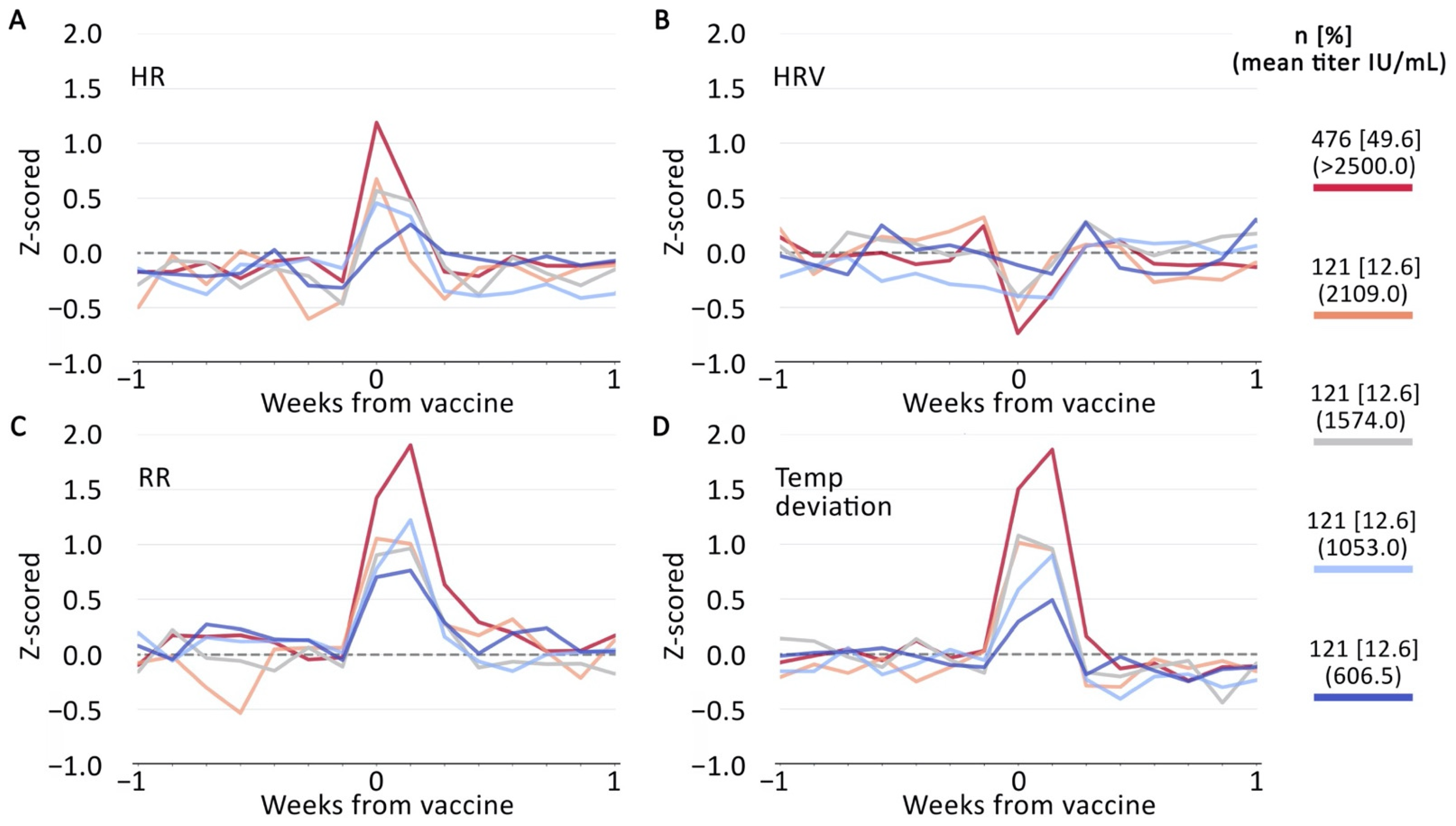
3. Results
3.1. Spearman Correlations
3.1.1. Device-Generated Metrics
3.1.2. Device-Generated Metrics Adjusted for Pre-Vaccination Baseline Period
3.1.3. Device-Generated Metrics during the Pre-Vaccination Baseline Period
3.2. Multivariate Models
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 1–9. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Helfritz, F.A.; et al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv 2021. [Google Scholar] [CrossRef]
- Spiegel, K.; Sheridan, J.F.; van Cauter, E. Effect of sleep deprivation on response to immunizaton. JAMA 2002, 288, 1471–1472. [Google Scholar] [CrossRef]
- Lange, T.; Perras, B.; Fehm, H.L.; Born, J. Sleep enhances the human antibody response to hepatitis a vaccination. Psychosom. Med. 2003, 65, 831–835. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. What to Expect after Getting a COVID-19 Vaccine. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html (accessed on 6 October 2021).
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Meza-López, C.; Wang, L.; Sulbarán, A.C.M.; Torres-Hernández, P.C.; Bedolla-Barajas, M.; de la O-Gómez, B.; Balcázar-Félix, P.; Hernández-Bello, J. Neutralizing antibodies titers and side effects in response to BNT162b2 vaccine in healthcare workers with and without prior SARS-CoV-2 infection. Vaccines 2021, 9, 742. [Google Scholar] [CrossRef]
- Debes, A.K.; Xiao, S.; Colantuoni, E.; Egbert, E.R.; Caturegli, P.; Gadala, A.; Milstone, A.M. Association of vaccine type and prior SARS-CoV-2 infection with symptoms and antibody measurements following vaccination among health care workers. JAMA Intern. Med. 2021, 181, 1660–1662. [Google Scholar] [CrossRef]
- Hajduczok, A.G.; DiJoseph, K.M.; Bent, B.; Thorp, A.K.; Mullholand, J.B.; MacKay, S.A.; Barik, S.; Coleman, J.J.; Paules, C.I.; Tinsley, A. Physiologic response to the Pfizer-BioNTech COVID-19 vaccine measured using wearable devices: Prospective observational study. JMIR Form. Res. 2021, 5, e28568. [Google Scholar] [CrossRef] [PubMed]
- 164090: SARS-CoV-2 Semi-Quantitative Total Antibody, Spike|Labcorp. Available online: https://www.labcorp.com/tests/164090/sars-cov-2-semi-quantitative-total-antibody-spike (accessed on 6 October 2021).
- Peterhoff, D.; Glück, V.; Vogel, M.; Schuster, P.; Schütz, A.; Neubert, P.; Albert, V.; Frisch, S.; Kiessling, M.; Pervan, P.; et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection 2021, 49, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cobas. Elecsys Anti-SARS-CoV-2 S. In Roche Diagnostics Gmbh. Available online: https://www.fda.gov/media/144037/download (accessed on 6 October 2021).
- Riester, E.; Findeisen, P.; Hegel, J.K.; Kabesch, M.; Ambrosch, A.; Rank, C.M.; Pessl, F.; Laengin, T.; Niederhauser, C. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J. Virol. Methods 2021, 297, 114271. [Google Scholar] [CrossRef] [PubMed]
- Scipy.Stats.Kendalltau—SciPy v1.7.1 Manual. Available online: https://docs.scipy.org/doc/scipy/reference/generated/scipy.stats.kendalltau.html (accessed on 22 November 2021).
- Arndt, S.; Turvey, C.; Andreasen, N.C. Correlating and predicting psychiatric symptom ratings: Spearmans r versus Kendalls tau correlation. J. Psychiatr. Res. 1999, 33, 97–104. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods; Griffin: Brighton, UK, 1948. [Google Scholar]
- Kendall, M.G. A new measure of rank correlation. Biometrika 1938, 30, 81–93. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [Green Version]
- The Cox Model|SpringerLink. Available online: https://link.springer.com/chapter/10.1007%2F978-1-4757-3294-8_3 (accessed on 20 November 2021).
- Prather, A.A.; Pressman, S.D.; Miller, G.E.; Cohen, S. Links between self-reported sleep and antibody responses to the influenza vaccine. Int. J. Behav. Med. 2021, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Cedernaes, J. Could a good night’s sleep improve COVID-19 vaccine efficacy? Lancet Respir. Med. 2021, 9, 447–448. [Google Scholar] [CrossRef]
- Der Holst, H.M.L.; jan Lammers, G.; van der Horst, G.T.J.; Chaves, I.; de Vries, R.D.; GeurtsvanKessel, C.H.; Koch, B.; van der Kuy, H.M. Understanding the association between sleep, shift work and COVID-19 vaccine immune response efficacy: Protocol of the S-CORE study. J. Sleep Res. 2021, e13496. [Google Scholar] [CrossRef]
- Saleh, E.; Moody, M.A.; Walter, E.B. Effect of antipyretic analgesics on immune responses to vaccination. Hum. Vaccines Immunother. 2016, 12, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B.; Cheremina, O.; Brune, K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Prymula, R.; Siegrist, C.l.; Chlibek, R.; Zemlickova, H.; Vackova, M.; Smetana, J.; Lommel, P.; Kaliskova, E.; Borys, D.; Schuerman, L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: Two open-label, randomised controlled trials. Lancet 2009, 374, 1339–1350. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 Vaccination. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/prepare-for-vaccination.html (accessed on 15 November 2021).
- Madison, A.A.; Shrout, M.R.; Renna, M.E.; Kiecolt-Glaser, J.K. Psychological and behavioral predictors of vaccine efficacy: Considerations for COVID-19. Perspect. Psychol. Sci. 2021, 16, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Kiecolt-Glaser, J.K.; Bonneau, R.H.; Malarkey, W.; Kennedy, S.; Hughes, J. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom. Med. 1992, 54, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gümüş, S.; Bektöre, B.; Bozkurt, H.; Gözalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2022, 94, 1060–1066. [Google Scholar] [CrossRef]

| Pfizer-BioNTech | Moderna-NIAID | Johnson & Johnson-Janssen | Overall | |
|---|---|---|---|---|
| N | 706 | 366 | 107 | 1179 |
| Age (M, SD) | 50.4 (11.4) | 52.8 (12.0) | 49.5 (9.9) | 51.0 (11.5) |
| Biological sex (N, %) Male Female Intersex | 334 (47.3) 371 (52.5) 1 (0.1) | 166 (45.4) 200 (54.6) 0 (0) | 53 (49.5) 54 (50.5) 0 (0) | 553 (46.9) 625 (53.0) 1 (0.1) |
| Race (N, %) African African American Caribbean Caucasian/White East Asian Middle Eastern Native American/Native Alaskan Native Hawaiian/Other Pacific Islander South AsianMore than one race Prefer not to answer/Unknown | 1 (0.1) 9 (1.3) 1 (0.1) 603 (85.4) 37 (5.2) 5 (0.7) 3 (0.4) 0 (0) 16 (2.3) 24 (3.4) 7 (1.0) | 1 (0.3) 6 (1.6) 1 (0.3) 325 (88.8) 9 (2.5) 3 (0.8) 0 (0) 0 (0) 3 (0.8) 15 (4.1) 3 (0.8) | 0 (0) 2 (1.9) 0 (0) 95 (88.8) 4 (3.7) 1 (0.9) 0 (0) 1 (0.9) 1 (0.9) 2 (1.9) 1 (0.9) | 2 (0.2) 17 (1.4) 2 (0.2) 1023 (86.8) 50 (4.2) 9 (0.8) 3 (0.3) 1 (0.1) 20 (1.7) 41 (3.5) 11 (0.9) |
| Hispanic/Latinx (N, %) Yes No Don’t Know/Not Sure Prefer not to answer | 30 (4.2) 671 (95.0) 4 (0.6) 1 (0.1) | 17 (4.6) 347 (94.8) 1 (0.3) 1 (0.3) | 7 (6.5) 100 (93.5) 0 (0) 0 (0) | 54 (4.6) 1118 (94.8) 5 (0.4) 2 (0.2) |
| Education (N, %) Less than a high school diploma High school diploma or GED Some college Associate Degree (e.g., AA, AS) Bachelor’s Degree (e.g., BA, BS) Master’s Degree (e.g., MA, MS) Advanced Degree (e.g., Ph.D., EdD, MD, JD) | 0 (0) 5 (0.7) 40 (5.7) 27 (3.8) 270 (38.2) 223 (31.6) 141 (20.0) | 0 (0) 3 (0.8) 27 (7.4) 13 (3.6) 131 (35.8) 119 (32.5) 73 (19.9) | 0 (0) 3 (2.8) 10 (9.3) 6 (5.6) 40 (37.4) 38 (35.5) 10 (9.3) | 0 (0) 11 (0.9) 77 (6.5) 46 (3.9) 441 (37.4) 380 (32.2) 224 (19.0) |
| RBD Value (N, %) Value of 0 to 2499 IU/mL Value of “>250 IU/mL” Value of “>2500 IU/mL” RBD Value (Median, IQR) Value of 0 to 2499 IU/mL Primary analysis data * | 458 (64.9) 33 (4.7) 215 (30.5) 1613.0 [778.4, 2500.0] | 89 (24.3) 18 (4.9) 259 (70.8) 2500.0 [2477.0, 2500.0] | 107 (100.0) 0 (0) 0 (0) 19.1 [6.1,50.1] | 654 (55.5) 51 (4.3) 474 (40.2) 1956.5 [753.3, 2500.0] |
| Metric | Injection 1 | Injection 1 | Injection 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J&J | Moderna | Pfizer | Moderna | Pfizer | ||||||||
| rho | p | rho | p | rho | p | rho | p | rho | p | |||
| Night Relative to Injection | −2 | Sleep Duration− | −0.127 | 0.207 | −0.031 | 0.571 | 0.018 | 0.649 | 0.069 | 0.218 | −0.023 | 0.564 |
| REM Sleep | 0.026 | 0.799 | −0.036 | 0.518 | 0.104 | 0.009 | 0.008 | 0.886 | 0.061 | 0.126 | ||
| Deep Sleep | −0.139 | 0.166 | −0.024 | 0.657 | 0.010 | 0.808 | 0.070 | 0.214 | 0.049 | 0.219 | ||
| HRV (RMSSD) | −0.119 | 0.236 | 0.013 | 0.812 | −0.046 | 0.251 | 0.007 | 0.908 | 0.025 | 0.536 | ||
| HR | −0.038 | 0.706 | 0.033 | 0.547 | 0.138 | 0.000 | 0.031 | 0.583 | 0.063 | 0.117 | ||
| RR | 0.072 | 0.477 | −0.048 | 0.381 | −0.067 | 0.092 | −0.130 | 0.020 | −0.045 | 0.260 | ||
| Temp Deviation | −0.028 | 0.784 | −0.012 | 0.830 | 0.052 | 0.193 | 0.043 | 0.439 | −0.005 | 0.891 | ||
| −1 | Sleep Duration | 0.036 | 0.723 | −0.022 | 0.683 | −0.044 | 0.270 | −0.059 | 0.289 | −0.068 | 0.088 | |
| REM Sleep | 0.018 | 0.856 | −0.062 | 0.255 | 0.033 | 0.403 | −0.086 | 0.126 | 0.001 | 0.976 | ||
| Deep Sleep | 0.012 | 0.908 | −0.048 | 0.375 | 0.047 | 0.236 | 0.005 | 0.933 | 0.048 | 0.235 | ||
| HRV (RMSSD) | −0.081 | 0.421 | 0.049 | 0.366 | 0.001 | 0.974 | 0.028 | 0.614 | 0.044 | 0.275 | ||
| HR | 0.056 | 0.574 | 0.021 | 0.704 | 0.103 | 0.009 | 0.020 | 0.726 | 0.093 | 0.020 | ||
| RR | 0.122 | 0.223 | −0.047 | 0.387 | −0.094 | 0.018 | −0.048 | 0.391 | −0.079 | 0.048 | ||
| Temp Deviation | −0.047 | 0.640 | −0.023 | 0.670 | 0.002 | 0.951 | 0.053 | 0.342 | −0.023 | 0.568 | ||
| 0 | Sleep Duration | 0.125 | 0.211 | 0.066 | 0.235 | −0.080 | 0.044 | −0.003 | 0.960 | −0.033 | 0.408 | |
| REM Sleep | 0.054 | 0.593 | 0.045 | 0.413 | −0.002 | 0.968 | 0.024 | 0.659 | 0.033 | 0.407 | ||
| Deep Sleep | −0.162 | 0.106 | −0.010 | 0.860 | 0.005 | 0.899 | −0.025 | 0.654 | −0.058 | 0.148 | ||
| HRV (RMSSD) | −0.047 | 0.638 | 0.011 | 0.838 | 0.006 | 0.873 | −0.056 | 0.312 | −0.092 | 0.022 | ||
| HR | 0.125 | 0.213 | 0.008 | 0.885 | 0.101 | 0.010 | 0.138 | 0.012 | 0.176 | 0.000 | ||
| RR | 0.123 | 0.222 | −0.053 | 0.340 | −0.058 | 0.141 | −0.021 | 0.711 | −0.004 | 0.925 | ||
| Temp Deviation | 0.155 | 0.122 | −0.009 | 0.870 | −0.003 | 0.935 | 0.123 | 0.026 | 0.152 | 0.000 | ||
| 1 | Sleep Duration | −0.202 | 0.044 | −0.053 | 0.334 | −0.002 | 0.957 | 0.069 | 0.221 | −0.009 | 0.823 | |
| REM Sleep | −0.056 | 0.580 | 0.023 | 0.674 | 0.045 | 0.258 | 0.068 | 0.231 | 0.082 | 0.041 | ||
| Deep Sleep | −0.149 | 0.138 | −0.006 | 0.912 | 0.042 | 0.290 | −0.090 | 0.110 | 0.026 | 0.522 | ||
| HRV (RMSSD) | −0.073 | 0.471 | 0.003 | 0.962 | −0.012 | 0.765 | 0.011 | 0.842 | −0.016 | 0.696 | ||
| HR | 0.049 | 0.631 | 0.002 | 0.968 | 0.087 | 0.028 | 0.053 | 0.352 | 0.116 | 0.004 | ||
| RR | 0.157 | 0.119 | −0.030 | 0.585 | −0.066 | 0.098 | −0.007 | 0.899 | 0.049 | 0.229 | ||
| Temp Deviation | 0.093 | 0.356 | 0.055 | 0.316 | −0.030 | 0.451 | 0.136 | 0.016 | 0.186 | 0.000 | ||
| 2 | Sleep Duration | 0.046 | 0.646 | −0.019 | 0.735 | −0.016 | 0.684 | −0.061 | 0.282 | −0.106 | 0.008 | |
| REM Sleep | 0.019 | 0.853 | −0.026 | 0.641 | 0.032 | 0.425 | −0.038 | 0.497 | −0.001 | 0.974 | ||
| Deep Sleep | 0.026 | 0.795 | −0.039 | 0.478 | −0.006 | 0.870 | 0.012 | 0.832 | −0.013 | 0.739 | ||
| HRV (RMSSD) | −0.074 | 0.465 | 0.038 | 0.491 | 0.005 | 0.891 | 0.004 | 0.942 | −0.041 | 0.312 | ||
| HR | 0.108 | 0.282 | 0.033 | 0.552 | 0.089 | 0.025 | 0.041 | 0.467 | 0.100 | 0.013 | ||
| RR | 0.144 | 0.152 | 0.000 | 0.997 | −0.090 | 0.023 | −0.048 | 0.394 | −0.027 | 0.496 | ||
| Temp Deviation | 0.187 | 0.062 | 0.001 | 0.987 | −0.019 | 0.641 | 0.081 | 0.153 | 0.021 | 0.594 | ||
| Metric | Device-Generated Metric | Adjusted for Baseline Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Injection 1 | Injection 2 | Injection 1 | Injection 2 | |||||||
| rho | p | rho | p | rho | p | rho | p | |||
| Night Relative to Injection | −2 | Sleep Duration | −0.001 | 0.982 | 0.021 | 0.519 | 0.001 | 0.988 | 0.062 | 0.057 |
| REM Sleep | 0.056 | 0.079 | 0.059 | 0.071 | 0.028 | 0.380 | 0.038 | 0.245 | ||
| Deep Sleep | −0.008 | 0.808 | 0.042 | 0.194 | −0.004 | 0.901 | 0.040 | 0.221 | ||
| HRV (RMSSD) | −0.031 | 0.338 | −0.007 | 0.819 | −0.065 | 0.043 | 0.004 | 0.904 | ||
| HR | 0.100 | 0.002 | 0.051 | 0.120 | 0.081 | 0.012 | −0.022 | 0.496 | ||
| RR | −0.041 | 0.197 | −0.071 | 0.030 | 0.058 | 0.071 | −0.011 | 0.735 | ||
| Temp Deviation | 0.017 | 0.603 | 0.001 | 0.967 | 0.044 | 0.167 | 0.023 | 0.488 | ||
| −1 | Sleep Duration | −0.011 | 0.729 | −0.042 | 0.199 | −0.010 | 0.764 | −0.023 | 0.488 | |
| REM Sleep | 0.036 | 0.264 | −0.015 | 0.648 | 0.008 | 0.798 | −0.050 | 0.123 | ||
| Deep Sleep | 0.017 | 0.594 | 0.031 | 0.340 | 0.056 | 0.079 | 0.024 | 0.463 | ||
| HRV (RMSSD) | −0.001 | 0.981 | 0.043 | 0.186 | 0.011 | 0.741 | 0.074 | 0.024 | ||
| HR | 0.070 | 0.029 | 0.052 | 0.114 | −0.009 | 0.773 | −0.011 | 0.746 | ||
| RR | −0.056 | 0.080 | −0.064 | 0.048 | 0.009 | 0.770 | −0.026 | 0.430 | ||
| Temp Deviation | 0.008 | 0.813 | −0.015 | 0.644 | 0.036 | 0.264 | 0.018 | 0.591 | ||
| 0 | Sleep Duration | −0.023 | 0.476 | −0.012 | 0.704 | −0.018 | 0.565 | 0.024 | 0.462 | |
| REM Sleep | 0.010 | 0.755 | 0.031 | 0.343 | −0.024 | 0.460 | 0.022 | 0.497 | ||
| Deep Sleep | −0.025 | 0.430 | −0.079 | 0.014 | −0.014 | 0.667 | −0.126 | 0.000 | ||
| HRV (RMSSD) | −0.010 | 0.749 | −0.118 | 0.000 | −0.007 | 0.816 | −0.199 | 0.000 | ||
| HR | 0.070 | 0.029 | 0.197 | 0.000 | 0.013 | 0.695 | 0.208 | 0.000 | ||
| RR | −0.039 | 0.221 | 0.038 | 0.241 | 0.069 | 0.030 | 0.177 | 0.000 | ||
| Temp Deviation | 0.006 | 0.850 | 0.238 | 0.000 | 0.023 | 0.471 | 0.244 | 0.000 | ||
| 1 | Sleep Duration | −0.018 | 0.573 | 0.065 | 0.047 | −0.016 | 0.625 | 0.125 | 0.000 | |
| REM Sleep | 0.034 | 0.289 | 0.081 | 0.014 | 0.010 | 0.757 | 0.084 | 0.011 | ||
| Deep Sleep | 0.014 | 0.655 | −0.012 | 0.710 | 0.028 | 0.381 | −0.021 | 0.522 | ||
| HRV (RMSSD) | −0.032 | 0.317 | −0.029 | 0.385 | −0.080 | 0.013 | −0.074 | 0.024 | ||
| HR | 0.072 | 0.024 | 0.100 | 0.002 | 0.035 | 0.281 | 0.083 | 0.012 | ||
| RR | −0.015 | 0.643 | 0.067 | 0.042 | 0.097 | 0.002 | 0.260 | 0.000 | ||
| Temp Deviation | 0.040 | 0.215 | 0.241 | 0.000 | 0.065 | 0.042 | 0.250 | 0.000 | ||
| 2 | Sleep Duration | 0.012 | 0.714 | −0.084 | 0.010 | 0.031 | 0.329 | −0.083 | 0.011 | |
| REM Sleep | 0.037 | 0.252 | −0.013 | 0.690 | 0.031 | 0.327 | −0.048 | 0.146 | ||
| Deep Sleep | −0.024 | 0.462 | −0.020 | 0.533 | −0.018 | 0.584 | −0.031 | 0.350 | ||
| HRV (RMSSD) | −0.001 | 0.980 | −0.031 | 0.337 | 0.008 | 0.799 | −0.051 | 0.120 | ||
| HR | 0.068 | 0.034 | 0.068 | 0.038 | 0.024 | 0.457 | 0.005 | 0.871 | ||
| RR | −0.051 | 0.111 | −0.008 | 0.816 | 0.054 | 0.092 | 0.117 | 0.000 | ||
| Temp Deviation | 0.013 | 0.693 | 0.068 | 0.039 | 0.021 | 0.507 | 0.076 | 0.020 | ||
| Metric | Injection 1 | Injection 1 | Injection 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J&J | Moderna | Pfizer | Moderna | Pfizer | ||||||||
| rho | p | rho | p | rho | p | rho | p | rho | p | |||
| Night Relative to Injection | −2 | Sleep Duration | −0.095 | 0.343 | −0.029 | 0.602 | 0.052 | 0.194 | 0.127 | 0.023 | 0.046 | 0.253 |
| REM Sleep | −0.052 | 0.605 | −0.051 | 0.356 | 0.080 | 0.043 | 0.014 | 0.800 | 0.047 | 0.244 | ||
| Deep Sleep | −0.137 | 0.173 | −0.011 | 0.841 | −0.008 | 0.847 | 0.073 | 0.194 | 0.028 | 0.478 | ||
| HRV (RMSSD) | −0.105 | 0.297 | −0.035 | 0.526 | −0.101 | 0.011 | 0.049 | 0.384 | 0.022 | 0.579 | ||
| HR | −0.111 | 0.268 | 0.028 | 0.607 | 0.100 | 0.012 | −0.028 | 0.624 | −0.057 | 0.155 | ||
| RR | −0.076 | 0.450 | −0.007 | 0.906 | 0.064 | 0.104 | −0.047 | 0.402 | 0.037 | 0.357 | ||
| Temp Deviation | −0.052 | 0.605 | 0.039 | 0.479 | 0.060 | 0.133 | 0.076 | 0.178 | −0.005 | 0.893 | ||
| −1 | Sleep Duration | 0.033 | 0.740 | −0.008 | 0.878 | −0.033 | 0.408 | −0.015 | 0.793 | −0.020 | 0.613 | |
| REM Sleep | −0.016 | 0.874 | −0.062 | 0.257 | −0.014 | 0.734 | −0.109 | 0.051 | −0.040 | 0.316 | ||
| Deep Sleep | 0.062 | 0.539 | −0.029 | 0.594 | 0.070 | 0.079 | 0.011 | 0.847 | 0.018 | 0.659 | ||
| HRV (RMSSD) | −0.077 | 0.441 | 0.035 | 0.522 | 0.004 | 0.920 | 0.054 | 0.339 | 0.049 | 0.220 | ||
| HR | 0.128 | 0.199 | 0.010 | 0.853 | −0.012 | 0.761 | −0.052 | 0.350 | 0.023 | 0.571 | ||
| RR | 0.007 | 0.947 | −0.007 | 0.902 | 0.009 | 0.816 | 0.055 | 0.325 | 0.004 | 0.914 | ||
| Temp Deviation | 0.004 | 0.971 | 0.031 | 0.567 | 0.003 | 0.944 | 0.072 | 0.201 | −0.017 | 0.678 | ||
| 0 | Sleep Duration | 0.166 | 0.098 | 0.097 | 0.079 | −0.050 | 0.207 | 0.015 | 0.784 | 0.022 | 0.589 | |
| REM Sleep | 0.106 | 0.293 | 0.051 | 0.355 | −0.044 | 0.269 | 0.037 | 0.501 | 0.012 | 0.763 | ||
| Deep Sleep | −0.173 | 0.083 | −0.010 | 0.863 | −0.009 | 0.816 | −0.054 | 0.326 | −0.120 | 0.003 | ||
| HRV (RMSSD) | −0.001 | 0.993 | −0.045 | 0.422 | 0.012 | 0.758 | −0.119 | 0.031 | −0.182 | 0.000 | ||
| HR | 0.125 | 0.212 | −0.026 | 0.636 | −0.018 | 0.642 | 0.148 | 0.007 | 0.124 | 0.002 | ||
| RR | 0.113 | 0.262 | 0.008 | 0.881 | 0.074 | 0.061 | 0.139 | 0.012 | 0.114 | 0.004 | ||
| Temp Deviation | 0.141 | 0.160 | 0.021 | 0.710 | 0.000 | 0.994 | 0.158 | 0.004 | 0.152 | 0.000 | ||
| 1 | Sleep Duration | −0.224 | 0.025 | −0.068 | 0.215 | 0.026 | 0.508 | 0.088 | 0.120 | 0.081 | 0.045 | |
| REM Sleep | −0.281 | 0.005 | 0.058 | 0.292 | −0.009 | 0.818 | 0.112 | 0.048 | 0.065 | 0.109 | ||
| Deep Sleep | −0.152 | 0.130 | 0.024 | 0.667 | 0.047 | 0.238 | −0.123 | 0.030 | 0.005 | 0.892 | ||
| HRV (RMSSD) | 0.002 | 0.986 | −0.042 | 0.439 | −0.060 | 0.131 | −0.004 | 0.946 | −0.058 | 0.147 | ||
| HR | 0.042 | 0.677 | −0.049 | 0.376 | −0.005 | 0.896 | 0.042 | 0.460 | 0.054 | 0.182 | ||
| RR | 0.057 | 0.573 | 0.103 | 0.061 | 0.041 | 0.299 | 0.168 | 0.003 | 0.259 | 0.000 | ||
| Temp Deviation | 0.067 | 0.511 | 0.094 | 0.085 | −0.009 | 0.814 | 0.169 | 0.003 | 0.182 | 0.000 | ||
| 2 | Sleep Duration | 0.030 | 0.769 | −0.006 | 0.912 | 0.034 | 0.391 | −0.066 | 0.246 | −0.064 | 0.111 | |
| REM Sleep | −0.125 | 0.214 | −0.018 | 0.740 | 0.016 | 0.685 | −0.080 | 0.155 | −0.031 | 0.446 | ||
| Deep Sleep | 0.052 | 0.602 | −0.018 | 0.738 | −0.013 | 0.742 | 0.046 | 0.415 | −0.065 | 0.106 | ||
| HRV (RMSSD) | −0.039 | 0.701 | 0.076 | 0.164 | −0.014 | 0.717 | 0.004 | 0.937 | −0.112 | 0.005 | ||
| HR | 0.111 | 0.269 | 0.010 | 0.858 | 0.021 | 0.602 | 0.003 | 0.963 | 0.033 | 0.408 | ||
| RR | 0.082 | 0.416 | 0.099 | 0.069 | 0.028 | 0.477 | 0.138 | 0.014 | 0.097 | 0.016 | ||
| Temp Deviation | 0.195 | 0.051 | 0.044 | 0.422 | −0.023 | 0.561 | 0.124 | 0.027 | 0.009 | 0.821 | ||
| Device-Generated Metric GFI –log2(p) = 44.95 | Coefficient (Beta) | 95% CI (LB, UB) | Z | p |
|---|---|---|---|---|
| HRV (RMSSD) | −0.002 | (−0.007, 0.004) | −0.584 | 0.559 |
| RR | 0.035 | (−0.024, 0.093) | 1.161 | 0.246 |
| HR | −0.019 | (−0.031, −0.007) | −3.178 | 0.002 |
| Deep | 0.000 | (0.000, 0.000) | 1.125 | 0.260 |
| Temp Deviation | −0.515 | (−0.707, −0.322) | −5.247 | <0.001 |
| Device-Generated Metric Adjusted for Baseline period GFI –log2(p) = 44.65 | ||||
| HRV (RMSSD) | 0.047 | (−0.023, 0.117) | 1.308 | 0.191 |
| RR | −0.042 | (−0.091, 0.008) | −1.632 | 0.103 |
| HR | −0.013 | (−0.073, 0.047) | −0.428 | 0.669 |
| Deep | 0.036 | (−0.032, 0.104) | 1.043 | 0.297 |
| Temp Deviation | −0.071 | (−0.109, −0.034) | −3.748 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, A.E.; Kasl, P.; Hartogensis, W.; Natale, J.L.; Dilchert, S.; Dasgupta, S.; Purawat, S.; Chowdhary, A.; Anglo, C.; Veasna, D.; et al. Metrics from Wearable Devices as Candidate Predictors of Antibody Response Following Vaccination against COVID-19: Data from the Second TemPredict Study. Vaccines 2022, 10, 264. https://doi.org/10.3390/vaccines10020264
Mason AE, Kasl P, Hartogensis W, Natale JL, Dilchert S, Dasgupta S, Purawat S, Chowdhary A, Anglo C, Veasna D, et al. Metrics from Wearable Devices as Candidate Predictors of Antibody Response Following Vaccination against COVID-19: Data from the Second TemPredict Study. Vaccines. 2022; 10(2):264. https://doi.org/10.3390/vaccines10020264
Chicago/Turabian StyleMason, Ashley E., Patrick Kasl, Wendy Hartogensis, Joseph L. Natale, Stephan Dilchert, Subhasis Dasgupta, Shweta Purawat, Anoushka Chowdhary, Claudine Anglo, Danou Veasna, and et al. 2022. "Metrics from Wearable Devices as Candidate Predictors of Antibody Response Following Vaccination against COVID-19: Data from the Second TemPredict Study" Vaccines 10, no. 2: 264. https://doi.org/10.3390/vaccines10020264
APA StyleMason, A. E., Kasl, P., Hartogensis, W., Natale, J. L., Dilchert, S., Dasgupta, S., Purawat, S., Chowdhary, A., Anglo, C., Veasna, D., Pandya, L. S., Fox, L. M., Puldon, K. Y., Prather, J. G., Gupta, A., Altintas, I., Smarr, B. L., & Hecht, F. M. (2022). Metrics from Wearable Devices as Candidate Predictors of Antibody Response Following Vaccination against COVID-19: Data from the Second TemPredict Study. Vaccines, 10(2), 264. https://doi.org/10.3390/vaccines10020264







