Duration of Hepatitis B Vaccine-Induced Protection among Medical Students and Healthcare Workers following Primary Vaccination in Infancy and Rate of Immunity Decline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expanded Immunization Program (EPI) in Thailand
2.2. Study Design and Methodology
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Anti-HBs Antibody Maintenance and Duration (Years) after Primary Vaccination
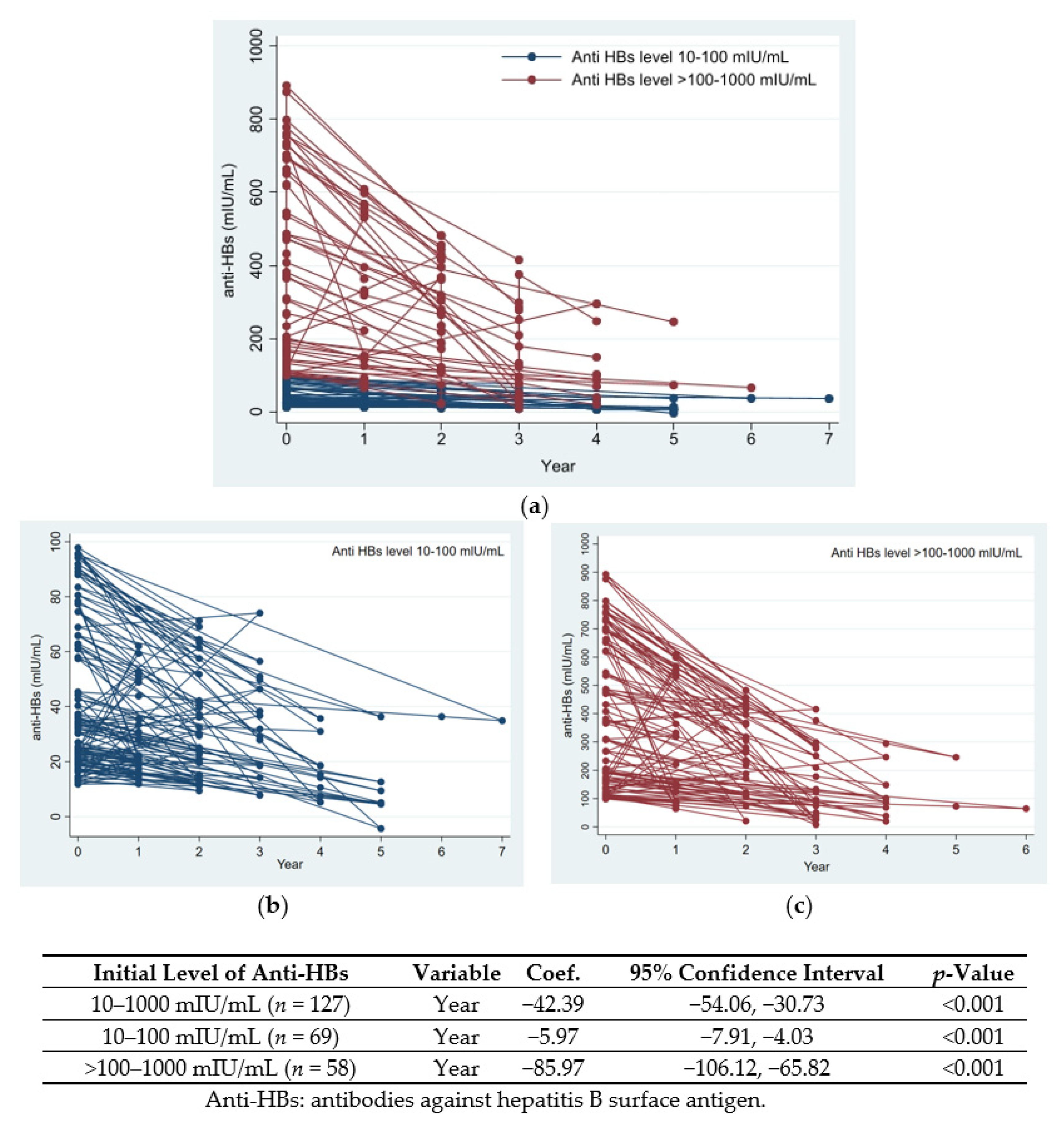
3.3. Changes in Anti-HBs Levels over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hepatitis B Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs204/en/ (accessed on 1 December 2021).
- MacLachlan, J.H.; Locarnini, S.; Cowie, B.C. Estimating the global prevalence of hepatitis B. Lancet 2015, 386, 1515–1517. [Google Scholar] [CrossRef]
- Guidelines for Viral Hepatitis Surveillance and Case Management. Available online: https://www.cdc.gov/hepatitis/statistics/surveillanceguidelines.htm (accessed on 1 December 2021).
- Thailand’s Achievement on Hepatitis B Control. Available online: https://www.who.int/thailand/news/detail/08-08-2019-thailand-achievement-on-hepatitis-b-control (accessed on 1 December 2021).
- WHO Hepatitis B Report, 2017. Executive Summary. Available online: https://www.who.int/publications/i/item/global-hepatitis-report-2017 (accessed on 1 December 2021).
- Chongsrisawat, V.; Yoocharoen, P.; Theamboonlers, A.; Tharmaphornpilas, P.; Warinsathien, P.; Sinlaparatsamee, S.; Paupunwatana, S.; Chaiear, K.; Khwanjaipanich, S.; Poovorawan, Y. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop. Med. Int. Health 2006, 11, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Poovorawan, Y.; Theamboonlers, A.; Sanpavat, S.; Pongpunlert, W.; Chumdermpadetsuk, S.; Safary, A.; Vandepapeliere, P. The Immunogenicity and Reactogenicity of Combined Tetravalent Diphtheria, Tetanus, Pertussis, and Hepatitis B Vaccine in Infants. In Viral Hepatitis and Liver Disease; Springer: Tokyo, Japan, 1994; pp. 526–529. [Google Scholar]
- Poovorawan, Y. Experience of combined tetravalent diphtheria, tetanus, whole-cell pertussis and hepatitis B vaccine in Thailand. Southeast Asian J. Trop Med. Public Health 1997, 28, 496–499. [Google Scholar] [PubMed]
- Cheang, H.K.; Wong, H.T.; Ho, S.C.; Chew, K.S.; Lee, W.S. Immune response in infants after universal hepatitis B vaccination: A community-based study in Malaysia. Singap. Med. J. 2013, 54, 224–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roznovsky, L.; Orsagova, I.; Kloudova, A.; Tvrdik, J.; Kabieszova, L.; Lochman, I.; Mrazek, J.; Hozakova, L.; Zjevikova, A.; Pliskova, L. Long-term protection against hepatitis B after newborn vaccination: 20-year follow-up. Infection 2010, 38, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Poovorawan, Y.; Chongsrisawat, V.; Theamboonlers, A.; Crasta, P.D.; Messier, M.; Hardt, K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: A 20-year follow-up study in Thailand. Hum. Vaccin Immunother. 2013, 9, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.C.; Wu, Z.W.; Zhou, H.S.; Gao, Z.; Hao, Z.Y.; Jin, F.; Zhang, Y.H.; Li, M.J.; Wang, F.; Li, Q.; et al. Long-term protection at 20–31 years after primary vaccination with plasma-derived hepatitis B vaccine in a Chinese rural community. Hum. Vaccin Immunother. 2020, 16, 16–20. [Google Scholar] [CrossRef]
- van der Sande, M.A.B.; Waight, P.; Mendy, M.; Rayco-Solon, P.; Hutt, P.; Fulford, T.; Doherty, C.; McConkey, S.J.; Jeffries, D.; Hall, A.J.; et al. Long-term protection against carriage of hepatitis B virus after infant vaccination. J. Infect. Dis. 2006, 193, 1528–1535. [Google Scholar] [CrossRef]
- Pileggi, C.; Papadopoli, R.; Bianco, A.; Pavia, M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine 2017, 35, 6302–6307. [Google Scholar] [CrossRef]
- Klinger, G.; Chodick, G.; Levy, I. Long-term immunity to hepatitis B following vaccination in infancy: Real-world data analysis. Vaccine 2018, 36, 2288–2292. [Google Scholar] [CrossRef]
- Posuwan, N.; Vorayingyong, A.; Jaroonvanichkul, V.; Wasitthankasem, R.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y. Implementation of hepatitis B vaccine in high-risk young adults with waning immunity. PLoS ONE 2018, 13, e0202637. [Google Scholar] [CrossRef] [Green Version]
- Doi, H.; Kanto, T. Factors influencing the durability of hepatitis B vaccine responses. Vaccine 2021, 39, 5224–5230. [Google Scholar] [CrossRef] [PubMed]
- Tharmaphornpilas, P.; Rasdjarmrearnsook, A.O.; Plianpanich, S.; Sa-nguanmoo, P.; Poovorawan, Y. Increased risk of developing chronic HBV infection in infants born to chronically HBV infected mothers as a result of delayed second dose of hepatitis B vaccination. Vaccine 2009, 27, 6110–6115. [Google Scholar] [CrossRef] [PubMed]
- Posuwan, N.; Wanlapakorn, N.; Sa-Nguanmoo, P.; Wasitthankasem, R.; Vichaiwattana, P.; Klinfueng, S.; Vuthitanachot, V.; Sae-Lao, S.; Foonoi, M.; Fakthongyoo, A.; et al. The Success of a Universal Hepatitis B Immunization Program as Part of Thailand’s EPI after 22 Years’ Implementation. PLoS ONE 2016, 11, e0150499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, A.D.; Hall, A.J.; Maine, N.; Mendy, M.; Whittle, H.C. What level of hepatitis B antibody is protective? J. Infect. Dis 1999, 179, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.; Wilson, R.; Patrick, G.; Andre, F.; Watson, V. Hepatitis B vaccines: Assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin. Ther. 2001, 23, 392–403. [Google Scholar] [CrossRef]
- Schillie, S.; Murphy, T.V.; Sawyer, M.; Ly, K.; Hughes, E.; Jiles, R.; de Perio, M.A.; Reilly, M.; Byrd, K.; Ward, J.W. Center for Disease Control and Prevention. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm. Rep. 2013, 62, 1–19. [Google Scholar]
- Poovorawan, Y.; Theamboonlers, A.; Vimolket, T.; Sinlaparatsamee, S.; Chaiear, K.; Siraprapasiri, T.; Khwanjaipanich, S.; Owatanapanich, S.; Hirsch, P.; Chunsuttiwat, S. Impact of hepatitis B immunisation as part of the EPI. Vaccine 2000, 19, 943–949. [Google Scholar] [CrossRef]
- Rao, T.V.; Suseela, I.J.; Sathiavathy, K.A. Estimation of antibodies to HBsAg in vaccinated health care workers. Indian J. Med. Microbiol. 2008, 26, 93–94. [Google Scholar] [CrossRef]
- Batra, V.; Goswami, A.; Dadhich, S.; Kothari, D.; Bhargava, N. Hepatitis B immunization in healthcare workers. Ann. Gastroenterol. 2015, 28, 276–280. [Google Scholar]
- Sahana, H.V.; Sarala, N.; Prasad, S.R. Decrease in anti-HBs antibodies over time in medical students and healthcare workers after hepatitis B vaccination. BioMed Res. Int. 2017, 2017, 1327492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Shim, K.S.; Lim, I.S.; Chae, S.A.; Yun, S.W.; Lee, N.M.; Choi, Y.B.; Yi, D.Y. Changes in hepatitis B virus antibody titers over time among children: A single center study from 2012 to 2015 in an urban of South Korea. BMC Pediatr. 2017, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q. A study on the relationship between maximal titers of anti-HBs antibodies and persistence of protective level after vaccination. Zhonghua Liu Xing Bing Xue Za Zhi 1992, 13, 134–137. [Google Scholar] [PubMed]
- Poovorawan, Y.; Chongsrisawat, V.; Theamboonlers, A.; Leroux-Roels, G.; Kuriyakose, S.; Leyssen, M.; Jacquet, J.M. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J. Viral. Hepat. 2011, 18, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Stramer, S.L.; Wend, U.; Candotti, D.; Foster, G.A.; Hollinger, F.B.; Dodd, R.Y.; Allain, J.P.; Gerlich, W. Nucleic acid testing to detect HBV infection in blood donors. N. Engl. J. Med. 2011, 364, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Simons, B.C.; Spradling, P.R.; Bruden, D.J.; Zanis, C.; Case, S.; Choromanski, T.L.; Apodaca, M.; Brogdon, H.D.; Dwyer, G.; Snowball, M.; et al. A Longitudinal Hepatitis B Vaccine Cohort Demonstrates Long-lasting Hepatitis B Virus (HBV) Cellular Immunity Despite Loss of Antibody Against HBV Surface Antigen. J. Infect. Dis. 2016, 214, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Bruce, M.G.; Bruden, D.; Hurlburt, D.; Zanis, C.; Thompson, G.; Rea, L.; Toomey, M.; Townshend-Bulson, L.; Rudolph, K.; Bulkow, L.; et al. Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. J. Infect. Dis. 2016, 214, 16–22. [Google Scholar] [CrossRef]
- Nelson, N.P.; Easterbrook, P.J.; McMahon, B.J. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin. Liver Dis. 2016, 20, 607–628, Erratum in Clin. Liver Dis. 2017, 21, xiii. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Franco, E.; Bagnato, B.; Marino, M.G.; Meleleo, C.; Serino, L.; Zaratti, L. Hepatitis B: Epidemiology and prevention in developing countries. World J. Hepatol. 2012, 4, 74–80. [Google Scholar] [CrossRef]
- McMahon, B.J.; Bruden, D.L.; Petersen, K.M.; Bulkow, L.R.; Parkinson, A.J.; Nainan, O.; Khristova, M.; Zanis, C.; Peters, H.; Margolis, H.S. Antibody levels and protection after hepatitis B vaccination: Results of a 15-year follow-up. Ann. Intern. Med. 2005, 142, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Viral Hepatitis, Q&As for Health Professionals. Available online: https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm (accessed on 1 December 2021).
- Verso, M.G.; Costantino, C.; Marrella, A.; Immordino, P.; Vitale, F.; Amodio, E. Kinetics of Anti-Hepatitis B Surface Antigen Titers in Nurse Students after a Two-Year Follow-Up. Vaccines 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Yamamoto, C.; Ko, K.; Chuon, C.; Sugiyama, A.; Ohisa, M.; Akita, T.; Katayama, K.; Yoshihara, M.; Tanaka, J. Acquisition rate of antibody to hepatitis B surface antigen among medical and dental students in Japan after three-dose hepatitis B vaccination. Vaccine 2019, 37, 145–151. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) |
|---|---|
| Median age, years (IQR 25th–75th percentile) | 23 (22–25) |
| Male | 72 (15) |
| Time since primary vaccination (years) | |
| 16–20 | 75 (16) |
| 21–25 | 319 (69) |
| 26–28 | 70 (15) |
| Initial level of anti-HBs (mIU/mL) | |
| <2 | 167 (36) |
| 2–<10 | 69 (15) |
| 10–1000 | 178 (38) |
| >1000 | 50 (11) |
| Duration after Primary Vaccination (Years) | n | Number of Participants with Initial Anti-HBs Level, n (%) | |||
|---|---|---|---|---|---|
| <10 (mIU/mL) | 10–1000 (mIU/mL) | >1000 (mIU/mL) | |||
| <2 (mIU/mL) | 2–<10 (mIU/mL) | ||||
| Group 1: 16–20 | 75 | 32 (42.7) | 22 (29.3) | 19 (25.3) | 2 (2.7) |
| Group 2: 21–25 | 319 | 112 (35.1) | 42 (13.2) | 124 (38.9) | 41 (12.8) |
| Group 3: 26–28 | 70 | 23 (32.9) | 5 (7.1) | 35 (50) | 7 (10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phattraprayoon, N.; Kakheaw, J.; Soonklang, K.; Cheirsilpa, K.; Ungtrakul, T.; Auewarakul, C.; Mahanonda, N. Duration of Hepatitis B Vaccine-Induced Protection among Medical Students and Healthcare Workers following Primary Vaccination in Infancy and Rate of Immunity Decline. Vaccines 2022, 10, 267. https://doi.org/10.3390/vaccines10020267
Phattraprayoon N, Kakheaw J, Soonklang K, Cheirsilpa K, Ungtrakul T, Auewarakul C, Mahanonda N. Duration of Hepatitis B Vaccine-Induced Protection among Medical Students and Healthcare Workers following Primary Vaccination in Infancy and Rate of Immunity Decline. Vaccines. 2022; 10(2):267. https://doi.org/10.3390/vaccines10020267
Chicago/Turabian StylePhattraprayoon, Nanthida, Jirapa Kakheaw, Kamonwan Soonklang, Kunsuda Cheirsilpa, Teerapat Ungtrakul, Chirayu Auewarakul, and Nithi Mahanonda. 2022. "Duration of Hepatitis B Vaccine-Induced Protection among Medical Students and Healthcare Workers following Primary Vaccination in Infancy and Rate of Immunity Decline" Vaccines 10, no. 2: 267. https://doi.org/10.3390/vaccines10020267
APA StylePhattraprayoon, N., Kakheaw, J., Soonklang, K., Cheirsilpa, K., Ungtrakul, T., Auewarakul, C., & Mahanonda, N. (2022). Duration of Hepatitis B Vaccine-Induced Protection among Medical Students and Healthcare Workers following Primary Vaccination in Infancy and Rate of Immunity Decline. Vaccines, 10(2), 267. https://doi.org/10.3390/vaccines10020267






