Effectiveness of COVID-19 Vaccines against Delta Variant (B.1.617.2): A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.1.1. Study Participants
2.1.2. Inclusion and Exclusion Criteria
2.1.3. Data Extraction
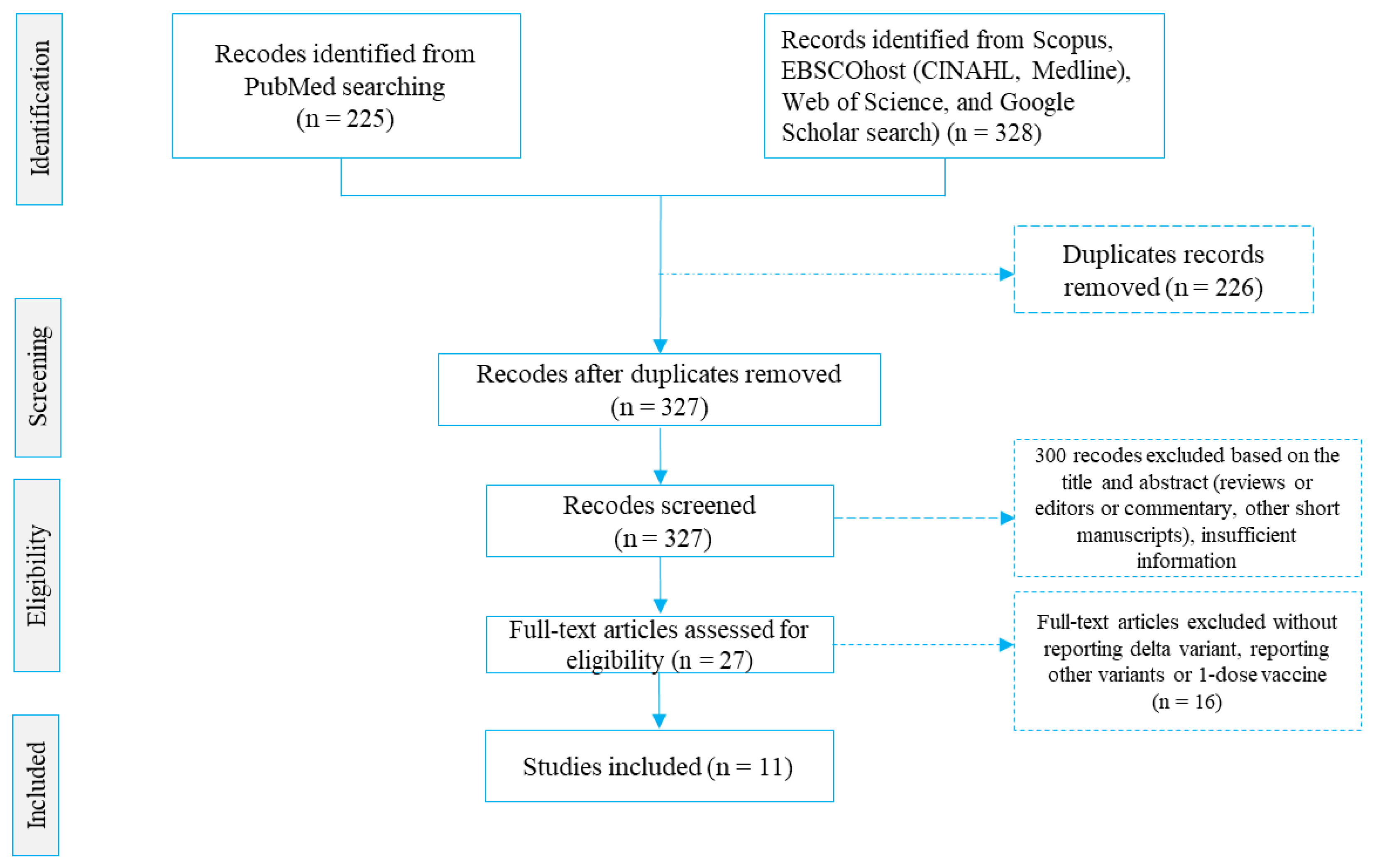
2.1.4. Study Screening and Selection
2.1.5. Quality Assessment
2.2. Data Analysis
3. Results
3.1. Description of Studies Included
3.2. Effectiveness of the COVID-19 Vaccines against the Delta Variant
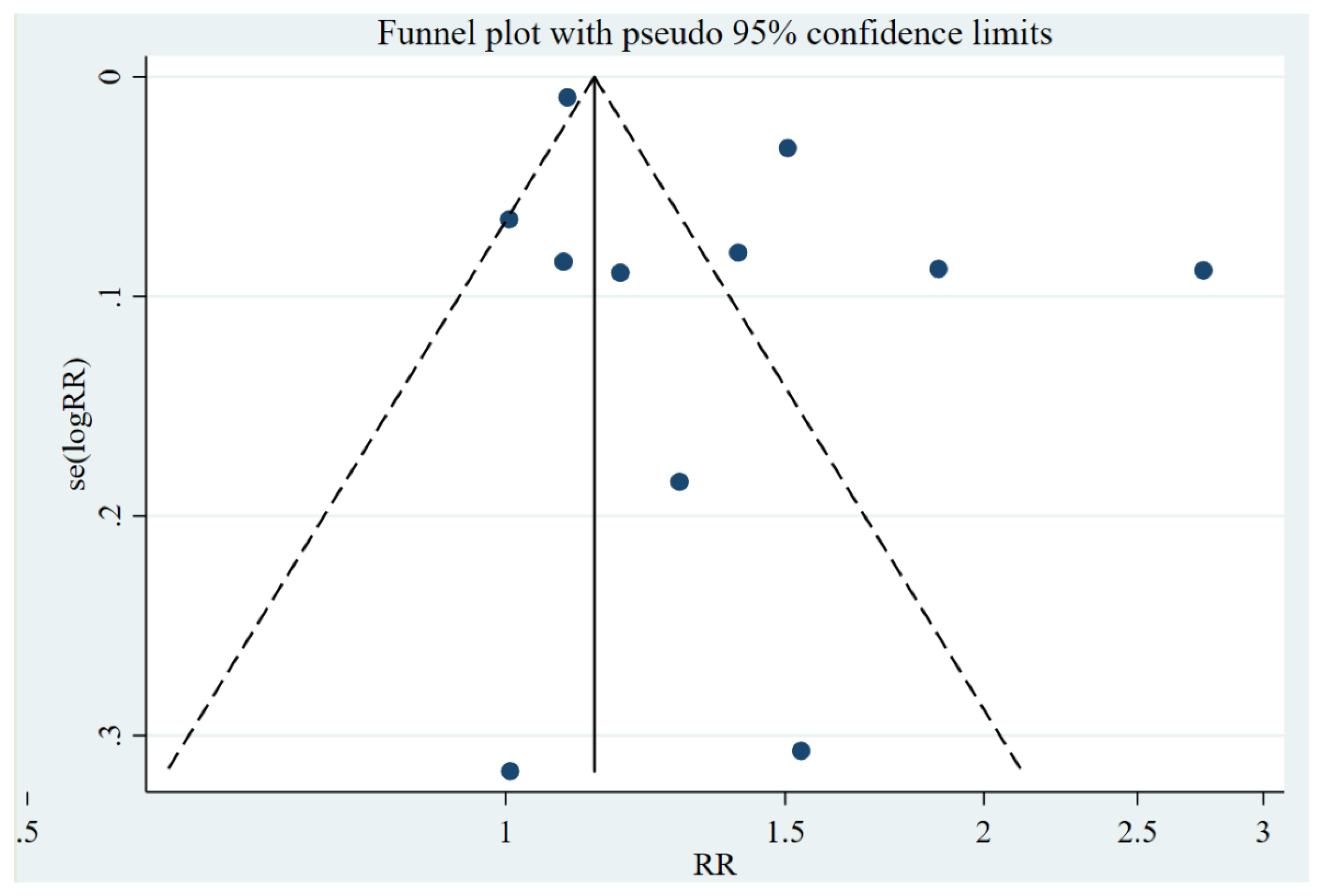
Quality of Included Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| # | Quality Assessment Indicators | Selected Cohort Studies | |||
|---|---|---|---|---|---|
| Fowlkes et al. (2021) [24] | Grannis et al. (2021) [25] | Jara et al. (2021) [26] | Nanduri et al. (2021) [27] | ||
| Q1 | Were the two groups similar and recruited from the same population? | ✓ | ✓ | ✓ | ✓ |
| Q2 | Were the exposures measured similarly to assign people to both exposed and unexposed groups? | ✓ | ✓ | ✓ | ✓ |
| Q3 | Was the exposure measured in a valid and reliable way? | ✓ | ✓ | ✓ | ✓ |
| Q4 | Were confounding factors identified? | ✓ | ✓ | ✓ | ✓ |
| Q5 | Were strategies to deal with confounding factors stated? | ✓ | ✓ | ✓ | ✓ |
| Q6 | Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | ✓ | ✓ | ✓ | ✓ |
| Q7 | Were the outcomes measured in a valid and reliable way? | ✓ | ✓ | ✓ | ✓ |
| Q8 | Was the follow-up time reported and sufficiently long enough for outcomes to occur? | ® | ® | ✓ | ® |
| Q9 | Was follow-up complete, and if not, were the reasons to loss to follow-up described and explored? | ✕ | ✕ | ® | ✕ |
| Q10 | Were strategies to address incomplete follow-up utilised? | ✕ | ✕ | ✕ | ✕ |
| Q11 | Was appropriate statistical analysis used? | ✓ | ✓ | ✓ | ✓ |
| Overall quality score | 8.5 (medium) | 8.5 (medium) | 9.5 (high) | 8.5 (medium) | |
| Overall appraisal | Included | Included | Included | Included | |
| # | Quality Assessment Indicators | Selected Case–Control Studies | |||||
|---|---|---|---|---|---|---|---|
| Barlow et al. (2021) [6] | Li et al. (2021) [28] | Hu et al. (2021) [29] | Bernal et al. (2021) [30] | Tang et al. (2021) [31] | Nasreen et al. (2021) [32] | ||
| Q1 | Were the groups comparable other than the presence of disease in cases or the absence of disease in controls? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Q2 | Were cases and controls matched appropriately? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Q3 | Were the same criteria used for the identification of cases and controls? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Q4 | Was exposure measured in a standard, valid and reliable way? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Q5 | Was exposure measured in the same way for cases and controls? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Q6 | Were confounding factors identified? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Q7 | Were strategies to deal with confounding factors stated? | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ |
| Q8 | Were outcomes assessed in a standard, valid and reliable way for cases and controls? | ✓ | ✓ | ✓ | ✓ | ✓ | ® |
| Q9 | Was the exposure period of interest long enough to be meaningful? | ® | ✕ | ✕ | ✕ | ✕ | ® |
| Q10 | Was appropriate statistical analysis used? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Overall quality score | 9.5 (high) | 8 (high) | 8 (medium) | 9 (high) | 8 (medium) | 8 (medium) | |
| Overall appraisal | Included | Included | Included | Included | Included | Included | |
| # | Quality Assessment Indicators for Case-Series Study | Kislaya et al. (2021) [33] |
|---|---|---|
| Q1 | Were there clear criteria for inclusion in the case series? | ✓ |
| Q2 | Was the condition measured in a standard, reliable way for all participants included in the case series? | ✓ |
| Q3 | Were valid methods used for identification of the condition for all participants included in the case series? | ✓ |
| Q4 | Did the case series have consecutive inclusion of participants? | ✓ |
| Q5 | Did the case series have the complete inclusion of participants? | ® |
| Q6 | Was there clear reporting of the demographics of the participants in the study? | ✓ |
| Q7 | Was there clear reporting of clinical information of the participants? | ✓ |
| Q8 | Were the outcomes or follow-up results of cases clearly reported? | ✓ |
| Q9 | Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | ✓ |
| Q10 | Was statistical analysis appropriate? | ✓ |
| Overall quality score | 9.5 (high) | |
| Overall appraisal | ||
References
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- GISAID. Delta Variant Detected Across 185 Countries. Global Initiative on Sharing Avian Influenza Data (GIAID). Phylodynamics Pandemic Coronavirus Var. VOC Delta G/478K.V1 First Detect. India. 2021. Available online: https://www.gisaid.org/hcov19-variants/ (accessed on 30 September 2021).
- Gallagher, J. COVID: Is There a Limit to How Much Worse Variants Can Get? How the R0 Numbers of COVID-19 Variants and Other Diseases Compare. 2021. Available online: https://www.bbc.com/news/health-57431420 (accessed on 30 September 2021).
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 6736, 21–24. [Google Scholar] [CrossRef]
- Callaway, E. COVID vaccine boosters: The most important questions. Nature 2021, 596, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Barlow, R.S.; Jian, K.; Larson, L.; Barlow, R.S. Effectiveness of COVID-19 Vaccines Against SARS-CoV-2 Infection during a Delta Variant Epidemic Surge in Multnomah County, Oregon, July 2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Public Health England. Effectiveness of COVID-19 Vaccines against Hospital Admission with the Delta (B.1.617.2) Variant. 2021. Available online: https://khub.net/web/phe-national/public-library/-/document_library/v2Ws-%0ARK3ZlEig/view/479607266 (accessed on 12 August 2021).
- Imai, N.; Hogan, A.B.; Williams, L.; Cori, A.; Mangal, T.D.; Winskill, P.; Whittles, L.K.; Watson, O.J.; Knock, E.S.; Baguelin, M.; et al. Interpreting estimates of coronavirus disease 2019 (COVID-19) vaccine efficacy and effectiveness to inform simulation studies of vaccine impact: A systematic review. Wellcome Open Res. 2021, 6, 185. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 495. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Hasan, M.R.; Malek, J.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. mRNA-1273 COVID-19 vaccine effectiveness against the B. 1.1. 7 and B. 1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021, 27, 1614–1621. [Google Scholar] [CrossRef]
- Chung, H.; He, S.; Nasreen, S.; Sundaram, M.E.; Buchan, S.A.; Wilson, S.E.; Chen, B.; Calzavara, A.; Fell, D.B.; Austin, P.C.; et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada. BMJ 2021, 374, 1–12. [Google Scholar] [CrossRef]
- Davis, C.; Logan, N.; Tyson, G.; Orton, R.; Harvey, W.; Haughney, J.; Perkins, J.; Peacock, T.P.; Barclay, W.S.; Cherepanov, P.; et al. Reduced neutralisation of the Delta (B. 1.617. 2) SARS-CoV-2 variant of concern following vaccination. PLoS Pathog. 2021, 17, e1010022. [Google Scholar] [CrossRef]
- Noori, M.; Nejadghaderi, S.A.; Arshi, S.; Carson-Chahhoud, K.; Ansarin, K.; Kolahi, A.A.; Safiri, S. Potency of BNT162b2 and mRNA-1273 vaccine-induced neutralizing antibodies against severe acute respiratory syndrome-CoV-2 variants of concern: A systematic review of in vitro studies. Rev. Med. Virol. 2021, e2277. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.T.; Bobos, P.; Odutayo, A.; Pai, M. Meta-Analysis of Risk of Vaccine-Induced Immune Thrombotic Thrombocytopenia Following ChAdOx1-S Recombinant Vaccine. medRxiv 2021. [Google Scholar] [CrossRef]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Ferri, N.; Toth, P.P.; Ruscica, M.; Corsini, A.; Cicero, A.F.G. Efficacy and Safety of Mipomersen: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2019, 79, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Tong, Y.; Du, F.; Lu, L.; Zhao, S.; Yu, K.; Piatek, S.J.; Larson, H.J.; Lin, L. Assessing COVID-19 vaccine hesitancy, confidence, and public engagement:a global social listening study. J. Med. Internet Res. 2021, 23, e27632. [Google Scholar] [CrossRef]
- Afolabi, A.A.; Ilesanmi, O.S. Dealing with vaccine hesitancy in Africa: The prospective COVID-19 vaccine context. Pan. Afr. Med. J. 2021, 38, 1–7. [Google Scholar] [CrossRef]
- Public Health England. SARS-CoV-2 Variants of Concern and Variants under Investigation in England: Technical Briefing 18. Public Health England, Wellington House, 133–155 Waterloo Road, London SE1 8UG: 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/990339/Variants_of_Concern_VOC_Technical_Briefing_13_England.pdf (accessed on 27 August 2021).
- Mahumud, R.A.; Kamara, J.K.; Renzaho, A.M.N. The epidemiological burden and overall distribution of chronic comorbidities in coronavirus disease-2019 among 202,005 infected patients: Evidence from a systematic review and meta-analysis. Infection 2020, 48, 813–833. [Google Scholar] [CrossRef]
- Munn, Z.; MClinSc, S.M.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based. Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Joanna Briggs Institute (JBI). JBI’s Critical Appraisal Tools. Faculty of Health and Medical Sciences; The University of Adelaide: Adelaide, Australia; Available online: https://jbi.global/critical-appraisal-tools (accessed on 27 August 2021).
- Fowlkes, A.; Gaglani, M.; Groover, K.; Thiese, M.S.; Tyner, H.; Ellingson, K. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance—Eight U.S. Locations, December 2020–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1167–1169. [Google Scholar] [CrossRef]
- Grannis, S.J.; Rowley, E.A.; Ong, T.C.; Stenehjem, E.; Klein, N.P.; DeSilva, M.B.; Naleway, A.L.; Natarajan, K.; Thompson, M.G. Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19–Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance—Nine States, June-August 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Pilishvili, T.; Derado, G.; Soe, M.M.; Dollard, P.; Wu, H.; Li, Q.; Bagchi, S.; Dubendris, H.; Link-Gelles, R.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—National Healthcare Safety Network, March 1–August. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-N.; Huang, Y.; Wang, W.; Jing, Q.; Zhang, C.; Qin, P.; Guan, W.; Gan, L.; Li, Y.; Liu, W.; et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case—control real-world study. Emerg. Microbes. Infect. 2021, 10, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tao, B.; Li, Z.; Song, Y.; Yi, C.; Li, J.; Zhu, M.; Yi, Y.; Huang, P.; Wang, J. Effectiveness of inactive COVID-19 vaccines against severe illness in B.1.617.2 2 (Delta) variant-infected patients in Jiangsu, China. Int. J. Infect. Dis. 2021, 116, 204–209. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; al Khatib, H.A.; AlMukdad, S.; Coyle, P.; Ayoub, H.H.; al Kanaani, Z.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. Nat. Med. 2021, 27, 2136–2143. [Google Scholar] [CrossRef]
- Nasreen, S.; He, S.; Chung, H.; Brown, K.A.; Gubbay, J.B.; Buchan, S.A.; Fell, D.B.; Austin, P.C.; Schwartz, K.L.; Sundaram, M.E.; et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. medRxiv 2021. [Google Scholar] [CrossRef]
- Kislaya, I.; Rodrigues, E.F.; Borges, V.; Gomes, J.P.; Sousa, C.; Almeida, J.P.; Peralta-Santos, A.; Nunes, B. Delta variant and mRNA COVID-19 vaccines effectiveness: Higher odds of vaccine infection breakthroughs. medRxiv 2021. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet. Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Ison, M.G.; Wolfe, C.; Boucher, H.W. Emergency use authorization of remdesivir: The need for a transparent distribution process. JAMA 2020, 323, 2365–2366. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Emergency Use Authorization. 2020. Available online: https://www.fda.gov/ (accessed on 27 August 2021).
- Burki, T.K. Lifting of COVID-19 restrictions in the UK and the Delta variant. Lancet Respir. Med. 2021, 9, e85. [Google Scholar] [CrossRef]
- Sander, A.-L.; Yadouleton, A.; Moreira-Soto, A.; Tchibozo, C.; Tchibozo, C.; Hounkanrin, G.; Badou, Y.; Fischer, C.; Krause, N.; Akogbeto, P.; et al. An Observational Laboratory-Based Assessment of SARS-CoV-2 Molecular Diagnostics in Benin, Western Africa. MSphere 2021, 6, e00979-20. [Google Scholar] [CrossRef]
- Mizrahi, B.; Lotan, R.; Kalkstein, N.; Peretz, A.; Perez, G.; Ben-Tov, A.; Chodick, G.; Gazit, S.; Patalon, T. Correlation of SARS-CoV-2 breakthrough infections to time-from-vaccine; Preliminary study. Nat Commun. 2021, 12, 6379. [Google Scholar] [CrossRef]

| Study | Country | Design | Fully Vaccinated Group | Unvaccinated Group | ||
|---|---|---|---|---|---|---|
| Number of Infected Persons, n (%) | Total Vaccinated Population | Number of Infected Persons, n (%) | Total Unvaccinated Population | |||
| Bernal et al. (2021) [30] | UK | Case–control | 143 (0.60) | 23,993 | 7313 (7.59) | 96,371 |
| Fowlkes et al. (2021) [24] | USA | Cohort study | 34 (1.14) | 2976 | 194 (4.69) | 4136 |
| Grannis et al. (2021) [25] | USA | Cohort study | 134 (2.94) | 4551 | 1185 (20.76) | 5708 |
| Barlow et al. (2021) [6] | USA | Case-control | 145 (29.00) | 500 | 279 (55.80) | 500 |
| Hu et al. (2021) [29] | China | Case–control | 187 (39.29) | 476 | 184 (38.66) | 476 |
| Jara et al. (2021) [26] | Chile | Cohort study | 12,286 (0.29) | 4,173,574 | 185,633 (3.39) | 5,471,728 |
| Kislaya et al. (2021) [33] | Portugal | Case-case study | 162 (11.89) | 1363 | 777 (46.00) | 1689 |
| Li et al. (2021) [28] | China | Case–control | 12 (12.37) | 97 | 37 (34.58) | 107 |
| Nanduri et al. (2021) [27] | USA | Cohort study | 2999 (0.06) | 5,011,746 | 1397 (0.15) | 953,861 |
| Nasreen et al. (2021) [32] | Canada | Case–control | 10 (0.12) | 8461 | 6325 (22.03) | 28,705 |
| Tang et al. (2021) [31] | Qatar | Case–control | 249 (0.02) | 1,286,395 | 4993 (4.06) | 122,928 |
| Total, n = 12 | N = 16,361 (0.16) | N = 10,514,132 | N = 208,317 (3.12) | N = 6,686,209 | ||
| Weighted pooled incidence of COVID-19 infection. | 8.88% (95% CI: 0.18–17.95) | 21.61% (95% CI: 8.50–34.72) | ||||
| Study Design | Number of Studies | Egger’s Regression Test | Small-Study Effect (p-Value) | |||
|---|---|---|---|---|---|---|
| RR, (95% CI) | p-Value | Bias (RR), 95% CI | p-Value | |||
| Case-control | 6 | 0.61 (0.02, 5.53) | 0.224 | 30.53 (0.89, 51.45) | 0.481 | p-value = 0.685 |
| Cohort study | 4 | 0.09 (0.04, 0.17) | 0.015 | 13.32 (0.98, 45.47) | 0.390 | p-value = 0.390 |
| Case-case study | 1 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahumud, R.A.; Ali, M.A.; Kundu, S.; Rahman, M.A.; Kamara, J.K.; Renzaho, A.M.N. Effectiveness of COVID-19 Vaccines against Delta Variant (B.1.617.2): A Meta-Analysis. Vaccines 2022, 10, 277. https://doi.org/10.3390/vaccines10020277
Mahumud RA, Ali MA, Kundu S, Rahman MA, Kamara JK, Renzaho AMN. Effectiveness of COVID-19 Vaccines against Delta Variant (B.1.617.2): A Meta-Analysis. Vaccines. 2022; 10(2):277. https://doi.org/10.3390/vaccines10020277
Chicago/Turabian StyleMahumud, Rashidul Alam, Mohammad Afshar Ali, Satyajit Kundu, Md Ashfikur Rahman, Joseph Kihika Kamara, and Andre M. N. Renzaho. 2022. "Effectiveness of COVID-19 Vaccines against Delta Variant (B.1.617.2): A Meta-Analysis" Vaccines 10, no. 2: 277. https://doi.org/10.3390/vaccines10020277
APA StyleMahumud, R. A., Ali, M. A., Kundu, S., Rahman, M. A., Kamara, J. K., & Renzaho, A. M. N. (2022). Effectiveness of COVID-19 Vaccines against Delta Variant (B.1.617.2): A Meta-Analysis. Vaccines, 10(2), 277. https://doi.org/10.3390/vaccines10020277







