Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review
Abstract
1. Introduction
2. Increased Risk of Infections in Patients with IMIDs
3. Vaccines and IMIDs
4. Immune Response Necessary to Generate Immunity
5. Approved Biologic Therapies and Their Impact on Responses to Vaccines
5.1. Integrins
5.1.1. Anti-α4 (Natalizumab)
5.1.2. Anti-α4β7 (Vedolizumab)
5.2. Co-Stimulators of T Cells
5.3. B Cells
5.3.1. Anti-CD20 (Rituximab)
5.3.2. Anti-BAFF (Belimumab)
5.4. Pro-Inflammatory Cytokines
5.4.1. Anti-TNF
5.4.2. Anti-IL-1
5.4.3. Anti-IL-17
5.4.4. Anti-IL-6
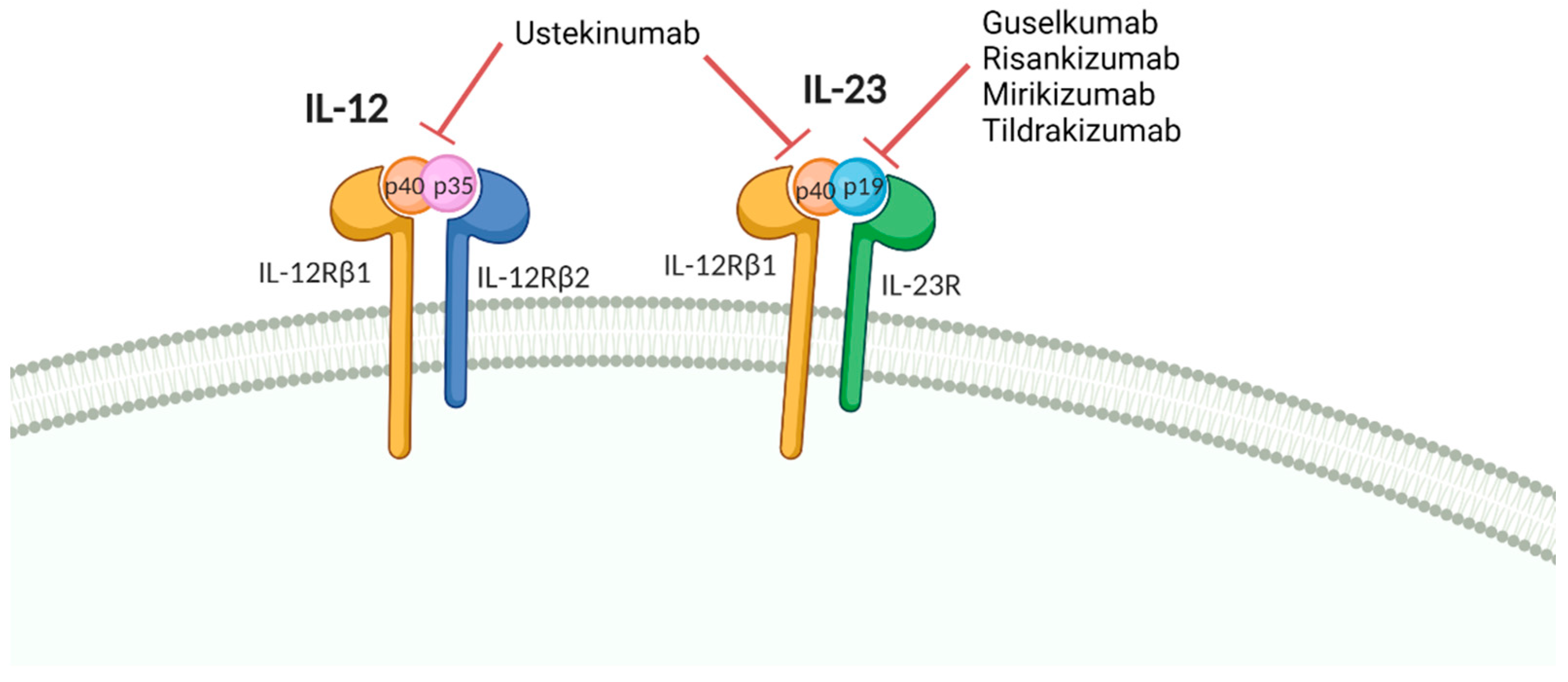
6. Vaccine Response in Patients Treated with IL12/23 and IL-23 Inhibitors
7. SARS-CoV-2 Vaccination in IMID Patients
7.1. Humoral Responses to SARS-CoV-2 in IMIDs
7.2. Cellular Responses to SARS-CoV-2 in IMIDs
7.3. Revaccination and New Variants
7.4. Vaccine Protection against SARS-CoV-2 Infection in IMIDs
7.5. Safety of SARS-CoV-2 Vaccines in IMIDs
7.6. Guidelines on SARS-CoV-2 Vaccination in IMIDs
7.7. Evidence Summary on SARS-CoV-2 Vaccination in IMIDs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- David, T.; Ling, S.F.; Barton, A. Genetics of immune-mediated inflammatory diseases. Clin. Exp. Immunol. 2018, 193, 3–12. [Google Scholar] [CrossRef]
- Wisniewski, A.; Kirchgesner, J.; Seksik, P.; Landman, C.; Bourrier, A.; Nion-Larmurier, I.; Marteau, P.; Cosnes, J.; Sokol, H.; Beaugerie, L.; et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur. Gastroenterol. J. 2020, 8, 303–313. [Google Scholar] [CrossRef]
- Jani, M.; Barton, A.; Hyrich, K. Prediction of infection risk in rheumatoid arthritis patients treated with biologics: Are we any closer to risk stratification? Curr. Opin. Rheumatol. 2019, 31, 285–292. [Google Scholar] [CrossRef]
- Yiu, Z.Z.N.; Sorbe, C.; Lunt, M.; Rustenbach, S.J.; Kuhl, L.; Augustin, M.; Mason, K.J.; Ashcroft, D.M.; Griffiths, C.E.M.; Warren, R.B.; et al. Development and validation of a multivariable risk prediction model for serious infection in patients with psoriasis receiving systemic therapy. Br. J. Dermatol. 2019, 180, 894–901. [Google Scholar] [CrossRef]
- Singh, J.A.; Guyatt, G.; Ogdie, A.; Gladman, D.D.; Deal, C.; Deodhar, A.; Dubreuil, M.; Dunham, J.; Husni, M.E.; Kenny, S.; et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res. 2019, 71, 2–29. [Google Scholar] [CrossRef]
- Menter, A.; Strober, B.E.; Kaplan, D.H.; Kivelevitch, D.; Prater, E.F.; Stoff, B.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 2019, 80, 1029–1072. [Google Scholar] [CrossRef]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Farraye, F.A.; Melmed, G.Y.; Lichtenstein, G.R.; Kane, S.V. ACG Clinical Guideline: Preventive care in inflammatory bowel disease. Am. J. Gastroenterol. 2017, 112, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Ehehalt, R.; Esteve, M.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Parvez, S. COVID-19: An overview of the current pharmacological interventions, vaccines, and clinical trials. Biochem. Pharmacol. 2020, 180, 114184. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 vaccine pipeline: An overview. Curr. Trop. Med. Rep. 2020, 7, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, K.W.; Li, L.; Peng, M.; Paris, M.; Shah, K.; Jick, S.S. Rates of cancers and opportunistic infections in patients with psoriatic arthritis compared with patients without psoriatic arthritis. J. Clin. Rheumatol. 2016, 22, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, A.; Navabi, S.; Williams, E.D.; Liu, G.; Kong, L.; Coates, M.D.; Clarke, K. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2019, 25, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Hudson, M.; Suissa, S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology 2007, 46, 1157–1160. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ahmed, M.; Conway, R.; Carey, J.J. Risk of infection with methotrexate therapy in inflammatory diseases: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 15. [Google Scholar] [CrossRef]
- Mazaud, C.; Fardet, L. Relative risk of and determinants for adverse events of methotrexate prescribed at a low dose: A systematic review and meta-analysis of randomized placebo-controlled trials. Br. J. Dermatol. 2017, 177, 978–986. [Google Scholar] [CrossRef]
- Baddley, J.W.; Cantini, F.; Goletti, D.; Gomez-Reino, J.J.; Mylonakis, E.; San-Juan, R.; Fernandez-Ruiz, M.; Torre-Cisneros, J. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (soluble immune effector molecules [I]: Anti-tumor necrosis factor-alpha agents). Clin. Microbiol. Infect. 2018, 24 (Suppl. S2), S10–S20. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Mariette, X.; Silva, J.T.; Benamu, E.; Calabrese, L.H.; Dumusc, A.; Smolen, J.S.; Aguado, J.M.; Fernandez-Ruiz, M. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (soluble immune effector molecules [II]: Agents targeting interleukins, immunoglobulins and complement factors). Clin. Microbiol. Infect. 2018, 24 (Suppl. S2), S21–S40. [Google Scholar]
- Gao, Q.; Zhao, Y.-X.; Wang, X.-J.; Shi, J.; Wang, H.-M. Efficacy and safety of IL-17 inhibitors for patients with psoriatic arthritis: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2958–2970. [Google Scholar]
- Redelman-Sidi, G.; Michielin, O.; Cervera, C.; Ribi, C.; Aguado, J.M.; Fernandez-Ruiz, M.; Manuel, O. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors). Clin. Microbiol. Infect. 2018, 24 (Suppl. S2), S95–S107. [Google Scholar]
- Mikulska, M.; Lanini, S.; Gudiol, C.; Drgona, L.; Ippolito, G.; Fernandez-Ruiz, M.; Salzberger, B. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin. Microbiol. Infect. 2018, 24 (Suppl. S2), S71–S82. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Armstrong, A.W.; Bell, S.; Anesi, G.L.; Blauvelt, A.; Calabrese, C.; Dommasch, E.D.; Feldman, S.R.; Gladman, D.; Kircik, L.; et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: Version 2-Advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J. Am. Acad. Dermatol. 2021, 84, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Bellinato, F.; Chiricozzi, A.; Girolomoni, G. The risk of COVID-19 pandemic in patients with moderate to severe plaque psoriasis receiving systemic treatments. Vaccines 2020, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Maconi, G.; Bosetti, C.; De Monti, A.; Boyapati, R.K.; Shelton, E.; Piazza, N.; Carvalhas Gabrielli, A.M.; Lenti, M.V.; Bezzio, C.; Ricci, C.; et al. Risk of COVID 19 in patients with inflammatory bowel diseases compared to a control population. Dig. Liver Dis. 2021, 53, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.A.; Melmed, G.Y.; McGovern, D.P.; Rai, V.; Krammer, F.; Rubin, D.T.; Abreu, M.T.; Dubinsky, M.C.; International Organization for the Study of Inflammatory Bowel Disease. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: Recommendations from an international consensus meeting. Gut 2021, 70, 635–640. [Google Scholar] [CrossRef]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology 2020, 159, 481–491.e483. [Google Scholar] [CrossRef]
- Mahil, S.K.; Dand, N.; Mason, K.J.; Yiu, Z.Z.N.; Tsakok, T.; Meynell, F.; Coker, B.; McAteer, H.; Moorhead, L.; Mackenzie, T.; et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis-insights from a global registry-based study. J. Allergy Clin. Immunol. 2021, 147, 60–71. [Google Scholar] [CrossRef]
- FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE Consortium and Contributors. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: Data from the French RMD COVID-19 cohort of 694 patients. Ann. Rheum. Dis. 2020, 80, 527–538. [Google Scholar]
- Gianfrancesco, M.; Hyrich, K.L.; Al-Adely, S.; Carmona, L.; Danila, M.I.; Gossec, L.; Izadi, Z.; Jacobsohn, L.; Katz, P.; Lawson-Tovey, S.; et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020, 79, 859–866. [Google Scholar] [CrossRef]
- Singh, A.K.; Jena, A.; Kumar, M.P.; Sharma, V.; Sebastian, S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2021, 9, 159–176. [Google Scholar] [CrossRef]
- Akiyama, S.; Hamdeh, S.; Micic, D.; Sakuraba, A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: A systematic review and meta-analysis. Ann. Rheum. Dis. 2021, 80, 384–391. [Google Scholar] [CrossRef]
- Schulze-Koops, H.; Krueger, K.; Vallbracht, I.; Hasseli, R.; Skapenko, A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann. Rheum. Dis. 2021, 80, e67. [Google Scholar] [CrossRef] [PubMed]
- Bossa, F.; Carparelli, S.; Latiano, A.; Palmieri, O.; Tavano, F.; Panza, A.; Pastore, M.; Marseglia, A.; D’Altilia, M.; Latiano, T.; et al. Impact of the COVID-19 outbreak and the serum prevalence of SARS-CoV-2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig. Liver Dis. 2021, 53, 277–282. [Google Scholar] [CrossRef]
- Michelena, X.; Borrell, H.; Lopez-Corbeto, M.; Lopez-Lasanta, M.; Moreno, E.; Pascual-Pastor, M.; Erra, A.; Serrat, M.; Espartal, E.; Anton, S.; et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin. Arthritis Rheum. 2020, 50, 564–570. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Systematic review on inflammatory bowel disease patients with coronavirus disease 2019: It is time to take stock. Clin. Gastroenterol. Hepatol. 2020, 18, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Gualdi, G.; Amerio, P. Effect of immunosuppressive drugs in immune-mediated inflammatory disease during the coronavirus pandemic. Dermatol. Ther. 2020, 33, e14204. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Gisondi, P.; Bellinato, F.; Girolomoni, G. Immune response to vaccination in patients with psoriasis treated with systemic therapies. Vaccines 2020, 8, 769. [Google Scholar] [CrossRef]
- Caso, F.; Ramonda, R.; Del Puente, A.; Darda, M.A.; Cantarini, L.; Peluso, R.; Esposito, C.; Ortolan, A.; Fiocco, U.; Punzi, L.; et al. Influenza vaccine with adjuvant on disease activity in psoriatic arthritis patients under anti-TNF-alpha therapy. Clin. Exp. Rheumatol. 2016, 34, 507–512. [Google Scholar]
- Kapetanovic, M.C.; Roseman, C.; Jönsson, G.; Truedsson, L.; Saxne, T.; Geborek, P. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum. 2011, 63, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Implications for management. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef]
- Carrascosa, J.M.; Galan, M.; de Lucas, R.; Perez-Ferriols, A.; Ribera, M.; Yanguas, I. Expert recommendations on treating psoriasis in special circumstances (part II). Actas Dermosifiliogr. 2016, 107, 712–729. [Google Scholar] [CrossRef] [PubMed]
- García-Serrano, C.; Mirada, G.; Marsal, J.R.; Ortega, M.; Sol, J.; Solano, R.; Artigues, E.M.; Estany, P. Compliance with the guidelines on recommended immunization schedule in patients with inflammatory bowel disease: Implications on public health policies. BMC Public Health 2020, 20, 713. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Jiskoot, W.; Kersten, G.F.A.; Mastrobattista, E.; Slutter, B. Vaccines. In Pharmaceutical Biotechnology: Fundamentals and Applications, 5th ed.; Commelin, D.J.A., Sindelar, R.D., Meibohm, B., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 281–304. [Google Scholar]
- Slifka, M.K.; Amanna, I.J. Role of multivalency and antigenic threshold in generating protective antibody responses. Front. Immunol. 2019, 10, 956. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Olberg, H.K.; Cox, R.J.; Nostbakken, J.K.; Aarseth, J.H.; Vedeler, C.A.; Myhr, K.M. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: An explorative study. Mult. Scler. 2014, 20, 1074–1080. [Google Scholar] [CrossRef]
- Olberg, H.K.; Eide, G.E.; Cox, R.J.; Jul-Larsen, A.; Lartey, S.L.; Vedeler, C.A.; Myhr, K.M. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur. J. Neurol. 2018, 25, 527–534. [Google Scholar] [CrossRef]
- Kaufman, M.; Pardo, G.; Rossman, H.; Sweetser, M.T.; Forrestal, F.; Duda, P. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2014, 341, 22–27. [Google Scholar] [CrossRef]
- Caldera, F.; Hillman, L.; Saha, S.; Wald, A.; Grimes, I.; Zhang, Y.; Sharpe, A.R.; Reichelderfer, M.; Hayney, M.S. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: A randomized clinical trial. Inflamm. Bowel Dis. 2020, 26, 593–602. [Google Scholar] [CrossRef]
- Wyant, T.; Leach, T.; Sankoh, S.; Wang, Y.; Paolino, J.; Pasetti, M.F.; Feagan, B.G.; Parikh, A. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: Randomised controlled trial results. Gut 2015, 64, 77–83. [Google Scholar] [CrossRef]
- Migita, K.; Akeda, Y.; Akazawa, M.; Tohma, S.; Hirano, F.; Ideguchi, H.; Kozuru, H.; Jiuchi, Y.; Matsumura, R.; Suematsu, E.; et al. Effect of abatacept on the immunogenicity of 23-valent pneumococcal polysaccharide vaccination (PPSV23) in rheumatoid arthritis patients. Arthritis Res. Ther. 2015, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Alten, R.; Bingham, C.O., 3rd; Cohen, S.B.; Curtis, J.R.; Kelly, S.; Wong, D.; Genovese, M.C. Antibody response to pneumococcal and influenza vaccination in patients with rheumatoid arthritis receiving abatacept. BMC Musculoskelet. Disord. 2016, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Crnkic Kapetanovic, M.; Saxne, T.; Jonsson, G.; Truedsson, L.; Geborek, P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R171. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Laurindo, I.M.; Guedes, L.K.; Saad, C.G.; Moraes, J.C.; Silva, C.A.; Bonfa, E. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res. 2013, 65, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Bingham, C.O., 3rd; Looney, R.J.; Deodhar, A.; Halsey, N.; Greenwald, M.; Codding, C.; Trzaskoma, B.; Martin, F.; Agarwal, S.; Kelman, A. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheum. 2010, 62, 64–74. [Google Scholar] [CrossRef]
- Richi, P.; Yuste, J.; Navío, T.; González-Hombrado, L.; Salido, M.; Thuissard-Vasallo, I.; Jiménez-Díaz, A.; Llorente, J.; Cebrián, L.; Lojo, L.; et al. Impact of biological therapies on the immune response after pneumococcal vaccination in patients with autoimmune inflammatory diseases. Vaccines 2021, 9, 203. [Google Scholar] [CrossRef]
- Richi, P.; Alonso, O.; Martin, M.D.; Gonzalez-Hombrado, L.; Navio, T.; Salido, M.; Llorente, J.; Andreu-Vazquez, C.; Garcia-Fernandez, C.; Jimenez-Diaz, A.; et al. Evaluation of the immune response to hepatitis B vaccine in patients on biological therapy: Results of the RIER cohort study. Clin. Rheumatol. 2020, 39, 2751–2756. [Google Scholar] [CrossRef]
- Arad, U.; Tzadok, S.; Amir, S.; Mandelboim, M.; Mendelson, E.; Wigler, I.; Sarbagil-Maman, H.; Paran, D.; Caspi, D.; Elkayam, O. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine 2011, 29, 1643–1648. [Google Scholar] [CrossRef]
- Oren, S.; Mandelboim, M.; Braun-Moscovici, Y.; Paran, D.; Ablin, J.; Litinsky, I.; Comaneshter, D.; Levartovsky, D.; Mendelson, E.; Azar, R.; et al. Vaccination against influenza in patients with rheumatoid arthritis: The effect of rituximab on the humoral response. Ann. Rheum. Dis. 2008, 67, 937–941. [Google Scholar] [CrossRef]
- Van Assen, S.; Holvast, A.; Benne, C.A.; Posthumus, M.D.; van Leeuwen, M.A.; Voskuyl, A.E.; Blom, M.; Risselada, A.P.; de Haan, A.; Westra, J.; et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010, 62, 75–81. [Google Scholar] [CrossRef]
- Westra, J.; van Assen, S.; Wilting, K.R.; Land, J.; Horst, G.; de Haan, A.; Bijl, M. Rituximab impairs immunoglobulin (Ig)M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients. Clin. Exp. Immunol. 2014, 178, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Richi, P.; Martin, M.D.; Navio, M.T.; Gonzalez-Hombrado, L.; Salido, M.; Llorente, J.; Thuissard-Vasallo, I.; Alcocer, P.; Saa-Requejo, C.M.; Jimenez-Diaz, A.; et al. Antibody responses to influenza vaccine in patients on biological therapy: Results of RIER cohort study. Med. Clin. 2019, 153, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, M.C.; Kristensen, L.E.; Saxne, T.; Aktas, T.; Morner, A.; Geborek, P. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis Res. Ther. 2014, 16, R2. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Krivine, A.; Weix, J.; Rozenberg, F.; Launay, O.; Huesler, J.; Guillevin, L.; Villiger, P.M. Protective effect of A/H1N1 vaccination in immune-mediated disease—A prospectively controlled vaccination study. Rheumatology 2012, 51, 695–700. [Google Scholar] [CrossRef]
- Nagel, J.; Saxne, T.; Geborek, P.; Bengtsson, A.A.; Jacobsen, S.; Svaerke Joergensen, C.; Nilsson, J.A.; Skattum, L.; Jonsen, A.; Kapetanovic, M.C. Treatment with belimumab in systemic lupus erythematosus does not impair antibody response to 13-valent pneumococcal conjugate vaccine. Lupus 2017, 26, 1072–1081. [Google Scholar] [CrossRef]
- Chatham, W.; Chadha, A.; Fettiplace, J.; Kleoudis, C.; Bass, D.; Roth, D.; Gordon, D. A randomized, open-label study to investigate the effect of belimumab on pneumococcal vaccination in patients with active, autoantibody-positive systemic lupus erythematosus. Lupus 2017, 26, 1483–1490. [Google Scholar] [CrossRef]
- Salinas, G.F.; De Rycke, L.; Barendregt, B.; Paramarta, J.E.; Hreggvidsdottir, H.; Cantaert, T.; van der Burg, M.; Tak, P.P.; Baeten, D. Anti-TNF treatment blocks the induction of T cell-dependent humoral responses. Ann. Rheum. Dis. 2013, 72, 1037–1043. [Google Scholar] [CrossRef]
- Mease, P.J.; Ritchlin, C.T.; Martin, R.W.; Gottlieb, A.B.; Baumgartner, S.W.; Burge, D.J.; Whitmore, J.B. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J. Rheumatol. 2004, 31, 1356–1361. [Google Scholar]
- Kapetanovic, M.C.; Saxne, T.; Sjoholm, A.; Truedsson, L.; Jonsson, G.; Geborek, P. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology 2006, 45, 106–111. [Google Scholar] [CrossRef]
- Kivitz, A.J.; Schechtman, J.; Texter, M.; Fichtner, A.; de Longueville, M.; Chartash, E.K. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: Results from a single-blind randomized phase IV trial. J. Rheumatol. 2014, 41, 648–657. [Google Scholar] [CrossRef]
- Kaine, J.L.; Kivitz, A.J.; Birbara, C.; Luo, A.Y. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J. Rheumatol. 2007, 34, 272–279. [Google Scholar] [PubMed]
- Visvanathan, S.; Keenan, G.F.; Baker, D.G.; Levinson, A.I.; Wagner, C.L. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J. Rheumatol. 2007, 34, 952–957. [Google Scholar] [PubMed]
- Kantso, B.; Halkjaer, S.I.; Thomsen, O.O.; Belard, E.; Gottschalck, I.B.; Jorgensen, C.S.; Krogfelt, K.A.; Slotved, H.C.; Ingels, H.; Petersen, A.M. Immunosuppressive drugs impairs antibody response of the polysaccharide and conjugated pneumococcal vaccines in patients with Crohn’s disease. Vaccine 2015, 33, 5464–5469. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Kim, H.S.; Ye, B.D.; Lee, K.M.; Kim, Y.S.; Rhee, S.Y.; Kim, H.J.; Yang, S.K.; Moon, W.; Koo, J.S.; et al. Patients with Crohn’s disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J. Crohns Colitis 2014, 8, 384–391. [Google Scholar] [CrossRef]
- Fiorino, G.; Peyrin-Biroulet, L.; Naccarato, P.; Szabo, H.; Sociale, O.R.; Vetrano, S.; Fries, W.; Montanelli, A.; Repici, A.; Malesci, A.; et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: A prospective study. Inflamm. Bowel Dis. 2012, 18, 1042–1047. [Google Scholar] [CrossRef]
- Van Aalst, M.; Garcia Garrido, H.M.; van der Leun, J.; Meek, B.; van Leeuwen, E.M.M.; Löwenberg, M.; D’Haens, G.R.; Ponsioen, C.Y.I.; Grobusch, M.P.; Goorhuis, A. Immunogenicity of the currently recommended pneumococcal vaccination schedule in patients with inflammatory bowel disease. Clin. Infect. Dis. 2020, 70, 595–604. [Google Scholar] [CrossRef]
- Rakoczi, E.; Perge, B.; Vegh, E.; Csomor, P.; Pusztai, A.; Szamosi, S.; Bodnar, N.; Szanto, S.; Szucs, G.; Szekanecz, Z. Evaluation of the immunogenicity of the 13-valent conjugated pneumococcal vaccine in rheumatoid arthritis patients treated with etanercept. Jt. Bone Spine 2016, 83, 675–679. [Google Scholar] [CrossRef]
- Andrade, P.; Santos-Antunes, J.; Rodrigues, S.; Lopes, S.; Macedo, G. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J. Gastroenterol. Hepatol. 2015, 30, 1591–1595. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Villagrasa, J.R.; Rodriguez-Nogueiras, A.; Chaparro, M. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2012, 107, 1460–1466. [Google Scholar] [CrossRef]
- Belle, A.; Baumann, C.; Bigard, M.A.; Zallot, C.; Gizard, E.; Gueant, J.L.; Bronowicki, J.P.; Peyrin-Biroulet, L. Impact of immunosuppressive therapy on hepatitis B vaccination in inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 2015, 27, 877–881. [Google Scholar] [CrossRef]
- Askling, H.H.; Rombo, L.; van Vollenhoven, R.; Hallen, I.; Thorner, A.; Nordin, M.; Herzog, C.; Kantele, A. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: A prospective, open-label, multi-centre study. Travel Med. Infect. Dis. 2014, 12, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, O.; Bashkin, A.; Mandelboim, M.; Litinsky, I.; Comaheshter, D.; Levartovsky, D.; Mendelson, E.; Wigler, I.; Caspi, D.; Paran, D. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin. Arthritis Rheum. 2010, 39, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Kobie, J.J.; Zheng, B.; Bryk, P.; Barnes, M.; Ritchlin, C.T.; Tabechian, D.A.; Anandarajah, A.P.; Looney, R.J.; Thiele, R.G.; Anolik, J.H.; et al. Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis Res. Ther. 2011, 13, R209. [Google Scholar] [CrossRef] [PubMed]
- Franca, I.L.; Ribeiro, A.C.; Aikawa, N.E.; Saad, C.G.; Moraes, J.C.; Goldstein-Schainberg, C.; Laurindo, I.M.; Precioso, A.R.; Ishida, M.A.; Sartori, A.M.; et al. TNF blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology 2012, 51, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Fomin, I.; Caspi, D.; Levy, V.; Varsano, N.; Shalev, Y.; Paran, D.; Levartovsky, D.; Litinsky, I.; Kaufman, I.; Wigler, I.; et al. Vaccination against influenza in rheumatoid arthritis: The effect of disease modifying drugs, including TNF alpha blockers. Ann. Rheum. Dis. 2006, 65, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Nii, T.; Nanki, T.; Kohsaka, H.; Harigai, M.; Komano, Y.; Sugihara, T.; Nonomura, Y.; Hirose, W.; Nagasaka, K.; et al. Anti-tumor necrosis factor therapy does not diminish the immune response to influenza vaccine in Japanese patients with rheumatoid arthritis. Mod. Rheumatol. 2007, 17, 531–533. [Google Scholar] [CrossRef]
- Polachek, A.; Korobko, U.; Mader-Balakirski, N.; Arad, U.; Levartovsky, D.; Kaufman, I.; Anouk, M.; Litinsky, I.; Wigler, I.; Mendelson, E.; et al. Immunogenicity and safety of vaccination against seasonal 2012 influenza virus among patients with psoriatic arthritis and psoriasis. Clin. Exp. Rheumatol. 2015, 33, 181–186. [Google Scholar]
- Hagihara, Y.; Ohfuji, S.; Watanabe, K.; Yamagami, H.; Fukushima, W.; Maeda, K.; Kamata, N.; Sogawa, M.; Shiba, M.; Tanigawa, T.; et al. Infliximab and/or immunomodulators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J. Crohns Colitis 2014, 8, 223–233. [Google Scholar] [CrossRef]
- Andrisani, G.; Frasca, D.; Romero, M.; Armuzzi, A.; Felice, C.; Marzo, M.; Pugliese, D.; Papa, A.; Mocci, G.; De Vitis, I.; et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-alpha agents: Effects of combined therapy with immunosuppressants. J. Crohns Colitis 2013, 7, 301–307. [Google Scholar] [CrossRef]
- Launay, O.; Abitbol, V.; Krivine, A.; Slama, L.B.; Bourreille, A.; Dupas, J.L.; Hebuterne, X.; Savoye, G.; Deplanque, D.; Bouhnik, Y.; et al. Immunogenicity and safety of influenza vaccine in inflammatory bowel disease patients treated or not with immunomodulators and/or biologics: A two-year prospective study. J. Crohns Colitis 2015, 9, 1096–1107. [Google Scholar] [CrossRef]
- Frasca, D.; Andrisani, G.; Diaz, A.; Felice, C.; Guidi, L.; Blomberg, B.B. AID in aging and autoimmune diseases. Autoimmunity 2013, 46, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gelinck, L.B.; van den Bemt, B.J.; Marijt, W.A.; van der Bijl, A.E.; Visser, L.G.; Cats, H.A.; Rimmelzwaan, G.F.; Kroon, F.P. Intradermal influenza vaccination in immunocompromized patients is immunogenic and feasible. Vaccine 2009, 27, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Gelinck, L.B.; van der Bijl, A.E.; Beyer, W.E.; Visser, L.G.; Huizinga, T.W.; van Hogezand, R.A.; Rimmelzwaan, G.F.; Kroon, F.P. The effect of anti-tumour necrosis factor alpha treatment on the antibody response to influenza vaccination. Ann. Rheum. Dis. 2008, 67, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Borte, S.; Liebert, U.G.; Borte, M.; Sack, U. Efficacy of measles, mumps and rubella revaccination in children with juvenile idiopathic arthritis treated with methotrexate and etanercept. Rheumatology 2009, 48, 144–148. [Google Scholar] [CrossRef]
- Scheinberg, M.; Guedes-Barbosa, L.S.; Mangueira, C.; Rosseto, E.A.; Mota, L.; Oliveira, A.C.; Lima, R.A. Yellow fever revaccination during infliximab therapy. Arthritis Care Res. 2010, 62, 896–898. [Google Scholar] [CrossRef]
- Chioato, A.; Noseda, E.; Stevens, M.; Gaitatzis, N.; Kleinschmidt, A.; Picaud, H. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: Results of an open-label, parallel-group, randomized single-center study. Clin. Vaccine Immunol. 2012, 19, 1597–1602. [Google Scholar] [CrossRef][Green Version]
- Richi, P.; Martín, M.; de Ory, F.; Gutiérrez-Larraya, R.; Casas, I.; Jiménez-Díaz, A.M.; Cava, F.; Muñoz-Fernández, S. Secukinumab does not impair the immunogenic response to the influenza vaccine in patients. RMD Open 2019, 5, e001018. [Google Scholar] [CrossRef]
- Gomez, E.V.; Bishop, J.L.; Jackson, K.; Muram, T.M.; Phillips, D. Response to tetanus and pneumococcal vaccination following administration of ixekizumab in healthy participants. BioDrugs 2017, 31, 545–554. [Google Scholar] [CrossRef]
- Bingham, C.O., 3rd; Rizzo, W.; Kivitz, A.; Hassanali, A.; Upmanyu, R.; Klearman, M. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: Results of a randomised controlled trial (VISARA). Ann. Rheum. Dis. 2015, 74, 818–822. [Google Scholar] [CrossRef]
- Mori, S.; Ueki, Y.; Akeda, Y.; Hirakata, N.; Oribe, M.; Shiohira, Y.; Hidaka, T.; Oishi, K. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann. Rheum. Dis. 2013, 72, 1362–1366. [Google Scholar] [CrossRef]
- Tsuru, T.; Terao, K.; Murakami, M.; Matsutani, T.; Suzaki, M.; Amamoto, T.; Nakashima, H.; Akiyama, A.; Nishimoto, N. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod. Rheumatol. 2014, 24, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Ueki, Y.; Hirakata, N.; Oribe, M.; Hidaka, T.; Oishi, K. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 2006–2010. [Google Scholar] [CrossRef] [PubMed]
- Shinoki, T.; Hara, R.; Kaneko, U.; Miyamae, T.; Imagawa, T.; Mori, M.; Yokota, S. Safety and response to influenza vaccine in patients with systemic-onset juvenile idiopathic arthritis receiving tocilizumab. Mod. Rheumatol. 2012, 22, 871–876. [Google Scholar] [CrossRef]
- Brodmerkel, C.; Wadman, E.; Langley, R.G.; Papp, K.A.; Bourcier, M.; Poulin, Y.; Ho, V.; Guenther, L.; Kunynetz, R.; Nigen, S.; et al. Immune response to pneumococcus and tetanus toxoid in patients with moderate-to-severe psoriasis following long-term ustekinumab use. J. Drugs Dermatol. 2013, 12, 1122–1129. [Google Scholar] [PubMed]
- Brodmerkel, C.; Zhu, Y.; Jiao, Q.; Cornacoff, J.; Treacy, G.; Mascelli, M.A.; Gottlieb, A.B. Effects of ustekinumab administration on primate/human antigen-recall and humoral immune response functions. J. Drugs Dermatol. 2010, 9, 677–683. [Google Scholar]
- Doornekamp, L.; Goetgebuer, R.L.; Schmitz, K.S.; Goeijenbier, M.; van der Woude, C.J.; Fouchier, R.; van Gorp, E.C.M.; de Vries, A.C. High immunogenicity to influenza vaccination in Crohn’s disease patients treated with ustekinumab. Vaccines 2020, 8, 455. [Google Scholar] [CrossRef]
- Ley, K.; Rivera-Nieves, J.; Sandborn, W.J.; Shattil, S. Integrin-based therapeutics: Biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 2016, 15, 173–183. [Google Scholar] [CrossRef]
- Keating, G.M. Abatacept: A review of its use in the management of rheumatoid arthritis. Drugs 2013, 73, 1095–1119. [Google Scholar] [CrossRef]
- Brunner, H.I.; Tzaribachev, N.; Cornejo, G.V.; Joos, R.; Gervais, E.; Cimaz, R.; Calvo Penades, I.; Cuttica, R.; Lutz, T.; Quartier, P.; et al. Maintenance of antibody response to diphtheria/tetanus vaccine in patients aged 2-5 years with polyarticular-course juvenile idiopathic arthritis receiving subcutaneous abatacept. Pediatr. Rheumatol. Online J. 2020, 18, 19. [Google Scholar] [CrossRef]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef]
- Baker, K.F.; Isaacs, J.D. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 2018, 77, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, Y.; Sharma, A. Antibody-based therapeutics in inflammatory diseases. In Pharmaceutical Biotechnology: Fundamentals and Applications, 5th ed.; Commelin, D.J.A., Sindelar, R.D., Meibohm, B., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 557–617. [Google Scholar]
- Wise, L.M.; Stohl, W. Belimumab and rituximab in systemic lupus erythematosus: A tale of two B cell-targeting agents. Front. Med. 2020, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Sadun, R.E.; et al. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: Version 1. Arthritis Rheumatol. 2021, 73, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Haraoui, B.; Kumar, D.; Marshall, J.K.; Bissonnette, R.; Bitton, A.; Bressler, B.; Gooderham, M.; Ho, V.; Jamal, S.; et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J. Cutan Med. Surg. 2019, 23, 50–74. [Google Scholar] [CrossRef]
- Mackay, F.; Browning, J.L. BAFF: A fundamental survival factor for B cells. Nat. Rev. Immunol. 2002, 2, 465–475. [Google Scholar] [CrossRef]
- Calero, I.; Sanz, I. Targeting B cells for the treatment of SLE: The beginning of the end or the end of the beginning? Discov. Med. 2010, 10, 416–424. [Google Scholar]
- Chatham, W.W.; Wallace, D.J.; Stohl, W.; Latinis, K.M.; Manzi, S.; McCune, W.J.; Tegzova, D.; McKay, J.D.; Avila-Armengol, H.E.; Utset, T.O.; et al. Effect of belimumab on vaccine antigen antibodies to influenza, pneumococcal, and tetanus vaccines in patients with systemic lupus erythematosus in the BLISS-76 trial. J. Rheumatol. 2012, 39, 1632–1640. [Google Scholar] [CrossRef]
- Steeland, S.; Libert, C.; Vandenbroucke, R.E. A new venue of TNF targeting. Int. J. Mol. Sci. 2018, 19, 1442. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Hua, C.; Barnetche, T.; Combe, B.; Morel, J. Effect of methotrexate, anti-tumor necrosis factor alpha, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care Res. 2014, 66, 1016–1026. [Google Scholar] [CrossRef]
- Subesinghe, S.; Bechman, K.; Rutherford, A.I.; Goldblatt, D.; Galloway, J.B. A systematic review and metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J. Rheumatol. 2018, 45, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Van Aalst, M.; Langedijk, A.C.; Spijker, R.; de Bree, G.J.; Grobusch, M.P.; Goorhuis, A. The effect of immunosuppressive agents on immunogenicity of pneumococcal vaccination: A systematic review and meta-analysis. Vaccine 2018, 36, 5832–5845. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, J.; Fonseca, K.; Ghosh, S.; Panaccione, R.; Gasia, M.F.; Ueno, A.; Kaplan, G.G.; Seow, C.H.; Wrobel, I. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: A randomized trial. Inflamm. Bowel Dis. 2016, 22, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Gelinck, L.B.; van der Bijl, A.E.; Visser, L.G.; Huizinga, T.W.; van Hogezand, R.A.; Rijkers, G.T.; Kroon, F.P. Synergistic immunosuppressive effect of anti-TNF combined with methotrexate on antibody responses to the 23 valent pneumococcal polysaccharide vaccine. Vaccine 2008, 26, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, F.; Delzell, E.; Chen, L.; Winthrop, K.L.; Lewis, J.D.; Saag, K.G.; Baddley, J.W.; Curtis, J.R. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012, 308, 43–49. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Richi, P.; Martin, M.D.; Andreu-Vazquez, C.; Jimenez-Diaz, A.; Steiner, M.; Munoz-Fernandez, S. Serological response to influenza vaccine in patients with autoimmune inflammatory diseases: Results of RIER study. Med. Clin. 2021, 156, 118–122. [Google Scholar] [CrossRef]
- Brogan, P.; Hofer, M.; Kuemmerle-Deschner, J.B.; Lauwerys, B.R.; Speziale, A.; Wei, X.; Laxer, R. Effectiveness of childhood vaccinations in CAPS patients treated with canakinumab: Results from an open-label phase III extension study [abstract THU0495]. Arthritis Rheum. 2015, 69, 393. [Google Scholar]
- Valeri, M.; Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 2016, 74, ftw111. [Google Scholar] [CrossRef]
- Iwakura, Y.; Nakae, S.; Saijo, S.; Ishigame, H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 2008, 226, 57–79. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kugyelka, R.; Kohl, Z.; Olasz, K.; Mikecz, K.; Rauch, T.A.; Glant, T.T.; Boldizsar, F. Enigma of IL-17 and Th17 cells in rheumatoid arthritis and in autoimmune animal models of arthritis. Mediat. Inflamm. 2016, 2016, 6145810. [Google Scholar] [CrossRef] [PubMed]
- Soare, A.; Weber, S.; Maul, L.; Rauber, S.; Gheorghiu, A.M.; Luber, M.; Houssni, I.; Kleyer, A.; von Pickardt, G.; Gado, M.; et al. Cutting edge: Homeostasis of innate lymphoid cells is imbalanced in psoriatic arthritis. J. Immunol. 2018, 200, 1249–1254. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Fauny, M.; Moulin, D.; D’Amico, F.; Netter, P.; Petitpain, N.; Arnone, D.; Jouzeau, J.Y.; Loeuille, D.; Peyrin-Biroulet, L. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 2020, 79, 1132–1138. [Google Scholar] [CrossRef]
- Favalli, E.G. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: A comprehensive review of IL-6 Inhibition for the management of rheumatoid arthritis. Rheumatol. Ther. 2020, 7, 473–516. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The immunobiology of the interleukin-12 family: Room for discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Kobayashi, M.; Fitz, L.; Ryan, M.; Hewick, R.M.; Clark, S.C.; Chan, S.; Loudon, R.; Sherman, F.; Perussia, B.; Trinchieri, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989, 170, 827–845. [Google Scholar] [CrossRef]
- Manetti, R.; Parronchi, P.; Giudizi, M.G.; Piccinni, M.P.; Maggi, E.; Trinchieri, G.; Romagnani, S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993, 177, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Floss, D.M.; Moll, J.M.; Scheller, J. IL-12 and IL-23-close relatives with structural homologies but distinct immunological functions. Cells 2020, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, N.; Bustamante, J.; Bourdery, L.; Bentebibel, S.E.; Boisson-Dupuis, S.; Hamlin, F.; Tran, M.V.; Blankenship, D.; Pascual, V.; Savino, D.A.; et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood 2013, 121, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, N.; Morita, R.; Bourdery, L.; Bentebibel, S.E.; Zurawski, S.M.; Banchereau, J.; Ueno, H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 2009, 31, 158–169. [Google Scholar] [CrossRef] [PubMed]
- De Boer, T.; van Dissel, J.T.; Kuijpers, T.W.; Rimmelzwaan, G.F.; Kroon, F.P.; Ottenhoff, T.H. Influenza virus vaccination induces interleukin-12/23 receptor beta 1 (IL-12/23R beta 1)-independent production of gamma interferon (IFN-gamma) and humoral immunity in patients with genetic deficiencies in IL-12/23R beta 1 or IFN-gamma receptor I. Clin. Vaccine Immunol. 2008, 15, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Barricarte, R.; Markle, J.G.; Ma, C.S.; Deenick, E.K.; Ramírez-Alejo, N.; Mele, F.; Latorre, D.; Mahdaviani, S.A.; Aytekin, C.; Mansouri, D.; et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 2018, 3, eaau6759. [Google Scholar] [CrossRef]
- Van de Vosse, E.; Haverkamp, M.H.; Ramirez-Alejo, N.; Martinez-Gallo, M.; Blancas-Galicia, L.; Metin, A.; Garty, B.Z.; Sun-Tan, C.; Broides, A.; de Paus, R.A.; et al. IL-12Rß1 deficiency: Mutation update and description of the IL12RB1 variation database. Hum. Mutat. 2013, 34, 1329–1339. [Google Scholar] [CrossRef]
- De Beaucoudrey, L.; Samarina, A.; Bustamante, J.; Cobat, A.; Boisson-Dupuis, S.; Feinberg, J.; Al-Muhsen, S.; Janniere, L.; Rose, Y.; de Suremain, M.; et al. Revisiting human IL-12Rbeta1 deficiency: A survey of 141 patients from 30 countries. Medicine 2010, 89, 381–402. [Google Scholar] [CrossRef]
- Odegard, J.M.; Marks, B.R.; DiPlacido, L.D.; Poholek, A.C.; Kono, D.H.; Dong, C.; Flavell, R.A.; Craft, J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J. Exp. Med. 2008, 205, 2873–2886. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Avery, D.T.; Chan, A.; Batten, M.; Bustamante, J.; Boisson-Dupuis, S.; Arkwright, P.D.; Kreins, A.Y.; Averbuch, D.; Engelhard, D.; et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 2012, 119, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Mustafa, F.; Rizvi, T.A.; Loney, T.; Suwaidi, H.A.; Al-Marzouqi, A.H.H.; Eldin, A.K.; Alsabeeha, N.; Adrian, T.E.; Stefanini, C.; et al. SARS-CoV-2/COVID-19: Viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses 2020, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Deepak, P.; Kim, W.; Paley, M.A.; Yang, M.; Carvidi, A.B.; El-Qunni, A.A.; Haile, A.; Huang, K.; Kinnett, B.; Liebeskind, M.J.; et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Simon, D.; Tascilar, K.; Fagni, F.; Kronke, G.; Kleyer, A.; Meder, C.; Atreya, R.; Leppkes, M.; Kremer, A.E.; Ramming, A.; et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann. Rheum. Dis. 2021, 80, 1312–1316. [Google Scholar] [CrossRef]
- Geisen, U.M.; Berner, D.K.; Tran, F.; Sumbul, M.; Vullriede, L.; Ciripoi, M.; Reid, H.M.; Schaffarzyk, A.; Longardt, A.C.; Franzenburg, J.; et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021, 80, 1306–1311. [Google Scholar] [CrossRef]
- Braun-Moscovici, Y.; Kaplan, M.; Braun, M.; Markovits, D.; Giryes, S.; Toledano, K.; Tavor, Y.; Dolnikov, K.; Balbir-Gurman, A. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann. Rheum. Dis. 2021, 80, 1317–1321. [Google Scholar] [CrossRef]
- Rubbert-Roth, A.; Vuilleumier, N.; Ludewig, B.; Schmiedeberg, K.; Haller, C.; von Kempis, J. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol. 2021, 3, e470–e472. [Google Scholar] [CrossRef]
- Bugatti, S.; De Stefano, L.; Balduzzi, S.; Greco, M.I.; Luvaro, T.; Cassaniti, I.; Bogliolo, L.; Mazzucchelli, I.; D’Onofrio, B.; di Lernia, M.; et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann. Rheum Dis. 2021. online ahead of print June 25. [Google Scholar] [CrossRef]
- Al-Janabi, A.; Littlewood, Z.; Griffiths, C.E.M.; Hunter, H.J.A.; Chinoy, H.; Moriarty, C.; Yiu, Z.Z.N.; Warren, R.B. Antibody responses to single-dose SARS-CoV-2 vaccination in patients receiving immunomodulators for immune-mediated inflammatory disease. Br. J. Dermatol. 2021, 185, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Venerito, V.; Stefanizzi, P.; Fornaro, M.; Cacciapaglia, F.; Tafuri, S.; Perniola, S.; Iannone, F.; Lopalco, G. Immunogenicity of BNT162b2 mRNA SARS-CoV-2 vaccine in patients with psoriatic arthritis on TNF inhibitors. RMD Open 2022, 8, e001847. [Google Scholar] [CrossRef]
- Haberman, R.H.; Herati, R.; Simon, D.; Samanovic, M.; Blank, R.B.; Tuen, M.; Koralov, S.B.; Atreya, R.; Tascilar, K.; Allen, J.R.; et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann. Rheum. Dis. 2021, 80, 1339–1344. [Google Scholar] [CrossRef]
- Cristaudo, A.; Graceffa, D.; Pimpinelli, F.; Sperati, F.; Spoletini, G.; Bonifati, C.; Pellini, R.; Lora, V.; Pontone, M.; Di Bella, O.; et al. Immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in psoriasis patients treated with biologic drugs. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mahil, S.K.; Bechman, K.; Raharja, A.; Domingo-Vila, C.; Baudry, D.; Brown, M.A.; Cope, A.P.; Dasandi, T.; Graham, C.; Lechmere, T.; et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: A cohort study. Lancet Rheumatol. 2021, 3, e627–e637. [Google Scholar] [CrossRef]
- Mahil, S.K.; Bechman, K.; Raharja, A.; Domingo-Vila, C.; Baudry, D.; Brown, M.A.; Cope, A.P.; Dasandi, T.; Graham, C.; Khan, H.; et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: A longitudinal cohort study. Lancet Rheumatol. 2022, 4, e42–e52. [Google Scholar] [CrossRef]
- Wong, S.Y.; Dixon, R.; Martinez Pazos, V.; Gnjatic, S.; Colombel, J.F.; Cadwell, K.; Group, I.-I.W. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology 2021, 161, 715–718.e714. [Google Scholar] [CrossRef]
- Classen, J.M.; Muzalyova, A.; Nagl, S.; Fleischmann, C.; Ebigbo, A.; Rommele, C.; Messmann, H.; Schnoy, E. Antibody response to SARS-CoV-2 vaccination in patients with inflammatory bowel disease-results of a single-center cohort study in a tertiary hospital in Germany. Dig. Dis. 2021, 10, 1–9. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Weaver, K.N.; Boccieri, M.; Firestine, A.; Zhang, X.; Long, M.D.; PREVENT-COVID Study Group. Humoral immune response to messenger RNA COVID-19 vaccines among patients with inflammatory bowel disease. Gastroenterology 2021, 161, 1340–1343.e1342. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Weaver, K.N.; Zhang, X.; Dai, X.; Watkins, R.; Adler, J.; Dubinsky, M.C.; Kastl, A.; Bousvaros, A.; Strople, J.A.; et al. Factors affecting initial humoral immune response to SARS-CoV-2 vaccines among patients with inflammatory bowel diseases. Am. J. Gastroenterol. 2021. online ahead of print December 29. [Google Scholar] [CrossRef]
- Charilaou, P.; Tricarico, C.; Battat, R.; Scherl, E.J.; Longman, R.S.; Lukin, D.J. Impact of inflammatory bowel disease therapies on durability of humoral response to SARS-CoV-2 vaccination. Clin. Gastroenterol. Hepatol. 2021. online ahead of print December 9. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Chowdhury, R.; Connolly, C.M.; Werbel, W.A.; Segev, D.L.; Parian, A.M.; IBD Group; Tsipotis, E.; Dudley-Brown, S.; Lazarev, M.; et al. Antibody response six months after SARS-CoV-2 mRNA vaccination in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2022. online ahead of print January 5. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Alfadhli, A.; Alotaibi, K.; Alsahli, A.; Mohammad, H.; Cherian, P.; Al-Khairi, I.; Alphonse Thanaraj, T.; Channanath, A.; et al. Serological response to BNT162b2 and ChAdOx1 nCoV-19 vaccines in patients with inflammatory bowel disease on biologic therapies. Vaccines 2021, 9, 1471. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef]
- Lin, S.; Kennedy, N.A.; Saifuddin, A.; Muñoz Sandoval, D.; Reynolds, C.; Castro Seoane, R.; Kottoor, S.; Pieper, F.; Lin, K.-M.; Butler, D.K.; et al. COVID-19 vaccine-induced antibodies are attenuated and decay rapidly in infliximab treated patients. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Reuken, P.A.; Andreas, N.; Grunert, P.C.; Glockner, S.; Kamradt, T.; Stallmach, A. T cell response after SARS-CoV-2 vaccination in immunocompromised patients with inflammatory bowel disease. J. Crohns Colitis 2021. online ahead of print Aug 11. [Google Scholar] [CrossRef]
- Li, D.; Xu, A.; Mengesha, E.; Elyanow, R.; Gittelman, R.M.; Chapman, H.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Pozdnyakova, V.; et al. The T-cell clonal response to SARS-CoV-2 vaccination in inflammatory bowel disease patients is augmented by anti-TNF therapy and often deficient in antibody-responders. medRxiv 2021. preprint. [Google Scholar]
- Ben-Tov, A.; Banon, T.; Chodick, G.; Kariv, R.; Assa, A.; Gazit, S.; Collaborators of the Maccabi Institute for Research & Innovation COVID-19 Task Force. BNT162b2 messenger RNA COVID-19 vaccine effectiveness in patients with inflammatory bowel disease: Preliminary real-world data during mass vaccination campaign. Gastroenterology 2021, 161, 1715–1717.e1711. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mahmud, N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology 2021, 161, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.L.; Caruso, G.; Trecarichi, A.C.; Micali, G. Safety of SARS-CoV-2 vaccines in psoriatic patients treated with biologics: A real life experience. Dermatol. Ther. 2022, 35, e15177. [Google Scholar] [CrossRef]
- Weaver, K.N.; Zhang, X.; Dai, X.; Watkins, R.; Adler, J.; Dubinsky, M.C.; Kastl, A.; Bousvaros, A.; Strople, J.A.; Cross, R.K.; et al. Impact of SARS-CoV-2 vaccination on inflammatory bowel disease activity and development of vaccine-related adverse events: Results from PREVENT-COVID. Inflamm. Bowel Dis. 2021, izab302. [Google Scholar] [CrossRef] [PubMed]
- Botwin, G.J.; Li, D.; Figueiredo, J.; Cheng, S.; Braun, J.; McGovern, D.P.B.; Melmed, G.Y. Adverse events following SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Li, D.; Debbas, P.; Cheng, S.; Braun, J.; McGovern, D.P.B.; Melmed, G.Y. Post-vaccination symptoms after a third dose of mRNA SARS-CoV-2 vaccination in patients with inflammatory bowel disease. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Goodhand, J.R.; Bewshea, C.; Nice, R.; Chee, D.; Lin, S.; Chanchlani, N.; Butterworth, J.; Cooney, R.; Croft, N.M.; et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021, 70, 865–875. [Google Scholar] [CrossRef]
- Hadjadj, J.; Planas, D.; Ouedrani, A.; Buffier, S.; Delage, L.; Nguyen, Y.; Bruel, T.; Stolzenberg, M.C.; Staropoli, I.; Ermak, N.; et al. Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in immunocompromised patients with systemic inflammatory diseases. Ann. Rheum. Dis. 2022. online ahead of print January 12. [Google Scholar] [CrossRef]
- Simon, D.; Tascilar, K.; Fagni, F.; Schmidt, K.; Kronke, G.; Kleyer, A.; Ramming, A.; Schoenau, V.; Bohr, D.; Knitza, J.; et al. Efficacy and safety of SARS-CoV-2 revaccination in non-responders with immune-mediated inflammatory disease. Ann. Rheum. Dis. 2021. online ahead of print November 24. [Google Scholar] [CrossRef]
- American Academy of Dermatology. COVID-19 Vaccine Administration Guidance. 2021. Available online: https://assets.ctfassets.net/1ny4yoiyrqia/2gTpp7G9GNSTtAHwPHNtbc/b23a62ab746ad4672158a0c9633ca7aa/COVID_vaccine_administration_guidance_2-15-21.pdf (accessed on 19 March 2021).
- European Academy of Dermatology and Venereology. COVID-19 Vaccination: Advice of the EADV Task Forces. 2021. Available online: https://www.eadv.org/index.php/cms-admin/showfile/COVID-19%20VACCINATION%20-%20TF%20ADVICE_02-10-2021-13-12-40.pdf (accessed on 19 March 2021).
- Alexander, J.L.; Moran, G.W.; Gaya, D.R.; Raine, T.; Hart, A.; Kennedy, N.A.; Lindsay, J.O.; MacDonald, J.; Segal, J.P.; Sebastian, S.; et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: A British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol. Hepatol. 2021, 6, 218–224. [Google Scholar] [CrossRef]
- American College of Rheumatology. COVID-19 Vaccine Clinical Guidance Summary for Patients with Rheumatic and Musculoskeletal Diseases. 2021. Available online: https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf (accessed on 19 March 2021).
- European Medicines Agency. Comirnaty and Spikevax: EMA Recommendations on Extra Doses and Boosters. 2021. Available online: https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters (accessed on 25 January 2022).
- Department of Health & Social Care. Joint Committee on Vaccination and Immunisation (JCVI) Advice on Third Primary Dose Vaccination. 2021. Available online: https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination (accessed on 25 January 2022).
- Centers for Disease Control and Prevention. Considerations for COVID-19 Vaccination in Moderately or Severely Immunocompromised People. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#considerations-covid19-vax-immunocopromised (accessed on 25 January 2022).
| Biologic Therapy | Vaccine | Population | Impact on Vaccine Response |
|---|---|---|---|
| Integrin inhibitors | |||
| Natalizumab (anti-α4β1) | Influenza | MS | Reduced humoral response [47,48] |
| Tetanus | MS | Normal humoral response [49] | |
| Vedolizumab (anti-α4β7) | Influenza | IBD | Normal humoral response [50] |
| Oral cholera | Healthy volunteers | Reduced humoral response [51] | |
| Hepatitis B | Healthy volunteers | Normal humoral response [51] | |
| CTLA-4 fusion protein | |||
| Abatacept | PPV-23 | RA | Reduced antibody titers, but normal functional antibodies [52] |
| Normal humoral response [53] | |||
| PCV-7 | RA | Impaired humoral response [54] | |
| Influenza | RA | Normal humoral response [53] | |
| Impaired humoral response [55] | |||
| CD20 inhibitor | |||
| Rituximab | PPV-23 | RA | Impaired humoral response [56] |
| IMIDs | Impaired humoral response [57] | ||
| PCV-7 | RA | Impaired humoral response [54] | |
| IMIDs | Impaired humoral response [57] | ||
| Hepatitis B | IMIDs | Impaired humoral response [58] | |
| Tetanus | RA | Preserved humoral response to recall antigen [56] | |
| Influenza | RA | Impaired humoral [59,60,61,62], but preserved cellular response [59] | |
| IMIDs | Impaired humoral response [63] | ||
| RA or vasculitis | Impaired humoral response [64,65] | ||
| BAFF inhibitor | |||
| Belimumab | PCV-13 | SLE | Normal humoral response [66] |
| PPV-23 | SLE | Normal humoral response [67] | |
| TNF inhibitors (aggregated) | |||
| PPV-23 | SpA | Reduced humoral response [68] | |
| PsA | Normal humoral response [69] | ||
| RA | Reduced humoral response [70] | ||
| Normal humoral response [71,72,73] | |||
| IBD | Reduced humoral response [74,75,76,77] | ||
| PCV-13 then PPV-23 | IBD | Impaired humoral response [77] | |
| PCV-13 | RA | Impaired humoral response [78] | |
| CD | Impaired humoral response [74] | ||
| Hepatitis B | SpA | Reduced humoral response [68] | |
| IBD | Impaired humoral response [79,80], or seroconversion rates similar to thiopurine or MTX [81] | ||
| Hepatitis A | RA | Impaired humoral response [82] | |
| Influenza | RA | Normal humoral response [71,72,83,84,85,86,87] | |
| AS | Normal humoral response [83] | ||
| SpA | Reduced humoral response [85] | ||
| PsA or PsO | Normal humoral response [88] | ||
| IBD | Reduced humoral response [89,90,91,92] | ||
| IMIDs | Reduced humoral response [93,94] | ||
| MMR | JIA | Normal humoral and cellular response [95] | |
| Yellow fever | RA | Preserved antibody response to revaccination [96] | |
| IL-17 inhibitors | |||
| Secukinumab | Meningococcal conjugate | Healthy volunteers | Normal humoral response [97] |
| Influenza | Healthy volunteers | Normal humoral response [97] | |
| PsA or AS | Normal humoral response [98] | ||
| Ixekizumab | PPV-23 | Healthy volunteers | Normal humoral response [99] |
| Tetanus | Healthy volunteers | Normal humoral response [99] | |
| IL-6 inhibitor | |||
| Tocilizumab | PPV-23 | RA | Normal humoral response [100,101,102] |
| PCV-13 then PPV-23 | IMIDs | Normal humoral response [57] | |
| PCV-7 | RA | Normal humoral response [54] | |
| Influenza | RA | Normal humoral response [64,102,103] | |
| SJIA | Normal humoral response [104] | ||
| Tetanus | RA | Normal humoral response [100] | |
| IL-12/IL-23 inhibitors | |||
| Ustekinumab | PPV-23 | PsO | Normal humoral response [105] |
| PsO or MS | Normal humoral response [106] | ||
| Influenza | CD | Normal humoral response [107] | |
| Tetanus | PsO or MS | Normal humoral response [106] | |
| PsO | Normal humoral response [105] | ||
| Biologic Therapy | Impact on Vaccine Response a | |
|---|---|---|
| CD20+ cell depletion | ||
| CTLA-4 fusion protein | ||
| TNF inhibitors | ||
| Integrin inhibitors | Oral cholera | |
| BAFF inhibitor | ||
| IL-17 inhibitors | ||
| IL-6 inhibitor | ||
| IL-12/IL-23 inhibitors | ||
| SARS-CoV-2 Vaccine | Patient Population | Biologic Therapy | Effect on Immunogenicity | Safety | Reference |
|---|---|---|---|---|---|
| mRNA-1273 and BNT162b2 | CID (n = 133) | 29% receiving anti- TNF, 9% anti-integrin, 8% anti-CD20, 8% anti-IL-12/23 or anti-IL-23, 2% anti-BAFF, 2% CTLA-4, and 1% each anti-IL-6 and anti-IL-1 | Corticosteroids and B cell-depleting therapies strongly impaired humoral response. JAKi and antimetabolites (e.g., MTX) blunted humoral responses. Anti- TNF, UST, and VDZ had minimal impact | Not reported | Deepak et al. 2021 [157] |
| BNT162b2 | IMID (n = 84) | 13% receiving anti-TNF, 8% anti-IL-17, 7% anti-IL-23, 4% anti-IL-6, 1% anti-IL-1, 1% anti-integrin | Impaired humoral response compared with healthy controls independent of treatment | AE similar to general population | Simon et al. 2021 [158] |
| mRNA-1273 and BNT162b2 | CID (n = 26) | 50% receiving anti-TNF, 12% anti-IL-17, and 4% each anti-IL-6, anti-IL-12/23, and anti-integrin | Slightly reduced humoral response | AE comparable to general population and no flares of CID | Geisen et al. 2021 [159] |
| BNT162b2 | IRD (n = 264) | 24% receiving anti-TNF, 18% anti-CD20, 15% anti-interleukins, and 3% CTLA-4 | Significant humoral response in majority of patients, except those receiving RTX | Minor adverse effects and no flares of IRD | Braun-Moscovici et al. 2021 [160] |
| mRNA-1273 and BNT162b2 | RA (n = 53) | 47% receiving biologic therapy | Significantly lower antibody response in patients with RA vs. healthy individuals. Lowest rate of response in patients receiving JAKi | Not reported | Rubbert-Roth et al. 2021 [161] |
| BNT162b2 | RA (n = 83) PsA (n = 29) SpA (n = 28) | 44% receiving anti-TNF, 21% CTLA-4, 14% anti-IL-12/23, and 10% anti-IL-6 | Impaired immunogenicity after one dose in patients treated with MTX, glucocorticoids, and abatacept. No effect of cytokine inhibitors | Not reported | Bugatti et al. 2021 [162] |
| BNT162b2 and ChAdOx1-S | IMID (n = 120), mostly PsO | 83% receiving biologic therapy | Impaired immunogenicity after one dose in patients treated with MTX compared to biologics | Not reported | Al-Janabi et al. 2021 [163] |
| BNT162b2 | PsA (n = 40) | 100% receiving anti-TNF | Anti-TNF numerically (not significantly) decreased humoral response vs. healthy controls; MTX, glucocorticoids, and sulfasalazine had no impact | AE similar to general population; no changes in clinical disease activity | Venerito et al. 2022 [164] |
| BNT162b2 | IMID (n = 51) mostly PsO and/or PsA (n = 24) RA (n = 22) | 55% receiving biologic therapy | Impaired humoral and cellular responses in patients receiving MTX vs. IMID patients on other DMARDS or healthy controls | Not reported | Haberman et al. 2021 [165] |
| BNT162b2 | PsO (n = 48) | 44% receiving anti-TNF, 27% anti-IL-23, 17% anti-IL-12/23, 13% anti-IL-17 | Reduced humoral response in patients receiving biologics in combotherapy vs. monotherapy | No increase in AEs and no flares | Cristaudo et al. 2021 [166] |
| BNT162b2 | PsO (n = 84) | 80% receiving biologic therapy | Impaired humoral response with MTX after first dose but preserved with biologics; after 2 doses, responses comparable to controls | Not reported | Mahil et al. 2021, 2022 [167,168] |
| mRNA-1273 and BNT162b2 | IBD (n = 48) | 33% receiving anti-TNF, 42% anti-integrin, 8% anti-IL-12/23, and 2% anti-IL-23 | 100% seropositivity after 2 doses but reduced serologic response in patients on anti-TNF or VDZ | Not reported | Wong et al. 2021 [169] |
| mRNA-1273, BNT162b2 and ChAdOx1-S | IBD (n = 72) Healthy controls (n = 72) | 37% receiving anti-TNF, 26% anti-integrin, and 19% anti-IL-12/23 | Slightly decreased antibody titers in IBD patients compared to healthy controls | Well tolerated with only mild side effects | Classen et al. 2021 [170] |
| mRNA-1273 and BNT162b2 | IBD (n = 317) | 42% receiving anti-TNF, 15% anti-integrin, and 12% anti-IL-12/23 | 95% had detectable antibodies | Not reported | Kappelman et al. 2021a [171] |
| mRNA-1273, BNT162b2 and Ad26.COV2.S | IBD (n = 1909) | 47% receiving anti-TNF, 15% anti-IL-12/23, and 12% anti-integrin | 96% achieved positive antibody response; age, corticosteroids, and anti-TNF + IMM associated with reduced odds of antibody response | Not reported | Kappelman et al. 2021b [172] |
| mRNA-1273 and BNT162b2 | IBD (n = 176) | 27% receiving anti-TNF, 27% anti-IL-12/23, and 11% anti-integrin | Significantly lower antibody titers and more rapid decay with anti-TNF ± IMM vs. UST, VDZ, or no therapy | Not reported | Charilaou et al. 2021 [173] |
| mRNA-1273 and BNT162b2 | IBD (n = 75) | 51% receiving anti-TNF, 23% anti-IL-12/23, and 8% anti-integrin | Detectable antibodies 6 months after 2-dose vaccination; patients receiving anti-TNF had lower antibody titers | Not reported | Frey et al. 2022 [174] |
| BNT162b2 and ChAdOx1-S | IBD (n = 126) | 76% receiving anti-TNF, 12% anti-IL-12/23, and 12% anti-integrin | Majority of patients receiving anti-TNF and VDZ and all on UST seroconverted; neutralizing antibody concentrations were higher with UST and VDZ | Not reported | Shehab et al. 2021 [175] |
| BNT162b2 and ChAdOx1-S | IBD (n = 2977) | 69% receiving anti-TNF and 31% anti-integrin | Five-fold reduction in humoral response with IFX vs. VDZ; more rapid decay in antibody levels in IFX-treated patients | Not reported | Kennedy et al. 2021 [176] Lin et al. 2021 [177] |
| BNT162b2 and ChAdOx1-S | IBD (n = 28) Healthy controls (n = 27) | 32% receiving anti-TNF, 29% anti-IL-12/23, 11% anti-integrin | Comparable T-cell responses between IBD patients and healthy controls | Not reported | Reuken et al. 2021 [178] |
| mRNA-1273, BNT162b2 and Ad26.COV2.S | IBD (n = 303) | 35% receiving anti-TNF, 43% anti-IL-12/23 or anti-integrin | Preserved T-cell clonal response with UST and VDZ and augmented by anti-TNF | Not reported | Li et al. 2021 [179] |
| BNT162b2 | IBD (n = 12,231) and matched patients (n = 36,254) | 11% receiving anti-TNF, 4% anti-integrin, and 2% anti-IL-12/23 | Similar risk of infection between controls and IBD patients, with no effect of immune-modifying therapies | Not reported | Ben-Tov et al. 2021 [180] |
| mRNA-1273 and BNT162b2 | IBD (n = 14,697) | Not reported | Two-dose vaccination reduced the hazard of infection by 69%, with an estimated efficacy of 80.4% | Not reported | Khan et al. 2021 [181] |
| mRNA-1273 and BNT162b2 | PsO (n = 50) | 100% receiving biologic therapy | Not reported | AE similar to general population | Musumeci et al. 2021 [182] |
| mRNA-1273, BNT162b2 and Ad26.COV2.S | IBD (n = 3316) | 46% receiving anti-TNF, 15% anti-IL-12/23, and 12% anti-integrin | Not reported | Low rates (2%) of IBD flare following vaccination and relatively few vaccine-related AEs | Weaver et al. 2021 [183] |
| mRNA-1273 and BNT162b2 | IBD (n = 246) | 37% receiving anti-TNF, 17% anti-IL-12/23, and 13% anti-integrin | Not reported | AE similar to general population | Botwin et al. 2021 [184] |
| mRNA-1273 and BNT162b2 | IBD (n = 524) | 89% receiving biologic therapy | Not reported | Post-vaccination symptoms after third dose are generally milder and less frequent than after second dose | Li et al. 2021 [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcillán, B.; Salavert, M.; Regueiro, J.R.; Díaz-Castroverde, S. Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review. Vaccines 2022, 10, 297. https://doi.org/10.3390/vaccines10020297
Garcillán B, Salavert M, Regueiro JR, Díaz-Castroverde S. Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review. Vaccines. 2022; 10(2):297. https://doi.org/10.3390/vaccines10020297
Chicago/Turabian StyleGarcillán, Beatriz, Miguel Salavert, José R. Regueiro, and Sabela Díaz-Castroverde. 2022. "Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review" Vaccines 10, no. 2: 297. https://doi.org/10.3390/vaccines10020297
APA StyleGarcillán, B., Salavert, M., Regueiro, J. R., & Díaz-Castroverde, S. (2022). Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review. Vaccines, 10(2), 297. https://doi.org/10.3390/vaccines10020297






