Clostridial Diseases of Horses: A Review
Abstract
:1. Introduction
2. Enteric and Enterotoxic Clostridial Infections
2.1. Diseases Produced by Clostridium perfringens
2.1.1. General Characteristics of Clostridium perfringens
2.1.2. Diseases Produced by Clostridium perfringens Type B
2.1.3. Diseases Produced by Clostridium perfringens Type C
2.1.4. NetF-Associated Diseases
2.1.5. Other C. perfringens-Related Diseases in Horses
2.2. Diseases Produced by Clostridioides difficile
2.2.1. Classic Clostridioides difficile-Associated Disease
2.2.2. Other Diseases Associated with Clostridioides difficile
2.3. Enteric Disease Produced by Paeniclostridium sordelli
3. Histotoxic Clostridial Infections
3.1. Gas Gangrene
3.2. Clostridial Hepatitis
3.2.1. Tyzzer Disease

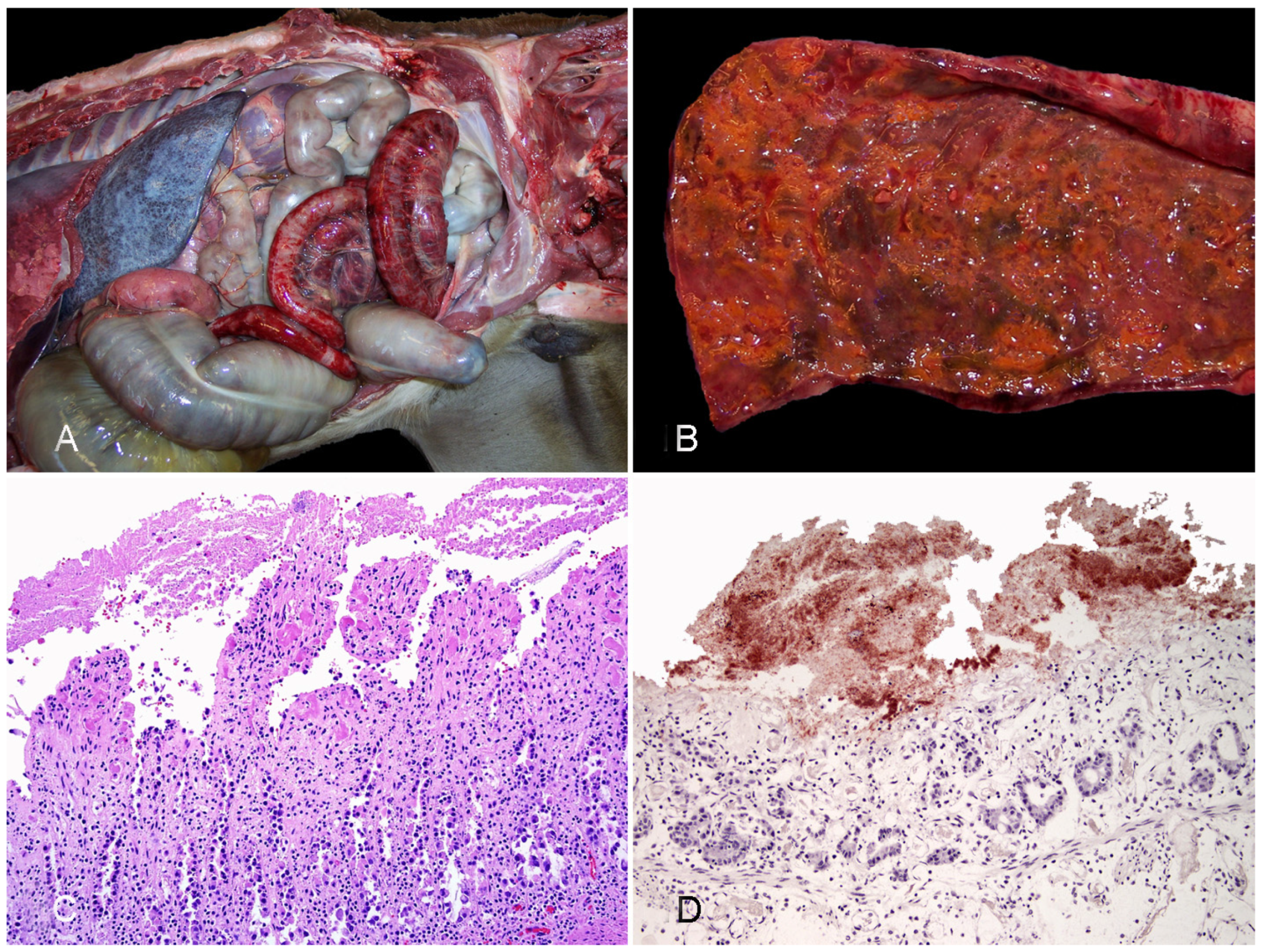
3.2.2. Infectious Necrotic Hepatitis
3.2.3. Bacillary Hemoglobinuria
4. Neurotoxic Clostridial Infections
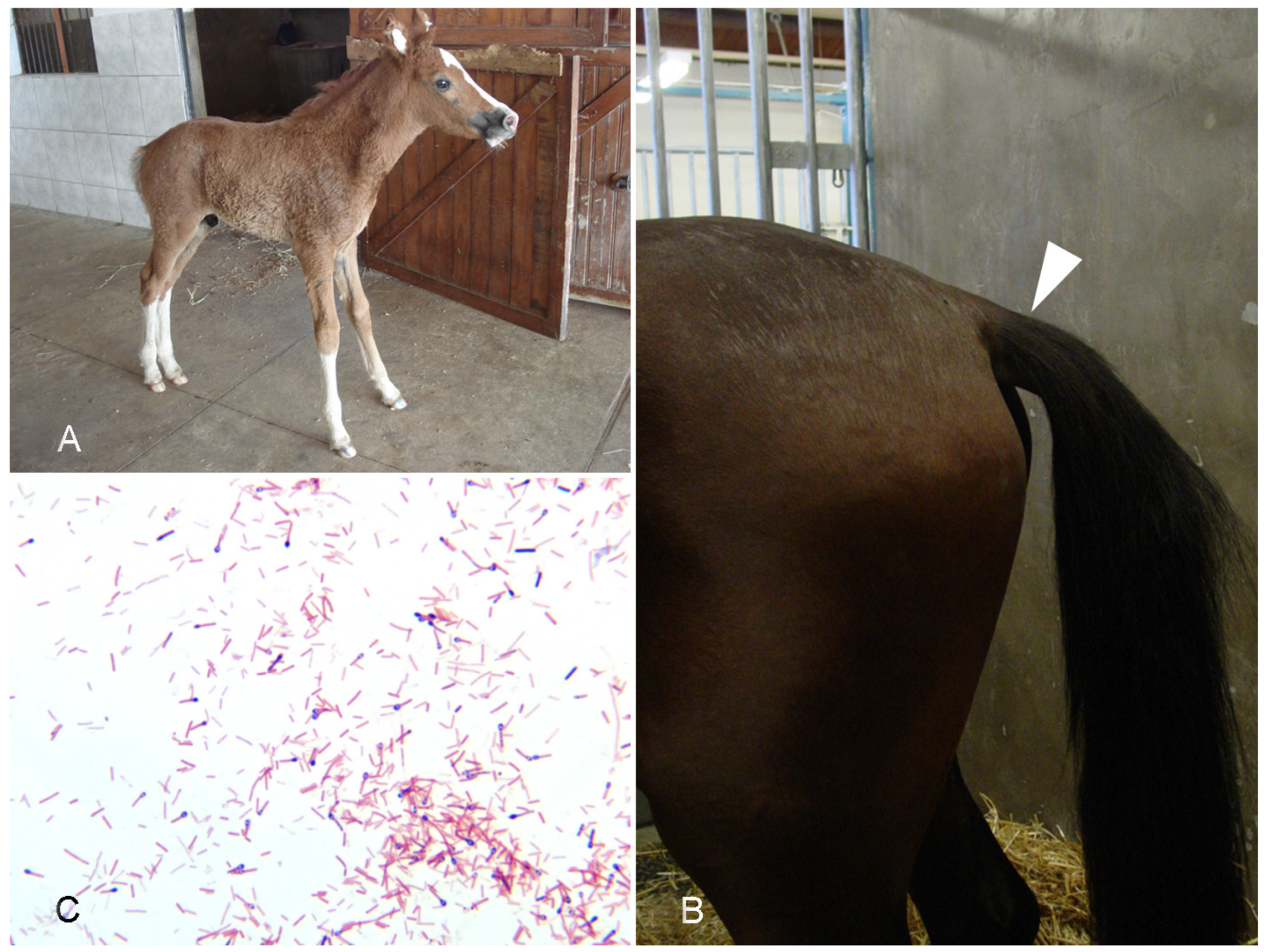
4.1. Tetanus
4.2. Botulism
5. General Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prescott, J.F.; MacInnes, J.I.; Wu, A.K.K. Taxonomic Relationships among the Clostridia. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–5. ISBN 978-1-118-72829-1. [Google Scholar]
- Rood, J.I. General Physiological and Virulence Properties of the Pathogenic Clostridia. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 7–12. ISBN 978-1-118-72829-1. [Google Scholar]
- Uzal, F.A.; Navarro, M.A.; Li, J.; Freedman, J.C.; Shrestha, A.; McClane, B.A. Comparative Pathogenesis of Enteric Clostridial Infections in Humans and Animals. Anaerobe 2018, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens Toxin-Based Typing Scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Miyakawa, M.E.; Fisher, D.J.; Poon, R.; Sayeed, S.; Adams, V.; Rood, J.I.; McClane, B.A.; Uzal, F.A. Both Epsilon-Toxin and Beta-Toxin Are Important for the Lethal Properties of Clostridium Perfringens Type B Isolates in the Mouse Intravenous Injection Model. Infect. Immun. 2007, 75, 1443–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzal, F.A.; Songer, J.G. Infections by Clostridium Perfringens Type B. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 139–142. ISBN 978-1-118-72829-1. [Google Scholar]
- Munday, J.S.; Bentall, H.; Aberdein, D.; Navarro, M.; Uzal, F.A.; Brown, S. Death of a Neonatal Lamb Due to Clostridium Perfringens Type B in New Zealand. N. Z. Vet. J. 2020, 68, 242–246. [Google Scholar] [CrossRef]
- Stubbings, D.P. Clostridium Perfringens Enterotoxaemia in Two Young Horses. Vet. Rec. 1990, 127, 431. [Google Scholar] [PubMed]
- Uzal, F.A.; Songer, J.G. Diagnosis of Clostridium Perfringens Intestinal Infections in Sheep and Goats. J. Vet. Diagn. Investig. 2008, 20, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Diab, S.S.; Kinde, H.; Moore, J.; Shahriar, M.F.; Odani, J.; Anthenill, L.; Songer, G.; Uzal, F.A. Pathology of Clostridium Perfringens Type C Enterotoxemia in Horses. Vet. Pathol. 2012, 49, 255–263. [Google Scholar] [CrossRef]
- Theoret, J.R.; McClane, B.A. Toxins of Clostridium perfringens. In Clostridial Diseases of Animals; Uzal, F.A., Songer, J.G., Prescott, J.F., Popoff, M.R., Eds.; Wiley Blackwell: Ames, IA, USA, 2016; pp. 45–69. [Google Scholar]
- Diab, S.S. Diseases Produced by Clostridium perfringens Type C. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 143–155. ISBN 978-1-118-72829-1. [Google Scholar]
- Uzal, F.A.; Arroyo, L.G.; Navarro, M.A.; Gomez, D.E.; Asín, J.; Henderson, E. Bacterial and Viral Enterocolitis in Horses: A Review. J. Vet. Diagn. Investig. 2021, 10406387211057469. [Google Scholar] [CrossRef]
- Mendonça, F.S.; Navarro, M.A.; Uzal, F.A. The Comparative Pathology of Enterocolitis Due to Clostridium perfringens Type C, Clostridioides difficile, Paeniclostridium sordellii, Salmonella enterica Subspecies Enterica Serovar Typhimurium and Non-Steroidal Antiinflammatory Drugs in Horses. J. Vet. Diagn. Investig. 2021, 10406387211041091. [Google Scholar] [CrossRef]
- Mehdizadeh-Gohari, I.; Parreira, V.R.; Nowell, V.J.; Nicholson, V.M.; Oliphant, K.; Prescott, J.F. A Novel Pore-Forming Toxin in Type A Clostridium Perfringens Is Associated with Both Fatal Canine Hemorrhagic Gastroenteritis and Fatal Foal Necrotizing Enterocolitis. PLoS ONE 2015, 10, e0122684. [Google Scholar] [CrossRef]
- Mehdizadeh-Gohari, I.; Parreira, V.R.; Timoney, J.F.; Fallon, L.; Slovis, N.; Prescott, J.F. NetF-Positive Clostridium Perfringens in Neonatal Foal Necrotising Enteritis in Kentucky. Vet. Rec. 2016, 178, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdizadeh-Gohari, I.M.; Parreira, V.R.; Prescott, J.F. NetF-Associated Necrotizing Enteritis of Foals and Canine Hemorrhagic Gastroenteritis. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 117–122. ISBN 978-1-118-72829-1. [Google Scholar]
- Mehdizadeh-Gohari, I.; Unterer, S.; Whitehead, A.E.; Prescott, J.F. NetF-Producing Clostridium Perfringens and Its Associated Diseases in Dogs and Foals. J. Vet. Diagn. Investig. 2020, 32, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Herholz, C.; Miserez, R.; Nicolet, J.; Frey, J.; Popoff, M.; Gibert, M.; Gerber, H.; Straub, R. Prevalence of Beta2-Toxigenic Clostridium Perfringens in Horses with Intestinal Disorders. J. Clin. Microbiol. 1999, 37, 358–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, S.S.; Songer, G.; Uzal, F.A. Clostridium difficile Infection in Horses: A Review. Vet. Microbiol. 2013, 167, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weese, J.S.; Toxopeus, L.; Arroyo, L. Clostridium difficile Associated Diarrhoea in Horses within the Community: Predictors, Clinical Presentation and Outcome. Equine Vet. J. 2006, 38, 185–188. [Google Scholar] [CrossRef]
- Madewell, B.R.; Tang, Y.J.; Jang, S.; Madigan, J.E.; Hirsh, D.C.; Gumerlock, P.H.; Silva, J. Apparent Outbreaks of Clostridium difficile-Associated Diarrhea in Horses in a Veterinary Medical Teaching Hospital. J. Vet. Diagn. Investig. 1995, 7, 343–346. [Google Scholar] [CrossRef] [Green Version]
- Weese, J.S. Clostridium (Clostridioides) difficile in Animals. J. Vet. Diagn. Investig. 2020, 32, 213–221. [Google Scholar] [CrossRef]
- Båverud, V.; Gustafsson, A.; Franklin, A.; Aspán, A.; Gunnarsson, A. Clostridium difficile: Prevalence in Horses and Environment, and Antimicrobial Susceptibility. Equine Vet. J. 2003, 35, 465–471. [Google Scholar] [CrossRef]
- Medina-Torres, C.E.; Weese, J.S.; Staempfli, H.R. Prevalence of Clostridium difficile in Horses. Vet. Microbiol. 2011, 152, 212–215. [Google Scholar] [CrossRef]
- Weese, J.S.; Staempfli, H.R.; Prescott, J.F. A Prospective Study of the Roles of Clostridium difficile and Enterotoxigenic Clostridium perfringens in Equine Diarrhoea. Equine Vet. J. 2001, 33, 403–409. [Google Scholar] [CrossRef]
- Schoster, A.; Staempfli, H.R.; Arroyo, L.G.; Reid-Smith, R.J.; Janecko, N.; Shewen, P.E.; Weese, J.S. Longitudinal Study of Clostridium difficile and Antimicrobial Susceptibility of Escherichia Coli in Healthy Horses in a Community Setting. Vet. Microbiol. 2012, 159, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Cartman, S.T.; Minton, N.P. Both, Toxin A and Toxin B, Are Important in Clostridium difficile Infection. Gut Microbes 2011, 2, 252–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, S.S.; Uzal, F.A.; Songer, J.G. Diseases Produced by Clostridium difficile. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 177–195. ISBN 978-1-118-72829-1. [Google Scholar]
- Thean, S.; Elliott, B.; Riley, T.V. Clostridium difficile in Horses in Australia—A Preliminary Study. J. Med. Microbiol. 2011, 60, 1188–1192. [Google Scholar] [CrossRef]
- Diab, S.S.; Rodriguez-Bertos, A.; Uzal, F.A. Pathology and Diagnostic Criteria of Clostridium difficile Enteric Infection in Horses. Vet. Pathol. 2013, 50, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Keel, M.K.; Songer, J.G. The Comparative Pathology of Clostridium difficile-Associated Disease. Vet. Pathol. 2006, 43, 225–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, K.W.; Jost, B.H.; Songer, J.G. Evaluation of a Test for Clostridium difficile Toxins A and B for the Diagnosis of Neonatal Swine Enteritis. J. Vet. Diagn. Investig. 2002, 14, 258–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorko, D.P.; Williams, E.C. Use of Cycloserine-Cefoxitin-Fructose Agar and L-Proline-Aminopeptidase (PRO Discs) in the Rapid Identification of Clostridium difficile. J. Clin. Microbiol. 1997, 35, 1258–1259. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, L.G.; Gomez, D.E.; Martins, C. Equine Duodenitis-Proximal Jejunitis: A Review. Can. Vet. J. 2018, 59, 510–517. [Google Scholar]
- Arroyo, L.G.; Costa, M.C.; Guest, B.B.; Plattner, B.L.; Lillie, B.N.; Weese, J.S. Duodenitis-Proximal Jejunitis in Horses After Experimental Administration of Clostridium difficile Toxins. J. Vet. Intern. Med. 2017, 31, 158–163. [Google Scholar] [CrossRef]
- Nyaoke, A.C.; Navarro, M.A.; Fresneda, K.; Diab, S.S.; Moore, J.; Lyras, D.; Awad, M.; Uzal, F.A. Paeniclostridium (Clostridium) sordellii-Associated Enterocolitis in 7 Horses. J. Vet. Diagn. Investig. 2020, 32, 239–245. [Google Scholar] [CrossRef]
- Peek, S.F.; Semrad, S.D.; Perkins, G.A. Clostridial Myonecrosis in Horses (37 Cases 1985–2000). Equine Vet. J. 2003, 35, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.S.; Uzal, F.A.; Oliveira, C.A.; Lobato, F.C.F. Gas Gangrene (Malignant Edema). In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 243–254. ISBN 978-1-118-72829-1. [Google Scholar]
- Sacco, S.C.; Ortega, J.; Navarro, M.A.; Fresneda, K.C.; Anderson, M.; Woods, L.W.; Moore, J.; Uzal, F.A. Clostridium sordellii-Associated Gas Gangrene in Eight Horses, 1998–2019. J. Vet. Diagn. Investig. 2019, 32, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Breuhaus, B.A.; Brown, C.M.; Scott, E.A.; Ainsworth, D.M.; Taylor, R.F. Clostridial Muscle Infections Following Intramuscular Injections in the Horse. J. Equine Vet. Sci. 1983, 3, 42–46. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Kang, M.-S.; Yoo, H.-S.; Lee, D.-Y.; Lee, H.-C.; Kim, D.-Y. Clostridium perfringens Type A Myonecrosis in a Horse in Korea. J. Vet. Med. Sci. 2003, 65, 1245–1247. [Google Scholar] [CrossRef] [Green Version]
- Coloe, P.J.; Ireland, L.; Vaudrey, J.C. Clostridium fallax as a Cause of Gas-Oedema Disease in a Horse. J. Comp. Pathol. 1983, 93, 597–601. [Google Scholar] [CrossRef]
- Hagemoser, W.A.; Hoffman, L.J.; Lundvall, R.L. Clostridium chauvoei Infection in a Horse. J. Am. Vet. Med. Assoc. 1980, 176, 631–633. [Google Scholar]
- Perdrizet, J.A.; Callihan, D.R.; Rebhun, W.C.; Shin, S.J. Successful Management of Malignant Edema Caused by Clostridium septicum in a Horse. Cornell Vet. 1987, 77, 328–338. [Google Scholar]
- Jeanes, L.; Magdesian, K.; Madigan, J. Clostridial Myonecrosis in Horses. Compend. Contin. Educ. Pract. Vet. 2001, 23, 577. [Google Scholar]
- Rebhun, W.C.; Shin, S.J.; King, J.M.; Baum, K.H.; Patten, V. Malignant Edema in Horses. J. Am. Vet. Med. Assoc. 1985, 187, 732–736. [Google Scholar]
- Vengust, M.; Arroyo, L.G.; Weese, J.S.; Baird, J.D. Preliminary Evidence for Dormant Clostridial Spores in Equine Skeletal Muscle. Equine Vet. J. 2003, 35, 514–516. [Google Scholar] [CrossRef]
- Hang’ombe, B.M.; Isogai, E.; Lungu, J.; Mubita, C.; Nambota, A.; Kirisawa, R.; Kimura, K.; Isogai, H. Detection and Characterization of Clostridium Species in Soil of Zambia. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 277–284. [Google Scholar] [CrossRef]
- Seifert, H.S.; Bader, K.; Cyplik, J.; González Salinas, J.; Roth, F.; Salinas Meléndez, J.A.; Sukop, U. Environment, Incidence, Aetiology, Epizootiology and Immunoprophylaxis of Soil-Borne Diseases in North-East Mexico. Zent. Vet. Reihe B 1996, 43, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Uzal, F.A.; Freedman, J.C.; Shrestha, A.; Theoret, J.R.; Garcia, J.; Awad, M.M.; Adams, V.; Moore, R.J.; Rood, J.I.; McClane, B.A. Towards an Understanding of the Role of Clostridium perfringens Toxins in Human and Animal Disease. Future Microbiol. 2014, 9, 361–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunagawa, K.; Sugitani, M. Post-Mortem Detection of Bacteremia Using Pairs of Blood Culture Samples. Leg. Med. 2017, 24, 92–97. [Google Scholar] [CrossRef]
- Parish, S.; Valberg, S. Clostridial Myonecrosis. In Large Animal Internal Medicine; Smith, B., Ed.; Mosby Elsevier: St. Louis, MO, USA, 2019; pp. 1432–1434. [Google Scholar]
- Ortega, J.; Daft, B.; Assis, R.A.; Kinde, H.; Anthenill, L.; Odani, J.; Uzal, F.A. Infection of Internal Umbilical Remnant in Foals by Clostridium sordellii. Vet. Pathol. 2007, 44, 269–275. [Google Scholar] [CrossRef]
- Farias, L.D.; Azevedo, M.D.S.; Trost, M.E.; De La Côrte, F.D.; Irigoyen, L.F.; de Vargas, A.C. Acute Myonecrosis in Horse Caused by Clostridium novyi Type A. Braz. J. Microbiol. 2014, 45, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.L. The Pathogenesis of Clostridial Myonecrosis. Int. J. Med. Microbiol. 2000, 290, 497–502. [Google Scholar] [CrossRef]
- Caplan, E.S.; Kluge, R.M. Gas Gangrene: Review of 34 Cases. Arch. Intern. Med. 1976, 136, 788–791. [Google Scholar] [CrossRef]
- Abreu, C.C.; Edwards, E.E.; Edwards, J.F.; Gibbons, P.M.; Leal de Araújo, J.; Rech, R.R.; Uzal, F.A. Blackleg in Cattle: A Case Report of Fetal Infection and a Literature Review. J. Vet. Diagn. Investig. 2017, 29, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Abreu, C.C.; Blanchard, P.C.; Adaska, J.M.; Moeller, R.B.; Anderson, M.; Navarro, M.A.; Diab, S.S.; Uzal, F.A. Pathology of Blackleg in Cattle in California, 1991–2015. J. Vet. Diagn. Investig. 2018, 30, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Aldape, M.J.; Bryant, A.E.; Stevens, D.L. Clostridium sordellii Infection: Epidemiology, Clinical Findings, and Current Perspectives on Diagnosis and Treatment. Clin. Infect. Dis. 2006, 43, 1436–1446. [Google Scholar] [CrossRef]
- Aronoff, D.M. Clostridium novyi, sordellii, and tetani: Mechanisms of Disease. Anaerobe 2013, 24, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Hatheway, C.L. Toxigenic Clostridia. Clin. Microbiol. Rev. 1990, 3, 66–98. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R.; Bouvet, P. Clostridial Toxins. Future Microbiol. 2009, 4, 1021–1064. [Google Scholar] [CrossRef] [PubMed]
- Almeida e Macedo, J.T.S.; Pires, P.S.; Pinheiro, E.E.G.; de Oliveira, R.S.; Silva, R.O.S.; Lobato, F.C.F.; Pedroso, P.M.O. Malignant Edema Caused by Clostridium chauvoei in a Horse. Acta Sci. Vet. 2013, 41, 24. [Google Scholar]
- Raymundo, D.L.; Pavarini, S.P.; Bezerra Junior, P.S.; Antoniassi, N.A.B.; Bandarra, P.M.; Bercht, B.S.; Gomes, M.J.P.; Driemeier, D. Mionecrose Aguda Por Clostridium septicum Em Equinos. Pesqui. Vet. Bras. 2010, 30, 637–640. [Google Scholar] [CrossRef]
- Bryant, A.E.; Bayer, C.R.; Aldape, M.J.; Wallace, R.J.; Titball, R.W.; Stevens, D.L. Clostridium perfringens Phospholipase C-Induced Platelet/Leukocyte Interactions Impede Neutrophil Diapedesis. J. Med. Microbiol. 2006, 55, 495–504. [Google Scholar] [CrossRef]
- Garofolo, G.; Galante, D.; Serrecchia, L.; Buonavoglia, D.; Fasanella, A. Development of a Real Time PCR Taqman Assay Based on the TPI Gene for Simultaneous Identification of Clostridium chauvoei and Clostridium septicum. J. Microbiol. Methods 2011, 84, 307–311. [Google Scholar] [CrossRef]
- Barnes, D.M.; Bergeland, M.E.; Higbee, J.M. Differential Diagnosis of Clostridial Myonecrosis. Can. Vet. J. 1975, 16, 357–359. [Google Scholar]
- Navarro, M.A.; Uzal, F.A. Pathobiology and Diagnosis of Clostridial Hepatitis in Animals. J. Vet. Diagn. Investig. 2020, 32, 192–202. [Google Scholar] [CrossRef]
- Barthold, S.W.; Griffey, S.M.; Percy, D.H. Mouse. In Pathology of Laboratory Rodents and Rabbits, 4th ed.; Barthold, S.W., Griffey, S.M., Percy, D.H., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2016; pp. 1–118. ISBN 978-1-118-92405-1. [Google Scholar]
- García, J.A.; Navarro, M.A.; Fresneda, K.; Uzal, F.A. Clostridium piliforme Infection (Tyzzer Disease) in Horses: Retrospective Study of 25 Cases and Literature Review. J. Vet. Diagn. Investig. 2021, 10406387211031213. [Google Scholar] [CrossRef] [PubMed]
- Swerczek, T.W. Tyzzer’s Disease in Foals: Retrospective Studies from 1969 to 2010. Can. Vet. J. 2013, 54, 876–880. [Google Scholar] [PubMed]
- Fosgate, G.T.; Hird, D.W.; Read, D.H.; Walker, R.L. Risk Factors for Clostridium piliforme Infection in Foals. J. Am. Vet. Med. Assoc. 2002, 220, 785–790. [Google Scholar] [CrossRef] [PubMed]
- St Denis, K.A.; Waddell-Parks, N.; Belanger, M. Tyzzer’s Disease in an 11-Day-Old Foal. Can. Vet. J. 2000, 41, 491–492. [Google Scholar]
- Fresneda, K.C.; Chaigneau, F.R.C. Tyzzer’s Disease. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 281–291. ISBN 978-1-118-72829-1. [Google Scholar]
- Cullen, J.M.; Stalker, M.J. Liver and Biliary System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Maxi, M.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 258–352. ISBN 978-0-7020-5318-4. [Google Scholar]
- Navarro, M.; Uzal, F.A. Infectious Necrotic Hepatitis. In Clostridial Diseases of Animals; Uzal, F.A., Songer, J.G., Prescott, J.F., Popoff, M.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 275–279. ISBN 978-1-118-72829-1. [Google Scholar]
- Aronoff, D.M.; Kazanjian, P.H. Historical and Contemporary Features of Infections Due to Clostridium novyi. Anaerobe 2018, 50, 80–84. [Google Scholar] [CrossRef]
- Nyaoke, A.C.; Navarro, M.A.; Beingesser, J.; Uzal, F.A. Infectious Necrotic Hepatitis Caused by Clostridium novyi Type B in a Horse: Case Report and Review of the Literature. J. Vet. Diagn. Investig. 2018, 30, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Bagadi, H.O.; Sewell, M.M. An Epidemiological Survey of Infectious Necrotic Hepatitis (Black Disease) of Sheep in Southern Scotland. Res. Vet. Sci. 1973, 15, 49–53. [Google Scholar] [CrossRef]
- Smith, G.W. Black Disease. In Large Animal Internal Medicine; Smith, B.P., Ed.; Mosby Elsevier: St. Louis, MO, USA, 2015; pp. 849–850. [Google Scholar]
- Oaks, J.L.; Kanaly, S.T.; Fisher, T.J.; Besser, T.E. Apparent Clostridium haemolyticum/Clostridium novyi Infection and Exotoxemia in Two Horses. J. Vet. Diagn. Investig. 1997, 9, 324–325. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, L.K.; Cypher, E.; Gordon, S.; Pauwels, F.; Ling, J.; Collett, M.G.; Uzal, F.A. Necrotic Hepatitis Associated with Clostridium novyi Infection (Black Disease) in a Horse in New Zealand. N. Z. Vet. J. 2015, 63, 177–179. [Google Scholar] [CrossRef]
- Nakamura, S.; Takematsu, K.; Nishida, S. Susceptibility to Mitomycin C and Lecithinase Activities of Clostridium oedematiens (C. novyi) Type B and D. J. Med. Microbiol. 1975, 8, 289–297. [Google Scholar] [CrossRef]
- Songer, J.G. Clostridium novyi (Myonecrosis, Black Disease, and Bacillary Hemoglobinuria) and Clostridium septicum (Braxy) Infections. In Food Animal Practice; Elsevier: Amsterdam, The Netherlands, 2009; pp. 58–61. ISBN 978-1-4160-3591-6. [Google Scholar]
- Sweeney, H.J.; Greig, A. Infectious Necrotic Hepatitis in a Horse. Equine Vet. J. 1986, 18, 150–151. [Google Scholar] [CrossRef]
- Davies, J.L.; Uzal, F.A.; Whitehead, A.E. Necrotizing Hepatitis Associated with Clostridium Novyi in a Pony in Western Canada. Can. Vet. J. 2017, 58, 285–288. [Google Scholar]
- Sasaki, Y.; Kojima, A.; Aoki, H.; Ogikubo, Y.; Takikawa, N.; Tamura, Y. Phylogenetic Analysis and PCR Detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi Types A and B, and Clostridium septicum Based on the Flagellin Gene. Vet. Microbiol. 2002, 86, 257–267. [Google Scholar] [CrossRef]
- Popoff, M.R. Tetanus. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 293–302. ISBN 978-1-118-72829-1. [Google Scholar]
- Megighian, A.; Pirazzini, M.; Fabris, F.; Rossetto, O.; Montecucco, C. Tetanus and Tetanus Neurotoxin: From Peripheral Uptake to Central Nervous Tissue Targets. J. Neurochem. 2021, 158, 1244–1253. [Google Scholar] [CrossRef]
- Furr, M. Disorders Associated with Clostridial Neurotoxins: Botulism and Tetanus. In Equine Neurology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 319–327. ISBN 978-1-118-99371-2. [Google Scholar]
- Ribeiro, M.G.; de Nardi Júnior, G.; Megid, J.; Franco, M.M.J.; Guerra, S.T.; Portilho, F.V.R.; Rodrigues, S.A.; Paes, A.C. Tetanus in Horses: An Overview of 70 Cases. Pesq. Vet. Bras. 2018, 38, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Green, S.L.; Little, C.B.; Baird, J.D.; Tremblay, R.R.; Smith-Maxie, L.L. Tetanus in the Horse: A Review of 20 Cases (1970 to 1990). J. Vet. Intern. Med. 1994, 8, 128–132. [Google Scholar] [CrossRef]
- Cook, T.M.; Protheroe, R.T.; Handel, J.M. Tetanus: A Review of the Literature. Br. J. Anaesth. 2001, 87, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Popoff, M.R. Tetanus in Animals. J. Vet. Diagn. Investig. 2020, 32, 184–191. [Google Scholar] [CrossRef]
- South, V. Clostridial Diseases of the Horse. Practice 2014, 36, 27–33. [Google Scholar] [CrossRef]
- Le Maréchal, C.; Woudstra, C.; Fach, P. Botulism. In Clostridial Diseases of Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 303–330. ISBN 978-1-118-72829-1. [Google Scholar]
- Ostrowski, S.R.; Kubiski, S.V.; Palmero, J.; Reilly, C.M.; Higgins, J.K.; Cook-Cronin, S.; Tawde, S.N.; Crossley, B.M.; Yant, P.; Cazarez, R.; et al. An Outbreak of Equine Botulism Type A Associated with Feeding Grass Clippings. J. Vet. Diagn. Investig. 2012, 24, 601–603. [Google Scholar] [CrossRef]
- Kinde, H.; Bettey, R.L.; Ardans, A.; Galey, F.D.; Daft, B.M.; Walker, R.L.; Eklund, M.W.; Byrd, J.W. Clostridium botulinum Type-C Intoxication Associated with Consumption of Processed Alfalfa Hay Cubes in Horses. J. Am. Vet. Med. Assoc. 1991, 199, 742–746. [Google Scholar]
- Semrad, S.; Peek, S. Equine Botulism. Compend. Contin. Educ. Pract. Vet. 2002, 24, 169–172. [Google Scholar]
- Whitlock, R.; McAdams, S. Equine Botulism. Clin. Tech. Equine Pract. 2006, 5, 37–42. [Google Scholar] [CrossRef]
- Galey, F.D. Botulism in the Horse. Vet. Clin. N. Am. Equine Pract. 2001, 17, 579–588. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R.; Sharma, S. Botulism Diagnostics: From Clinical Symptoms to in Vitro Assays. Crit. Rev. Microbiol. 2007, 33, 109–125. [Google Scholar] [CrossRef]
- Rosen, O.; Feldberg, L.; Dor, E.; Zichel, R. New Approach for the Rational Selection of Markers to Identify Botulinum Toxins. Arch. Toxicol. 2021, 95, 1503–1516. [Google Scholar] [CrossRef]
- Morineaux, V.; Mazuet, C.; Hilaire, D.; Enche, J.; Popoff, M.R. Characterization of Botulinum Neurotoxin Type A Subtypes by Immunocapture Enrichment and Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 5559–5570. [Google Scholar] [CrossRef]
- Plößl, T.; Vujtovic-Ockenga, N.; Kehrenberg, C.; Klaubert, B. Multi-Dimensional Nanoscale Liquid Chromatography and Nano-Electrospray Ion-Trap Mass Spectrometry for Detection of Clostridium botulinum Type C and the Produced Botulinum Neurotoxin Type C Complex. J. Microbiol. Methods 2021, 193, 106397. [Google Scholar] [CrossRef]
- Centurioni, D.A.; Egan, C.T.; Perry, M.J. Current Developments in Diagnostic Assays for Laboratory Confirmation and Investigation of Botulism. J. Clin. Microbiol. 2021, JCM0013920. [Google Scholar] [CrossRef]
- Diamant, E.; Torgeman, A.; Epstein, E.; Mechaly, A.; David, A.B.; Levin, L.; Schwartz, A.; Dor, E.; Girshengorn, M.; Barnea, A.; et al. A Cell-Based Alternative to the Mouse Potency Assay for Pharmaceutical Type E Botulinum Antitoxins. ALTEX 2021, 39, 113–122. [Google Scholar] [CrossRef]




| Type | Toxin Produced | |||||
|---|---|---|---|---|---|---|
| CPA | CPB | ETX | ITX | CPE | NetB | |
| A | + | − | − | − | − | − |
| B | + | + | + | − | − | − |
| C | + | + | − | − | +/− | − |
| D | + | − | + | − | +/− | − |
| E | + | − | − | + | +/− | − |
| F | + | − | − | − | + | − |
| G | + | − | − | − | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzal, F.A.; Navarro, M.A.; Asin, J.; Henderson, E.E. Clostridial Diseases of Horses: A Review. Vaccines 2022, 10, 318. https://doi.org/10.3390/vaccines10020318
Uzal FA, Navarro MA, Asin J, Henderson EE. Clostridial Diseases of Horses: A Review. Vaccines. 2022; 10(2):318. https://doi.org/10.3390/vaccines10020318
Chicago/Turabian StyleUzal, Francisco A., Mauricio A. Navarro, Javier Asin, and Eileen E. Henderson. 2022. "Clostridial Diseases of Horses: A Review" Vaccines 10, no. 2: 318. https://doi.org/10.3390/vaccines10020318
APA StyleUzal, F. A., Navarro, M. A., Asin, J., & Henderson, E. E. (2022). Clostridial Diseases of Horses: A Review. Vaccines, 10(2), 318. https://doi.org/10.3390/vaccines10020318






