Non-Inferiority Field Study Comparing the Administrations by Conventional Needle-Syringe and Needle-Free Injectors of a Trivalent Vaccine Containing Porcine Circovirus Types 2a/2b and Mycoplasma hyopneumoniae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm
2.2. Experimental Design
2.3. Post-Vaccination Skin Reaction
2.4. Clinical Observations
2.5. Average Daily Weight Gain
2.6. Serology
2.7. Quantification of PCV2d DNA in Serum
2.8. Quantification of M. hyopneumoniae DNA in Larynx
2.9. Pathology
2.10. Statistical Analysis
3. Results
3.1. Post-Vaccination Skin Reactions
3.2. Clinical Signs
3.3. Mortality
3.4. Average Daily Weight Gain
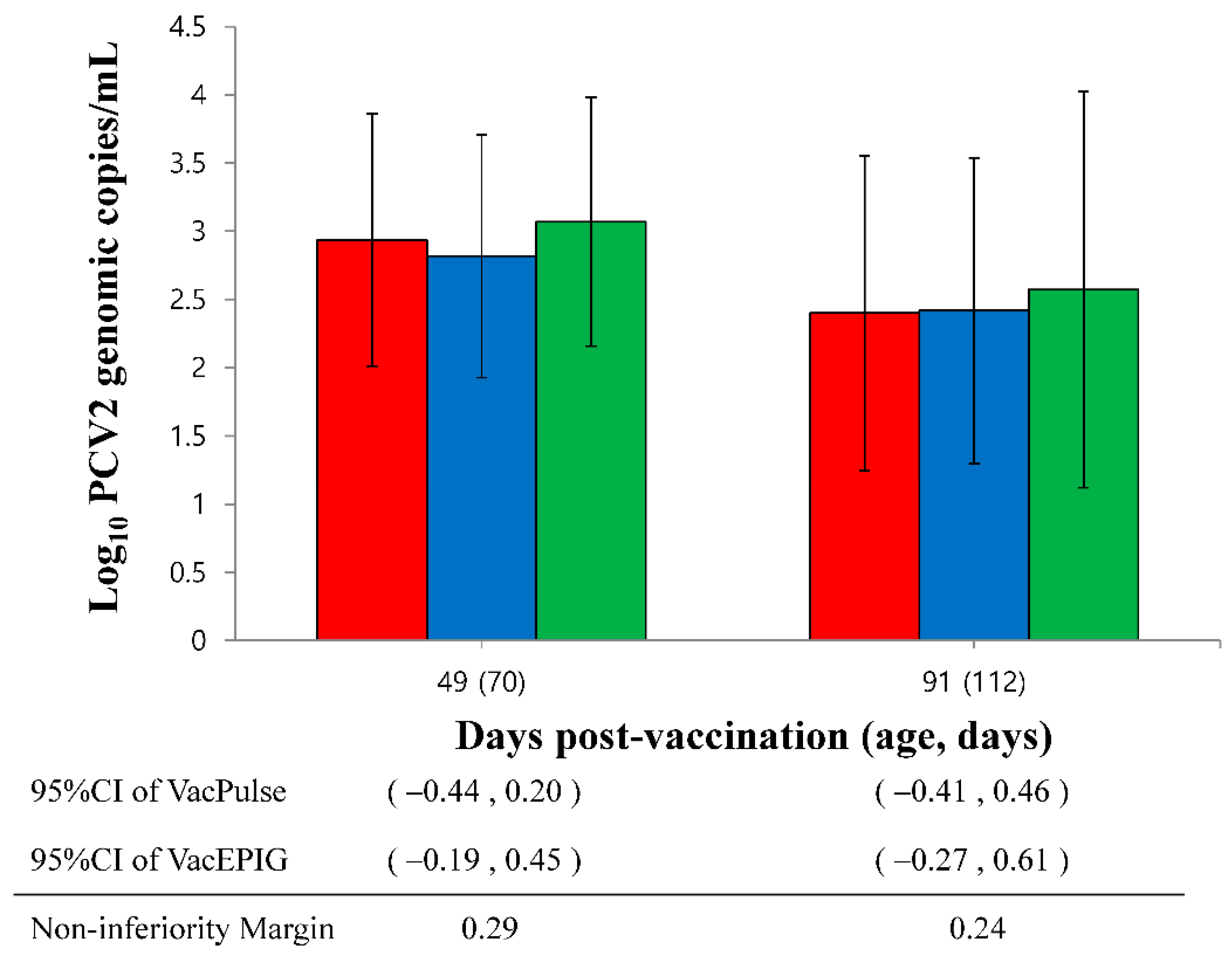
3.5. Quantification of PCV2d DNA in Serum
3.6. Quantification of M. hyopneumoniae DNA in Larynx
3.7. PCV2 Serology
3.8. Mycoplasma hyopneumoniae Serology
3.9. Pathology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chae, C. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 2005, 169, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Postweaning multisystemic wasting syndrome: A review of aetiology, diagnosis and pathology. Vet. J. 2004, 168, 41–49. [Google Scholar] [CrossRef]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.; Sibila, M.; Kuhnert, P.; Segalés, J.; Haesebrouck, F.; Pieters, M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound. Emerg. Dis. 2018, 65, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Chung, H.K.; Chae, C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet. J. 2003, 166, 251–256. [Google Scholar] [CrossRef]
- Martinez-Miro, S.; Tecles, F.; Ramon, M.; Escribano, D.; Hemandez, F.; Madrid, J.; Orengo, J.; Martinez-Subiela, S.; Manteca, X.; Ceron, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Chase, C.C.L.; Daniels, C.S.; Garcia, R.; Milward, F.; Nation, T. Needle-free injection technology in swine: Progress toward vaccine efficacy and pork quality. J. Swine Health Prod. 2008, 16, 254–261. [Google Scholar]
- Otake, S.; Dee, S.A.; Rossow, K.D.; Joo, H.S.; Deen, J.; Molitor, T.W.; Pijoan, C. Transmission of porcine reproductive and respiratory syndrome virus by needles. Vet. Rec. 2002, 150, 114–115. [Google Scholar] [CrossRef]
- Baker, S.R.; Mondaca, E.; Polson, D.; Dee, S.D. Evaluation of a needle-free device to prevent hematogenous transmission of porcine reproductive and respiratory syndrome virus. J. Swine Health Prod. 2012, 20, 123–128. [Google Scholar]
- Imeah, B.; Penz, E.; Rana, M.; Trask, C. For the Needle-less Injector Study Team Economic analysis of new workplace technology including productivity and injury: The case of needle-less injection in swine. PLoS ONE 2020, 15, e0233599. [Google Scholar]
- Yang, S.; Oh, T.; Park, K.H.; Cho, H.; Suh, J.; Chae, C. Experimental efficacy of a trivalent vaccine containing porcine types 2a/b and Mycoplasma hyopneumoniae against PCV2d and M. hyopneumoniae challenges. Vet. Microbiol. 2021, 258, 109100. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Cortey, M.; Segalés, J.; Hughes, J.; Drigo, M. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol. Phylogenet. Evol. 2016, 100, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Chae, C. The prevalence of porcine circovirus type 2e (PCV2e) in Korean slaughter pig lymph nodes when compared with other PCV2 genotypes. Transbound. Emerg. Dis. 2021, 68, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Um, H.; Yang, S.; Oh, T.; Park, K.; Cho, H.; Suh, J.; Min, K.-D.; Chae, C. Comparative evaluation of growth performance between bivalent and trivalent vaccines containing porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae in a herd with subclinical PCV2d infection and enzootic pneumonia. Vaccines 2021, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef]
- Dubosson, C.R.; Conzelmann, C.; Miserez, R.; Boerlin, P.; Frey, J.; Zimmermann, W.; Häni, H.; Kuhnert, P. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet. Microbiol. 2004, 102, 55–65. [Google Scholar] [CrossRef]
- Opriessnig, T.; Thacker, E.L.; Yu, S.; Fenaux, M.; Meng, X.-J.; Halbur, P.G. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 2004, 41, 624–640. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Chae, C. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J. Comp. Pathol. 2004, 131, 121–126. [Google Scholar] [CrossRef]
- Jones, G.F.; Rapp-Gabrielson, V.; Wilke, R.; Thacker, E.L.; Thacker, B.J.; Gergen, L.; Sweeney, D.; Wasmoen, T. Intradermal vaccination for Mycoplasma hyopneumoniae. J. Swine Health Prod. 2005, 13, 19–27. [Google Scholar]
- Bennett, C.R.; Mundell, R.D.; Monheim, L.M. Studies on tissue penetration characteristics produced by jet injection. J. Am. Dent. Assoc. 1971, 83, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Undi, M.; Rodriguez-Lecompte, J.; Joseph, T.; Morrison, J.; Yitbarek, A.; Wittenberg, K.; Tremblay, R.; Corw, G.; Ominski, K. A study of the effectiveness of a needle-free injection device compared with a needle and syringe used to vaccinate calves against bovine viral diarrhea and infectious bovine rhinotracheitis viruses. Vet. J. 2013, 198, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Giudice, E.; Campbell, D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Trask, C.; Bath, B.; Milosavljevic, S.; Kociolek, A.M.; Predicala, B.; Penz, E.; Adebayo, O.; Whittington, L. Evaluating swine injection technologies as a workplace musculoskeletal injury intervention: A study protocol. BioMed Res. Int. 2017, 2017, 5094509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafer, A.L.; Langley, R.L.; Morrow, W.E.M.; Tulis, J.J. Occupational hazards reported by swine veterinarians in the United States. J. Swine Health Prod. 1996, 4, 128–141. [Google Scholar]







| Groups | No. of Pigs | Injection Instrument | Dosage | Age (Days) |
|---|---|---|---|---|
| VacS | 60 | Syringe | One (2.0 mL) | 21 |
| VacPulse | 60 | Needle-free Pulse FX device | One (2.0 mL) | 21 |
| VacEPIG | 60 | Needle-free EPIG device | One (2.0 mL) | 21 |
| UnVac | 60 | Syringe | One (2.0 mL) | 21 |
| Age (Days) | Groups | ||||
|---|---|---|---|---|---|
| VacS | VacPulse | VacEPIG | UnVac | ||
| Body weight | 21 | 6.02 ± 0.33 | 5.96 ± 0.30 | 5.99 ± 0.32 | 5.93 ± 0.35 |
| (Kg) | 175 | 105.65 ± 2.46 a | 105.32 ± 2.24 a | 104.93 ± 1.99 a | 99.30 ± 2.68 b |
| ADWG | 21–70 | 392.82 ± 39.44 | 388.71 ± 31.96 | 391.87 ± 31.50 | 376.50 ± 33.16 |
| (gram/pig/day) | 70–175 | 765.21 ± 31.16 a | 764.78 ± 23.91 a | 759.41 ± 22.76 a | 713.04 ± 26.84 b |
| 21–175 | 647.03 ± 15.32 a | 645.14 ± 15.04 a | 642.55 ± 13.12 a | 606.36 ± 17.86 b | |
| Groups | ||||
|---|---|---|---|---|
| VacS | VacPulse | VacEPIG | UnVac | |
| Macroscopic | 16.92 ± 9.72 a | 18.08 ± 10.34 a | 19.66 ± 9.87 a | 31.62 ± 11.05 b |
| lung lesions | ||||
| Microscopic | 0.73 ± 0.62 a | 0.82 ± 0.71 a | 0.92 ± 0.68 a | 2.06 ± 0.59 b |
| lung lesions | ||||
| Microscopic | 0.56 ± 0.57 a | 0.51 ± 0.47 a | 0.60 ± 0.52 a | 1.76 ± 0.63 b |
| lymphoid lesions | ||||
| Macroscopic Lung Lesions | Microscopic Lung Lesions | Microscopic Lymphoid Lesions | ||||
|---|---|---|---|---|---|---|
| OR | (95%CI) | OR | (95%CI) | OR | (95%CI) | |
| VacPulse | 0.96 | (0.47–2.00) | 1.34 | (0.63–2.89) | 0.56 | (0.23–1.32) |
| VacEPIG | 1.19 | (0.57–2.46) | 1.50 | (0.70–3.24) | 1.03 | (0.28–1.52) |
| VacS | 1.00 | (Reference) | 1.00 | (Reference) | 1.00 | (Reference) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Ahn, Y.; Oh, T.; Suh, J.; Chae, C. Non-Inferiority Field Study Comparing the Administrations by Conventional Needle-Syringe and Needle-Free Injectors of a Trivalent Vaccine Containing Porcine Circovirus Types 2a/2b and Mycoplasma hyopneumoniae. Vaccines 2022, 10, 358. https://doi.org/10.3390/vaccines10030358
Cho H, Ahn Y, Oh T, Suh J, Chae C. Non-Inferiority Field Study Comparing the Administrations by Conventional Needle-Syringe and Needle-Free Injectors of a Trivalent Vaccine Containing Porcine Circovirus Types 2a/2b and Mycoplasma hyopneumoniae. Vaccines. 2022; 10(3):358. https://doi.org/10.3390/vaccines10030358
Chicago/Turabian StyleCho, Hyejean, Yongjun Ahn, Taehwan Oh, Jeongmin Suh, and Chanhee Chae. 2022. "Non-Inferiority Field Study Comparing the Administrations by Conventional Needle-Syringe and Needle-Free Injectors of a Trivalent Vaccine Containing Porcine Circovirus Types 2a/2b and Mycoplasma hyopneumoniae" Vaccines 10, no. 3: 358. https://doi.org/10.3390/vaccines10030358
APA StyleCho, H., Ahn, Y., Oh, T., Suh, J., & Chae, C. (2022). Non-Inferiority Field Study Comparing the Administrations by Conventional Needle-Syringe and Needle-Free Injectors of a Trivalent Vaccine Containing Porcine Circovirus Types 2a/2b and Mycoplasma hyopneumoniae. Vaccines, 10(3), 358. https://doi.org/10.3390/vaccines10030358






