Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects
Abstract
:1. Introduction
2. The Origin and Transmission of YF
2.1. The Origin of YF
2.2. The Transmission of YF
3. YF Epidemiology and Transmission Cycles
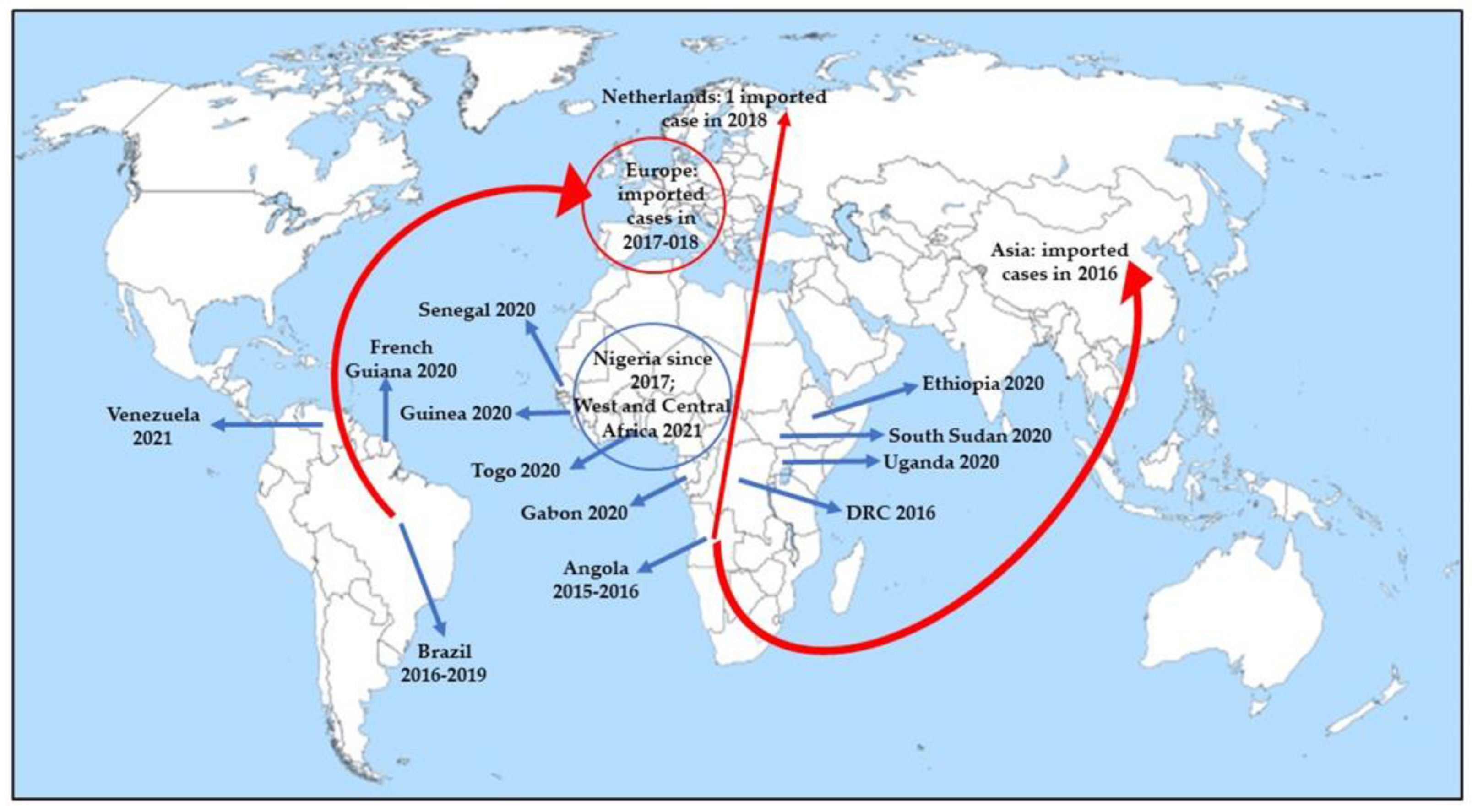
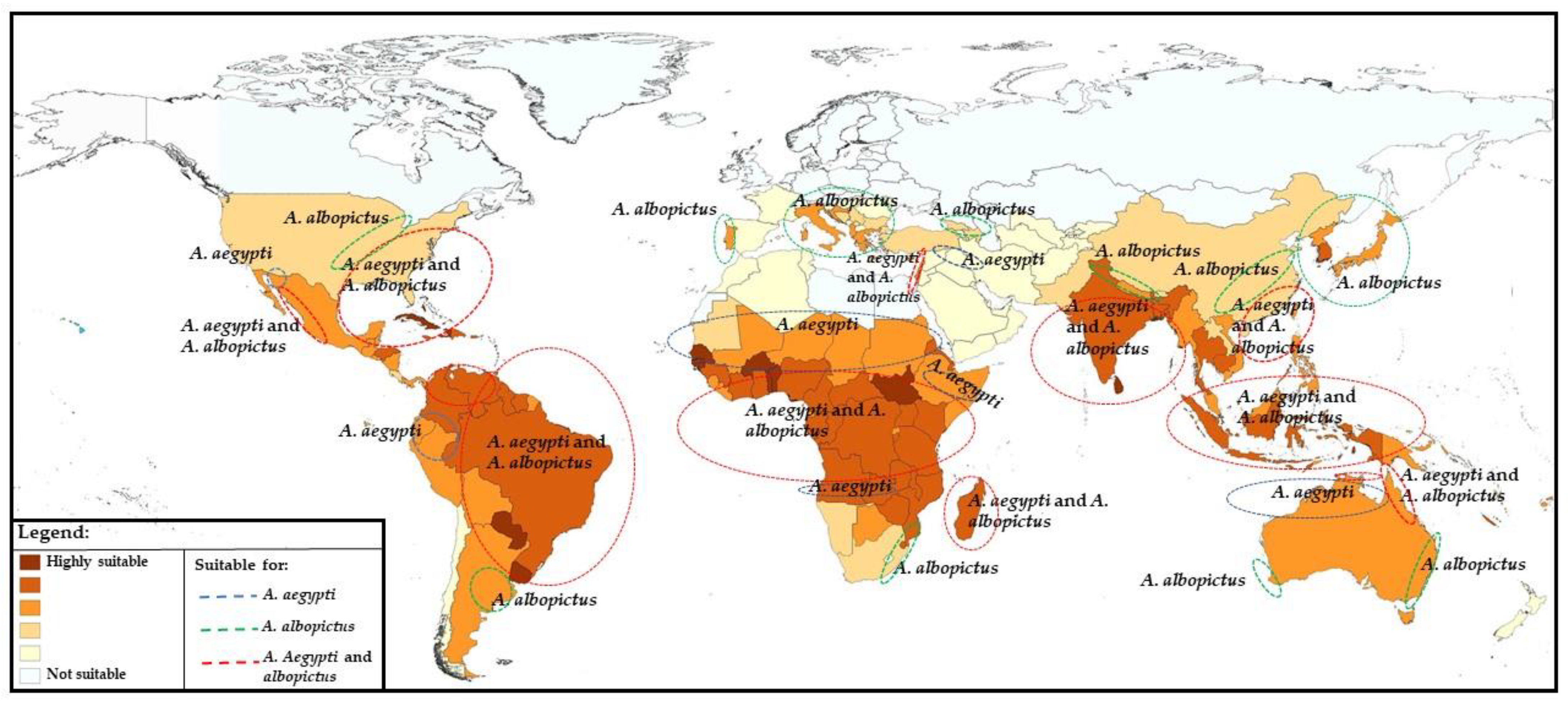
4. Prospects for Changing Epidemiology in Future
5. YF Vaccine
5.1. The History of the YF Vaccine
5.2. YF Vaccine
5.3. YF Vaccine Efficacy
5.4. Common Symptoms and Adverse Effects to Yellow Fever Vaccine
5.5. The Issue of YF Vaccine Supply: The Use of Fractioning Doses
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staples, J.E.; Monath, T.P. Yellow fever: 100 years of discovery. JAMA 2008, 300, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Litvoc, M.N.; Novaes, C.T.G.; Lopes, M.I.B.F. Yellow fever. Rev. Assoc. Med. Bras. 2018, 64, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.A.; Vasconcelos, P.F.C.; Staples, J.E. The whole iceberg: Estimating the incidence of yellow fever virus infection from the number of severe cases. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellow Fever. Available online: https://www.who.int/news-room/fact-sheets/detail/yellow-fever (accessed on 29 December 2021).
- Garske, T.; Van Kerkhove, M.D.; Yactayo, S.; Ronveaux, O.; Lewis, R.F.; Staples, J.E.; Perea, W.; Ferguson, N.M. Yellow Fever Expert Committee. Yellow Fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014, 11, e1001638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chippaux, J.P.; Chippaux, A. Yellow fever in Africa and the Americas: A historical and epidemiological perspective. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2018, 24, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, J.E.; Holmes, E.C.; Barrett, A.D. Out of Africa: A molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007, 3, e75. [Google Scholar] [CrossRef] [Green Version]
- White, C.R. Yellow fever; history of the disease in the eighteenth and nineteenth century. J. Kans. Med. Soc. 1959, 60, 298–302. [Google Scholar]
- Thomas, H. The slave trade: The story of the Atlantic slave trade. Touchstone N. Y. 1999, 1440–1870. [Google Scholar]
- Fontenille, D.; Diallo, M.; Mondo, M.; Ndiaye, M.; Thonnon, J. First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 533–535. [Google Scholar] [CrossRef]
- Sternberg, G.M. Report of the Etiology and Prevention of Yellow Fever; Government Printing Office: Washington, DC, USA, 1890; pp. 49–65.
- Patterson, K.D. Yellow fever epidemics and mortality in the United States, 1693–1905. Soc. Sci. Med. 1992, 34, 855–865. [Google Scholar] [CrossRef]
- Bollet, A.J. Military medicine in the Spanish-American War. Perspect Biol. Med. Spring. 2005, 48, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Guardia, C.A. Public health and sanitation in the Panama Canal Zone: A historical account, 1880–1915. Bol. Oficina Sanit. Panam. 1983, 95, 62–73. [Google Scholar] [PubMed]
- Reed, W.; Carroll, J.; Agramonte, A. The etiology of yellow fever: An additional note. JAMA 1901, 36, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Bryan, C.S.; Moss, S.W.; Kahn, R.J. Yellow fever in the Americas. Infect. Dis. Clin. N. Am. 2004, 18, 275–292. [Google Scholar] [CrossRef]
- Strode, G.K.; Bugher, J.C.; Austin-Kerr, J.; Smith, H.H.; Smithburn, K.C.; Taylor, R.M.; Theiler, M.; Warren, A.J.; Whitman, L. Yellow Fever; McGraw-Hill Book Co.: New York, NY, USA, 1951. [Google Scholar]
- Beck, A.; Guzmán, H.; Li, L.; Ellis, B.; Tesh, R.B.; Barret, A.D.T. Phylogeographic reconstruction of African yellow fever virus isolates indicates recent simultaneous dispersal into East and West Africa. PLoS Negl. Trop. Dis. 2013, 7, e1910. [Google Scholar] [CrossRef] [Green Version]
- Yellow Fever. Available online: https://www.cdc.gov/globalhealth/newsroom/topics/yellowfever/index.html (accessed on 29 December 2021).
- Johansson, M.A.; Arana-Vizcarrondo, N.; Biggerstaff, B.J.; Gallagher, N.; Marano, N.; Staples, J.E. Assessing the risk of international spread of yellow fever virus: A mathematical analysis of an urban outbreak in Asuncion, 2008. Am. J. Trop. Med. 2012, 86, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.A.; Santos, F.B.D.; Vazeille, M.; Vasconcelos, P.; Lourenço-de-Oliveira, R.; Failloux, A.B. Potential risk of re-emergence of urban transmission of yellow fever virus in Brazil facilitated by competent aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef]
- Simon, L.V.; Hashmi, M.F.; Torp, K.D. Yellow Fever; Stat Pearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Monath, T.P. Facing up to re-emergence of urban yellow fever. Lancet 1999, 353, 1541. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/emergencies/disease-outbreak-news (accessed on 23 December 2021).
- World Health Organization. Yellow Fever Outbreak Angola, Democratic Republic of the Congo and Uganda 2016–2017; World Health Organization: Geneva, Switzerland, 2017; Available online: http://www.who.int/emergencies/yellowfever/en (accessed on 1 February 2022).
- Ahmed, Q.A.; Memish, Z.A. Yellow fever from Angola and Congo: A storm gathers. Trop. Dr. 2017, 47, 92–96. [Google Scholar] [CrossRef]
- Ortiz-Martinez, Y.; Patiño-Barbosa, A.M.; Rodriguez-Morales, A.J. Yellow fever in the Americas: The growing concern about new epidemics. F1000Research 2017, 6, 398. [Google Scholar] [CrossRef]
- Gaythorpe, K.A.; Abbas, K.; Huber, J.; Karachaliou, A.; Thakkar, N.; Woodruff, K.; Li, X.; Echeverria-Londono, S.; VIMC Working Group on COVID-19 Impact on Vaccine Preventable Disease; Ferrari, M.; et al. Impact of COVID-19-related disruptions to measles, meningococcal A, and yellow fever vaccination in 10 countries. Elife 2021, 10, e67023. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Eliminate Yellow fever Epidemics (EYE): A global strategy, 2017–2026. Wkly. Epidemiol. Rec. 2017, 16, 193–204. [Google Scholar]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cathey, J.T.; Marr, J.S. Yellow fever, Asia and the East African slave trade. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J.; Wallis, G.P.; Aitken, T.H.; Miller, B.R.; Amato, G.D.; Lorenz, L.; Powell, J.R.; Beaty, B.J. Oral infection of Aedes aegypti with yellow fever virus: Geographic variation and genetic considerations. Am. J. Trop. Med. Hyg. 1985, 34, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Brent, S.E.; Watts, A.; Cetron, M.; German, M.; Kraemer, M.U.G.; Bogoch, I.I.; Brady, O.J.; Hay, S.I.; Creatore, M.I.; Khan, K. Identifying Global Vulnerabilities to Urban Transmission of Yellow Fever Virus. Bull. World Health Organ. 2018, 96, 343. [Google Scholar] [CrossRef]
- Gubler, D.J. Potential yellow fever epidemics in unexposed populations. Bull. World Health Organ. 2018, 96, 299. [Google Scholar] [CrossRef]
- Wasserman, S.; Tambyah, P.A.; Lim, P.L. Yellow fever cases in Asia: Primed for an epidemic. Int. J. Infect. Dis. 2016, 489, 8–103. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar]
- Cracknell, D.B.; Gaythorpe, K.; Imai, N.; Dorigatti, I. Yellow fever in Asia-a risk analysis. J. Travel Med. 2021, 28, taab015. [Google Scholar] [CrossRef]
- Kuno, G. The absence of yellow fever in Asia: History, hypotheses, vector dispersal, possibility of YF in Asia, and other enigmas. Viruses 2020, 12, 1349. [Google Scholar]
- Abrao, E.P.; Da Fonseca, B.A. Infection of Mosquito Cells (C6/36) by Dengue-2 Virus Interferes with Subsequent Infection by Yellow Fever Virus. Vector Borne Zoonotic Dis. 2016, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.P.A.; Fonseca, B.A.L. Dengue-2 Virus Almost Abolish Yellow Fever Virus Replication in C6/36 Cells; Amer Soc Trop Med & Hygiene: Atlanta, GA, USA, 2006. [Google Scholar]
- Kumm, H. Seasonal variations in rainfall: Prevalence of Haemagogus and incidence of jungle yellow fever in Brazil and Colombia. Trans. R. Soc. Trop. Med. Hyg. 1950, 43, 673–682. [Google Scholar] [CrossRef]
- Chen, L.H.; Wilson, M.E. Yellow fever control: Current epidemiology and vaccination strategies. Trop. Dis. Travel Med. Vaccines 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Hamlet, A.; Gaythorpe, K.A.M.; Garske, T.; Ferguson, N.M. Seasonal and inter-annual drivers of yellow fever transmission in South America. PLoS Negl. Trop. Dis. 2021, 15, e0008974. [Google Scholar] [CrossRef] [PubMed]
- Faust, C.L.; McCallum, H.I.; Bloomfield, L.S.P.; Gottdenker, N.L.; Gillespie, T.R.; Torney, C.J.; Dobson, A.P.; Plowright, R.K. Pathogen spillover during land conversion. Ecol. Lett. 2018, 21, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, T.L.; Gillespie, T.R.; Rwego, I.B.; Estoff, E.L.; Chapman, C.A. Forest Fragmentation as Cause of Bacterial Transmission among Primates, Humans, and Livestock, Uganda. Emerg. Infect. Dis. 2008, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- Gottdenker, N.L.; Chaves, L.F.; Calzada, J.E.; Saldana, A.; Carroll, C.R. Host Life History Strategy, Species Diversity, and Habitat Influence Trypanosoma cruzi Vector Infection in Changing Landscapes. PLoS Negl. Trop. Dis. 2012, 6, e1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seltmann, A.; Czirjak, G.A.; Courtiol, A.; Bernard, H.; Struebig, M.J.; Voigt, C.C. Habitat disturbance results in chronic stress and impaired health status in forest-dwelling paleotropical bats. Conserv. Physiol. 2017, 5, cox020. [Google Scholar] [CrossRef] [Green Version]
- Burkett-Cadena, N.D.; Vittor, A.Y. Deforestation and vector-borne disease: Forest conversion favors important mosquito vectors of human pathogens. Basic Appl. Ecol. 2018, 26, 101–110. [Google Scholar] [CrossRef]
- Sadeghieh, T.; Sargeant, J.M.; Greer, A.L.; Berke, O.; Dueymes, G.; Gachon, P.; Ogden, N.H.; Ng, V. Yellow fever virus outbreak in Brazil under current and future climate. Infect. Dis. Model. 2021, 6, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.H.; Childs, M.L.; Caldwell, J.M.; Mordecai, E.A. Seasonal temperature variation influences climate suitability for dengue, chikungunya, and Zika transmission. PLoS Negl. Trop. Dis. 2018, 12, e0006451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslwanter, D.; Lasso, G.; Wec, A.Z.; Furtado, N.D.; Raphael, L.M.S.; Tse, A.L.; Sun, Y.; Stransky, S.; Pedreño-Lopez, N.; Correia, C.A.; et al. Genotype-specific features reduce the susceptibility of South American yellow fever virus strains to vaccine-induced antibodies. Cell Host Microbe 2022, 30, 248–259.e6. [Google Scholar] [CrossRef] [PubMed]
- Jácome, R.; Carrasco-Hernández, R.; Campillo-Balderas, J.A.; López-Vidal, Y.; Lazcano, A.; Wenzel, R.P.; Ponce De León, S. A yellow flag on the horizon: The looming threat of yellow fever to North America. Int. J. Infect. Dis. 2019, 87, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Ogden, N.H.; Milka, R.; Caminade, C.; Gachon, P. Recent and projected future climatic suitability of North America for the Asian tiger mosquito Aedes albopictus. Parasit. Vectors 2014, 7, 532. [Google Scholar] [CrossRef]
- Lowe, A.M.; Forest-Bérard, K.; Trudel, R.; Lo, E.; Gamache, P.; Tandonnet, M.; Kotchi, S.O.; Leighton, P.; Dibernardo, A.; Lindsay, R.; et al. Mosquitoes Know No Borders: Surveillance of Potential Introduction of Aedes Species in Southern Québec, Canada. Pathogens 2021, 10, 998. [Google Scholar] [CrossRef]
- Fontenille, D.; Cruaud, A.; Vial, L.; Garros, C. Understanding the role of arthropod vectors in the emergence and spread of plant, animal and human diseases. A chronicle of epidemics foretold in South of France. Comptes Rendus Biol. 2021, 343, 311–344. [Google Scholar] [CrossRef]
- Soper, F.L. The newer epidemiology of yellow fever. Am. J. Public Health Nations Health 1937, 27, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Monath, T.P. Yellow fever: Victor, Victoria? Conqueror, Conquest? Epidemics and research in the last forty years and prospects for the future. Am. J. Trop. Med. Hyg. 1991, 45, 1–43. [Google Scholar] [CrossRef]
- Monath, T.P.; Cetron, M.S.; Teuwen, D.E. Yellow Fever. In Vaccines, 5th ed.; Plotkin, S., Orenstein, W.A., Offit, P., Eds.; Elsevier: Philadelphia, PA, USA, 2008; pp. 959–1056. [Google Scholar]
- World Health Organization. Assessment of yellow fever epidemic risk—A decision-making tool for preventive immunization campaigns. Wkly. Epidemiol. Rec. 2007, 82, 153–160. [Google Scholar]
- Rezende, I.M.; Sacchetto, L.; Munhoz de Mello, E.; Alves, P.A.; Campos de Melo Iani, F.; Adelino, T.E.R.; Duarte, M.M.; Cury, A.L.F.; Bernardes, A.F.L.; Santos, T.A.; et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl. Trop. Dis. 2018, 12, e0006538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussallem, T.M.; Gava, C.; Ardisson, K.S.; Soares Marques, C.; Graceli, G.C.; Aline Da Penha Valadares Koski, A.V.; Almada, G.L.; Rodrigues Da Silva, A.; Alves De Jesus, F.A.; Rodrigues, G.A.P.; et al. Yellow fever outbreak in a rural-urban mixed community of Espirito Santo, Brazil: Epidemiological aspects. Rev. Panam. Salud. Publ. 2019, 43, e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearer, F.M.; Moyes, C.L.; Pigott, D.M.; Brady, O.J.; Marinho, F.; Deshpande, A.; Longbottom, J.; Browne, A.J.; Kraemer, M.U.G.; O’Reilly, K.M.; et al. Global yellow fever vaccination coverage from 1970 to 2016: An adjusted retrospective analysis. Lancet Infect. Dis. 2017, 17, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- Monath, T.P. Yellow fever vaccine. In Vaccines, 4th ed.; Plotkin, S.A., Orenstein, W.A., Eds.; WB Saunders: Philadelphia, PA, USA, 2004; pp. 1095–1176. [Google Scholar]
- WHO. Vaccines and vaccination against yellow fever. Wkly. Epidemiol. Rec. 2013, 88, 269–283. [Google Scholar]
- Collins, N.D.; Barrett, A.D. Live attenuated yellow fever 17D vaccine: A legacy vaccine still controlling outbreaks in modern day. Curr. Infect. Dis. Rep. 2017, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.C.; Campi-Azevedo, A.C.; Peruhype-Megalhaes, V.; Costa-Pereira, C.; Albuquerque, C.P.; Muniz, L.F.; Yokoy De Souza, T.; Oliveira, A.C.V.; Martins-Filho, O.A.; Da Mota, L.M.H. The 17D-204 and 17DD yellow fever vaccines: An overview of major similarities and subtle differences. Expert Rev. Vaccines 2018, 17, 79–90. [Google Scholar] [CrossRef]
- Post, P.R.; Santos, C.N.; Carvalho, R.; Cruz, A.C.; Rice, C.M.; Galler, R. Heterogeneity in envelope protein sequence and N-linked glycosylation among yellow fever virus vaccine strains. Virology 1992, 188, 160–167. [Google Scholar] [CrossRef]
- Barrett, A.D.T. Yellow fever live attenuated vaccine: A very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine 2017, 35, 5951–5955. [Google Scholar] [CrossRef]
- Staples, J.E.; Bocchini, J.A., Jr.; Rubin, L.; Fischer, M. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine booster doses: Recommendations of the advisory committee on immunization practices, 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 647–650. [Google Scholar]
- Leder, K.; Chen, L.H.; Wilson, M.E. Aggregate travel vs. single trip assessment: Arguments for cumulative risk analysis. Vaccine 2012, 30, 2600–2604. [Google Scholar] [CrossRef]
- Lown, B.A.; Chen, L.H.; Wilson, M.E.; Sisson, E.; Gershman, M.; Yanni, E.; Jentes, E.S.; Hochberg, N.S.; Hamer, D.; Barnett, E.D. Vaccine administration decision making: The case of yellow fever vaccine. Clin. Infect. Dis. 2012, 55, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lown, B.A.; Chen, L.H.; Han, P.V.; Jentes, E.S.; Wilson, M.E.; Benoit, C.M.; Avery, K.A.; Ooi, W.; Hamer, D.H.; Barnett, E.D. Preferences and decision needs of Boston-area travelers to countries with risk of yellow fever transmission: Implications for health care providers. J. Travel Med. 2014, 21, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poland, J.D.; Calisher, C.H.; Monath, T.P.; Downs, W.G.; Murphy, K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull. World Health Organ. 1981, 59, 895–900. [Google Scholar]
- Advisory Committee on Immunization Practices (ACIP). Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for Use of Yellow Fever Vaccine Booster Doses. Available online: https://www.cdc.gov/vaccines/acip/recs/grade/yf-vac-boost.html2015 (accessed on 29 December 2021).
- Elliott, M. Yellow fever in the recently inoculated. Trans. R. Soc. Trop. Med. Hyg. 1944, 38, 231–234. [Google Scholar] [CrossRef]
- Mason, R.A.; Tauraso, N.M.; Spertzel, R.O.; Ginn, R.K. Yellow fever vaccine: Direct challenge of monkeys given graded doses of 17D vaccine. Appl. Microbiol. 1973, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Gershman, M.; Fischer, M. Centers for Disease Control & Prevention. Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 2010, 59, 1–27. [Google Scholar]
- Fox, J.P.; Elveback, L.; Scott, W.; Gatewood, L.; Ackerman, E. Herd immunity: Basic concept and relevance to public health immunization practises. Am. J. Epidemiol. 1995, 141, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Gee, F.L. Immunity to yellow fever nine years after vaccination with 17D vaccine. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 449–458. [Google Scholar] [CrossRef]
- Courtois, G. Duration of immunity after yellow vaccination. Ann. Soc. Belg. Med. Trop. 1954, 34, 9–12. [Google Scholar]
- Groot, H.; Riberiro, R.B. Neutralizing and haemmaglutination-inhibiting anti-bodies to yellow fever 17 years after vaccination with 17D vaccine. Bull. World Health Organ. 1962, 27, 699–707. [Google Scholar]
- Rosenzweig, E.C.; Babione, R.W.; Wisseman, C.L., Jr. Immunological studies with group B arthropod-borne viruses. IV. Persistence of yellow fever antibodies following vaccination with 17D strain yellow fever vaccine. Am. J. Trop. Med. Hyg. 1963, 12, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Julander, J.G.; Trent, D.W.; Monath, T.P. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine 2011, 29, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines for Plaque Reduciton Neutralization Testing of Human Antibodies to Dengue Viruses. 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/69687/who_ivb_07.07_eng.pdf;jsessionid=19BF765D9B3FDAD85EF3F217B0165953?sequence=1 (accessed on 23 December 2021).
- Roehrig, J.T.; Hombach, J.; Barrett, A.D. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Gotuzzo, E.; Yactayo, S.; Córdova, E. Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013, 89, 434–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collaborative group for studies on yellow fever vaccines. Duration of post-vaccination immunity against yellow fever in adults. Vaccine 2014, 32, 4977–4984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanna, I.J.; Slifka, M.K. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert Rev. Vaccines 2016, 15, 1519–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieten, R.W.; Jonker, E.F.; Van Leeuwen, E.M.; Remmerswaal, E.B.; Ten Berge, I.J.; De Visser, A.W.; Van Genderen, P.J.J.; Goorhuis, A.; Visser, L.G.; Grobusch, M.P.; et al. A single 17D yellow fever vaccination provides lifelong immunity; Characterization of Yellow-Fever-Specific Neutralizing Antibody and T-Cell Responses after Vaccination. PLoS ONE 2016, 11, e0149871. [Google Scholar] [CrossRef] [Green Version]
- Visser, L.G.; Veit, O.; Chen, L.H. Waning immunity after single-dose yellow fever vaccination: Who needs a second shot? J. Travel Med. 2019, 26, tay134. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.C.; Barrett, A.D.T. Are booster doses of yellow fever vaccine needed? Lancet Infect. Dis. 2019, 19, 1275–1276. [Google Scholar] [CrossRef] [Green Version]
- Group for Studies of Yellow Fever Vaccine. A randomized double-blind clinical trial of two yellow fever vaccines prepared with substrains 17DD and 17D-213/77 in children nine-23 months old. Mem. Inst. Oswaldo Cruz 2015, 110, 771–780. [Google Scholar] [CrossRef]
- Goujon, C.; Gougeon, M.L.; Tondeur, L.; Poirier, B.; Seffer, V.; Desprès, P.; Consigny, P.H.; Vray, M.; Study Group. A study of the immune response to yellow fever vaccine among infants previously immunized against measles. Vaccine 2017, 35, 6166–6171. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Fraissinet, J.; Ansah, P.O.; Kelly, C.; Bhat, N.; Sow, S.O.; Mejía, J.E. Long-term immunity against yellow fever in children vaccinated during infancy: A longitudinal cohort study. Lancet Infect. Dis. 2019, 19, 1363–1370. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.; Zuckerman, J.; Clarke, P.; Barrett, P.; Kirkpatrick, C.; Blondeau, C. Comparison of the immunogenicity and safety of two 17D yellow fever vaccines. Am. J. Trop. Med. Hyg. 1999, 60, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Monath, T.P.; Nichols, R.; Archambault, W.R.; Moore, L.; Marchesani, R.; Tian, J.; Shope, R.E.; Thomas, N.; Schrader, N.; Furby, D.; et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and yellow fever-VAX) in a Phase III multicenter, double-blind clinical trial. Am. J. Trop. Med. Hyg. 2002, 66, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Kelso, J.M.; Mootrey, G.T.; Tsai, T.F. Anaphylaxis from yellow fever vaccine. J. Allergy Clin. Immunol. 1999, 103, 698–701. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 2002, 51, 4–6. [Google Scholar]
- Centers for Disease Control and Prevention. Yellow Fever Vaccine Information Statement. 2004. Available online: http://www.cdc.gov/nip/publications/VIS/vis-yf.pdf (accessed on 25 January 2007).
- Gerasimon, G.; Lowry, K. Rare case of fatal yellow fever vaccine-associated viscerotropic disease. South. Med. J. 2005, 98, 653–656. [Google Scholar] [CrossRef]
- Martin, M.; Weld, L.H.; Tsai, T.F.; Mootrey, G.T.; Chen, R.T.; Niu, M.; Cetron, M.S. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg. Infect. Dis. 2001, 7, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Eidex, B.R. Yellow Fever Vaccine Safety Working Group. History of thymoma and yellow fever vaccination. Lancet 2004, 364, 936. [Google Scholar] [CrossRef]
- World Health Organization. Fractional Dose Yellow Fever Vaccine as a Dose-Sparing Option for Outbreak Response; WHO Secretariat Information Paper; World Health Organization: Geneva, Switzerland, 2016; Available online: http://apps.who.int/iris/bitstream/10665/246236/1 (accessed on 29 December 2021).
- Roukens, A.H.; Vossen, A.C.; Bredenbeek, P.J.; Van Dissel, J.T.; Visseret, L.G. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: A randomized controlled non-inferiority trial. PLoS ONE 2008, 3, e1993. [Google Scholar] [CrossRef] [Green Version]
- Siegrist, C.A. Vaccine immunology. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2018; p. 150. [Google Scholar]
- Martins, R.M.; Maia, M.L.; Farias, R.H.; Camacho, L.A.B.; Freire, M.S.; Galler, R.; Yamamura, A.M.Y.; Almeida, L.F.C.; Lima, S.M.B.; Nogueira, R.M.R.; et al. 17DD yellow fever vaccine: A double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum. Vaccines Immunother. 2013, 9, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Roukens, A.H.E.; Visser, L.G. Fractional-dose yellow fever vaccination: An expert review. J. Travel Med. 2019, 26, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannice, K.; Wilder-Smith, A.; Hombach, J. Fractional-Dose Yellow Fever Vaccination—Advancing the Evidence Base. N. Engl. J. Med. 2018, 379, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.M.; Harris, J.B.; Ahuka-Mundeke, S.; Dixon, M.G.; Kizito, G.M.; Nsele, P.M.; Umutesi, G.; Laven, J.; Kosoy, O.; Paluku, G.; et al. Immunogenicity of Fractional-Dose Vaccine during a Yellow Fever Outbreak—Final Report. N. Engl. J. Med. 2019, 381, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Yellow Fever Supply and Procurement Roadmap. Available online: https://www.gavi.org/about/market-shaping/supply-and-procurement-roadmaps/ (accessed on 29 December 2021).
- Tottey, S.; Shoji, Y.; Jones, R.M.; Chichester, J.A.; Green, B.J.; Musiychuk, K.; Si, H.; Manceva, S.D.; Rhee, A.; Shamloul, M.; et al. Plant-produced subunit vaccine candidates against yellow fever induce virus neutralizing antibodies and confer protection against viral challenge in animal models. Am. J. Trop. Med. Hyg. 2018, 98, 420–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massad, E.; Miguel, M.M.; Coutinho, F.A.B. Is vaccinating monkeys against yellow fever the ultimate solution for the Brazilian recurrent epizootics? Epidemiol. Infect. 2018, 146, 1622–1624. [Google Scholar] [CrossRef] [Green Version]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Gonçalo Seixas, G.; Pinto, J.; Lee Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. Dis. 2019, 13, e0006822. [Google Scholar]
- Shearer, F.M.; Longbottom, J.; Browne, A.J.; Pigott, D.M.; Brady, O.J.; Kraemer, M.U.G.; Marinho, F.; Yactayo, S.; De Araújo, V.E.M.; Da Nóbrega, A.A.; et al. Existing and potential infection risk zones of yellow fever worldwide: A modelling analysis. Lancet Glob. Health 2018, 6, e270–e278. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines 2022, 10, 372. https://doi.org/10.3390/vaccines10030372
Gianchecchi E, Cianchi V, Torelli A, Montomoli E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines. 2022; 10(3):372. https://doi.org/10.3390/vaccines10030372
Chicago/Turabian StyleGianchecchi, Elena, Virginia Cianchi, Alessandro Torelli, and Emanuele Montomoli. 2022. "Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects" Vaccines 10, no. 3: 372. https://doi.org/10.3390/vaccines10030372
APA StyleGianchecchi, E., Cianchi, V., Torelli, A., & Montomoli, E. (2022). Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines, 10(3), 372. https://doi.org/10.3390/vaccines10030372




