Abstract
We investigated Omicron infections among healthcare workers (HCW) presenting with symptoms of SARS-CoV-2 infection and evaluated the protective effect of vaccination or prior infection. Between 24 November and 31 December 2021, HCW in Johannesburg, South Africa, were tested for SARS-CoV-2 infection by Nucleic Acid Amplification Test (NAAT). Blood samples collected either at the symptomatic visit or in the 3 months prior, were tested for spike protein immunoglobulin G (IgG). Overall, 433 symptomatic HCW were included in the analysis, with 190 (43.9%) having an Omicron infection; 69 (16.7%) were unvaccinated and 270 (62.4%) received a single dose of the Ad26.COV.2 vaccine. There was no difference in the odds of identifying Omicron between unvaccinated and Ad26.COV.2 vaccinated HCW (adjusted odds ratio (aOR) 0.81, 95% confidence interval (CI): 0.46, 1.43). One-hundred and fifty-four (35.3%) HCW had at least one SARS-CoV-2 NAAT-confirmed prior infection; these had lower odds of Omicron infection compared with those without past infection (aOR 0.55, 95%CI: 0.36, 0.84). Anti-spike IgG concentration of 1549 binding antibody unit/mL was suggestive of significant reduction in the risk of symptomatic Omicron infection. We found high reinfection and vaccine breakthrough infection rates with the Omicron variant among HCW. Prior infection and high anti-spike IgG concentration were protective against Omicron infection.
1. Introduction
The Omicron (B.1.1.529/21K) severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variant was reported in South Africa on 25 November 2021, following investigation of a rapid increase in coronavirus disease (COVID-19) cases in the Gauteng province, and identification of a spike gene target failure (SGTF) on the Taqpath assay (ThermoFisher™), which also includes two other gene targets [1]. The Omicron variant has now been described to contain mutations that confer more infectious (double mutation in nucleocapsid, R203K, G204R), more transmissible (H655Y, N679K, P681H mutations in the spike protein), and higher ability to evade host immunity (including ∆105–107 mutation affecting nonstructural proteins and multiple other mutations affecting the spike protein receptor binding domain (RBD) and N-terminal domain) compared with the ancestral virus [1,2]. In December 2021, the Omicron variant constituted 98% of all SARS-CoV-2 infections in South Africa, and has now spread globally [3].
Healthcare workers (HCW) in South Africa were offered the Ad26.COV.2 COVID-19 vaccine as part of the Sisonke trial from 17 February 2021 as a single dose schedule, and subsequently, a booster dose was offered since 8 November 2021 [4,5]. From May 2021, HCW could also access the BNT162b2 COVID-19 vaccine as part of the national vaccine rollout in South Africa.
Here, we describe the Omicron infections among HCW who presented with symptoms suggestive of SARS-CoV-2 infection from 24 November to 31 December 2021. We also detailed breakthrough infections in vaccinated HCW and reinfections in previous Nucleic Acid Amplification Test (NAAT)-confirmed SARS-CoV-2 cases. In addition, blood samples collected either at the symptomatic visit or in the 3 months prior were tested for full-length SARS-CoV-2 spike protein immunoglobulin G (IgG) to assess the potential protective effect of these antibodies against Omicron infection.
2. Methods
2.1. Study Design
Healthcare workers (HCW) working at the Chris Hani Baragwanath Academic Hospital (CHBAH) in Johannesburg, Gauteng province, South Africa were enrolled from April to July 2020, into a longitudinal cohort surveillance study; this cohort has been previously described [6]. Due to participants discontinuing the study, enrolments into the longitudinal cohort were restarted on 16 February 2021 and closed on 10 August 2021. Among the longitudinal cohort participants, routine study visits (every 1 to 2 weeks for nasal swab collection and approximately every 4 weeks for nasal swab and venous blood collection) and visits when COVID-like symptoms are present are still ongoing.
From 22 June 2021, HCW from CHBAH, not enrolled into the longitudinal cohort, who presented with COVID-like symptoms could enroll into a test negative case-control (TNC) study and be tested for SARS-CoV-2 infection by Nucleic Acid Amplification Test (NAAT) using a nasal swab. On 14 December 2021, this was expanded to two other Johannesburg hospitals: Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) and Helen Joseph Hospital (HJH). HCW enrolled into the TNC could be present for multiple study visits. Enrolments are still ongoing at the three hospitals. HCW in the longitudinal cohort who were investigated for symptomatic illness were also eligible for inclusion in the TNC study.
Symptoms considered to be consistent with COVID-19 included any of the following: fever/feeling feverish, cough, sore throat, rhinitis, myalgia, shortness of breath, acute gastroenteritis/vomiting/nausea, impaired sense of smell or taste, fatigue, or headache. If an HCW had multiple symptomatic study visits between 24 November and 31 December 2021, only the visit with a positive NAAT SARS-CoV-2 result was included, or the first symptomatic visit if there was no positive NAAT result.
Demographic, health, and behavioral questionnaires collected personal information including COVID-19 vaccination history; previous SARS-CoV-2 infection was determined by documented NAAT positivity in the cohort participants or self-reporting.
2.2. Laboratory Methods
Total nucleic acids were extracted from nasal swabs using an automated NucliSENS-easyMAG nucleic acid extraction platform. NAAT was performed using the TaqPath COVID-19 diagnostic test from ThermoFisher that uses a triple-target (orf1ab, N gene, spike gene) design. Results were classified as positive for SARS-CoV-2 when the three targets or when both orf1ab and N gene had cycle threshold (Ct) values < 37 and inconclusive if only one target was detected with Ct values < 37. Results were classified as Omicron variant when orf1ab and N gene were detected but not the spike gene (SGTF).
Serum or plasma samples were collected at approximately monthly intervals from the longitudinal cohort participants. Participants enrolled into the TNC study had blood samples collected at enrolment. SARS-CoV-2 full-length spike protein immunoglobulin G (IgG) was measured by a quantitative assay on the Luminex platform as described [7,8]. The assay was evaluated for detection of antibodies against SARS-CoV-2 using COVID-19 convalescent plasma panel NIBSC 20/118. Based on differences in IgG titers from pre-COVID-19 and baseline samples when compared to post-infection samples of participants who were SARS-CoV-2 NAAT positive, 32 binding antibody units (BAU)/mL was selected as the threshold value indicative of seropositivity for full-length spike.
2.3. Statistical Analysis
Participants’ categorical characteristics were described as percentages and compared between NAAT-confirmed Omicron infected and uninfected HCW by Chi-square test. Continuous variables were represented as means with standard deviations (SD) or median with interquartile range (IQR) and compared by Student’s t-test or Mann–Whitney test, respectively. The association between Omicron infection and vaccination or previous SARS-CoV-2 infection was estimated by univariate and multivariate logistic regression. Participants were considered fully vaccinated if they received at least one dose of Ad26.COV.2 vaccine ≥14 days before the symptomatic visit or two doses of BNT162b2 vaccine, with the last dose ≥14 days before the symptomatic visit. Prior SARS-CoV-2 infections were categorized under the three previous pandemic waves: April to October 2020, November 2020 to April 2021, and May to September 2021. Differences in geometric mean units for spike IgG between NAAT-confirmed Omicron infected and uninfected HCW were analyzed on log10-transformed data.
A recursive partitioning approach was performed in the form of conditional inference tree. This method is particularly good at finding conditional thresholds in covariates by means of significance tests. Significance tests of the null hypothesis, that Omicron infection risk is identical on either side of the proposed threshold, are performed at each node of the tree in a recursive way. These tests are done by means of the conditional distribution of linear statistics in the permutation test framework, as outlined by Hothorn et al., which for two categorical variables corresponds to a Chi-square test [9]. Recursion is stopped when the obtained Bonferroni-adjusted p-values meets the user input significance level (in this case 90%). The final tree outlines all the splits for which the null hypothesis was rejected, i.e., for which a difference in outcome is statistically significant with 90% confidence across merging branches. With this type of conditional partitioning, a training set can be used to find the relevant splits, after which a validation portion of the data can be used to evaluate the method’s predictive power, in particularly type I errors (this method predicts the outcome to be a symptomatic infection, when in reality, none was recorded for that person) obtained with this method [9]. This analysis was performed using the rpart, party, and caret R packages.
For the purposes of the analyses presented throughout, serological measurements were restricted to blood samples collected within 3 months of symptomatic visits. If multiple samples were available, the sample collected closest to the symptomatic visit was used. Participants were excluded from the serology analysis if they received any vaccine between the last blood draw and the symptomatic visit or if the last blood draw was <14 days after the last vaccination. Since IgG antibodies after natural infection among vaccinated and previous infected individuals can rise quickly, we did a sub-analysis excluding the samples collected on the day of the symptomatic visit.
3. Results
The first case of SARS-CoV-2 with SGTF among HCW in our study was detected on 24 November 2021, prior to which the last confirmed SARS-CoV-2 infection was on 20 September 2021. From 24 November to 31 December 2021, 445 HCW had at least one symptomatic visit where nasal swabs were collected and tested by Taqpath NAAT for SARS-CoV-2 infection. Nine HCW had inconclusive results and were excluded from the analysis, and all but three (also excluded from the analysis) of the SARS-CoV-2 infections detected during this period had NAAT results with SGTF and putatively were Omicron variant.
Among the 433 symptomatic HCW included in the analysis, 190 (43.9%) had a positive NAAT. There were no reported Omicron infection-related hospital admissions among the study participants. Overall, 270 (62.4%) received a single dose of the Ad26.COV.2 vaccine (median 280 days; interquartile range (IQR): 257, 287), and 49 (11.8%) received a booster dose (median of 22 days; IQR: 18, 33) ≥14 days before the symptomatic visit. Only 26 (6.3%) HCW received two doses of BNT162b2 ≥ 14 days before the visit, and 69 (16.7%) were unvaccinated. Vaccination coverage was similar among HCW with symptomatic illness in whom Omicron was and was not identified (Table 1). Additionally, there was no difference in the odds of identifying Omicron between unvaccinated and vaccinated HCW, although the numbers for BNT162b recipients were low (Table 2).

Table 1.
Healthcare workers with at least one symptomatic study visit between 24 November and 31 December 2021.

Table 2.
Protection against Omicron infection by vaccination or previous SARS-CoV-2 NAAT-confirmed infection.
Overall, 154 (35.6%) HCW had at least one SARS-CoV-2 NAAT-confirmed infection prior to November 2021, 53 (34.4%) of whom were reinfected with Omicron, compared with 137 Omicron infections among the 279 (49.1% p = 0.003) HCW without previous NAAT-confirmed infection. Participants with previous NAAT-confirmed infection had lower odds of Omicron infection compared with those without past infection (adjusted odds ratio (aOR) 0.55, 95% confidence interval (CI): 0.36, 0.84). Stratifying by timing of previous infection, infection during the preceding third wave was associated with lower odds of symptomatic Omicron illness relative to HCW without any previous NAAT-confirmed infection (aOR 0.40, 95%CI: 0.20, 0.80); likewise, individuals who were infected during the second wave had similar lower odds of being infected with Omicron during the study period (aOR 0.49, 95%CI: 0.20, 1.23), although not significant (Table 2).
Anti-spike IgG geometric mean units (measured in 267 participants) were lower in HCW who eventually had an Omicron infection compared with those who never tested positive (577 binding antibody unit (BAU)/mL, vs. 968 BAU/mL, p = 0.009) (Table 1). Excluding blood samples collected at the time of the current visit, a similar trend in IgG levels was observed (Table 1).
To further investigate which combinations of covariates significantly modulate Omicron infection, a conditional inference tree was built (Figure 1A). Significance was detected in previous SARS-CoV-2 NAAT-confirmed cases and those with spike IgG levels > 1549 BAU/mL (Figure 1B), each with only 33% probability of infection. The boxplots in Figure 1C represent the anti-spike IgG levels by prior SARS-CoV-2 NAAT-confirmed infection and vaccination status. Overall, IgG concentrations were higher among HCW with prior infection (p = 0.00015), and in the group not previously infected in those with more vaccine doses (p = 0.000057). A lower significance was detected among the groups with different vaccination status for those who had a prior confirmed SARS-CoV-2 infection (p = 0.038).
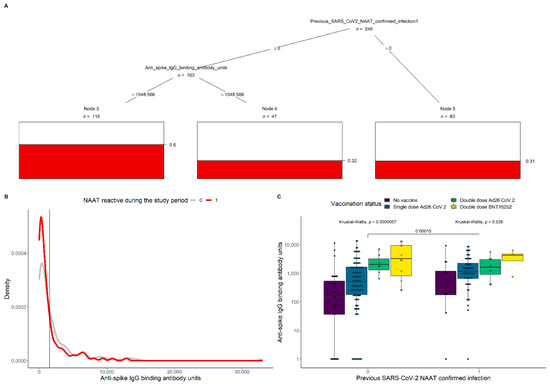
Figure 1.
Conditional inference of Omicron infection probability and anti-spike IgG levels by prior SARS-CoV-2 NAAT-confirmed infection. (A) Inferred significant splits in previous SARS-CoV-2 NAAT-confirmed cases and spike IgG levels impact on the probability of having an Omicron infection during the study period (indicated by the red bars). The tree was generated from a training set composed of 90% of all visits with a known serological result. The algorithms infection predictive power was measured to be 72% in the remaining 10% of the data, with 23% type I error. (B) Antibody density distributions for participants with either NAAT-confirmed Omicron infection (red line) or no infection (grey line) during the study period. The vertical black line corresponds to the threshold 1549 anti-spike IgG binding units that emerged from the analysis on panel A. (C) Anti-spike IgG levels by prior SARS-CoV-2 NAAT-confirmed infection and vaccination status. Kruskal–Wallis tests indicate significant differences in IgG concentrations between participants with different levels of prior infection (p = 0.00015), and with more doses of vaccination in those who were not previously exposed (p = 0.000057). A lower significance in differences in IgG concentration for groups with different vaccination status for those who had a prior confirmed SARS-CoV-2 infection (p = 0.038).
4. Discussion
We show that prior SARS-CoV-2 infection prevented symptomatic reinfection with Omicron at 45–60%, which is consistent with results from a large population study in Qatar [10]. Although it has been suggested that Omicron is evasive to neutralizing antibodies induced by natural infection from previous variants or vaccine-elicited [11], we show that HCW who were not infected by Omicron during the five week period of this analysis had higher concentration of anti-spike IgG prior the symptomatic visit, with >1549 BAU/mL being the threshold suggestive of significant reduction in the risk of symptomatic Omicron infection. This might be due to some residual neutralization activity, or that anti-spike IgG recognizes the virus beyond neutralization via Fc-effector mechanisms, as recently suggested [12]. Protection can be achieved by prior infection (irrespective of the vaccination status) or by recent vaccination, with participants who were unvaccinated or those who received a single dose of Ad26.COV.2, most of whom more than eight months prior, showing significantly lower levels of anti-spike IgG. Although we did not evaluate cell-mediated immunity and immune memory, it has been reported that after natural infection, the T-cell-mediated responses are targeted across a larger variety of epitopes than the humoral response, and therefore, might be more durable to genetic changes in viral epitopes [13]. Moreover, studies showed that the majority of CD4+ and CD8+ T-cell response to the spike protein induced by vaccination or prior natural infection cross-recognized the Omicron variant, thereby likely contributing to protection against severe disease [14,15]. In addition, Omicron-infected patients had similar T-cell responses to ancestral spike, nucleocapsid, and membrane proteins to those found in patients hospitalized in previous waves [15].
A limitation of our study was that prior infection was assessed by NAAT only, with some unvaccinated participants with no previous NAAT-confirmed infection being seropositive for anti-spike antibody, demonstrating exposure to SARS-CoV-2 before blood collection. A high SARS-CoV-2 seropositivity in South Africa prior to the Omicron wave has actually been suggested as a plausible explanation for the disconnection between hospitalization/death rates and infection rates associated with Omicron in the country [7]. Other limitations include the relatively small sample sizes of our groups and the fact that we did not perform any functional antibody assays, and as such, we cannot identify the exact protection mechanism.
We found high reinfection and vaccine breakthrough infection rates with the Omicron variant among HCW at three hospitals in Johannesburg, South Africa. Inherently, either natural infection or vaccination elicits immune responses that decays over time, with the specificity (cross-immunity), quality (neutralization), and magnitude (absolute amount) of circulating antibodies determining the likelihood of future symptomatic infections. Although a study from the United Kingdom showed limited protection against symptomatic Omicron illness after BNT162b2 or ChAdOx1 vaccination [16], recent results on the vaccine effectiveness of Ad26.COV.2 booster dose in South Africa against Omicron hospitalization also demonstrates the value of booster vaccinations [4].
Author Contributions
M.C.N.: conceptualization the study and the analysis, overall supervision of the project, data interpretation, writing of the first draft of the manuscript; S.M.-S.: sample analysis, data analysis, manuscript revision; V.L.B.: sample analysis, data analysis, manuscript revision; G.K., data interpretation, manuscript revision; R.A.: data analysis, data interpretation, writing; S.A.M.: conceptualization of the study, manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the European & Developing Countries Clinical Trials Partnership (grant number RIA2020EF-3020) and The Bill & Melinda Gates Foundation (grant number INV018148_2020). There was also partial support from the Department of Science and Technology and National Research Foundation: South African Research Chair Initiative in Vaccine Preventable Diseases; and the South African Medical Research Council.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Research Ethics Committee of the University of the Witwatersrand (protocol code 200405, approved on 17 April 2020).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification will be shared upon request. Researchers who wish to use the data to address any specific questions not directly addressed under the study objectives and which the data would lend itself to, who provide a methodologically sound proposals that has been approved by an independent review committee, may request the data. Requests should be directed to marta.nunes@wits-vida.org.
Acknowledgments
The authors would like to express special appreciation to the study participants and all the healthcare workers at Chris Hani Baragwanath Academic Hospital, Charlotte Maxeke Johannesburg Academic Hospital, and Helen Joseph Hospital and to the Wits VIDA staff. Wits VIDA HCW Study Group: Yasmin Adam, Ziyaad Dangor, David P. Moore, Charl Verwey, Sithembiso C. Velaphi, Firdose Nakwa, Rudo Mathivha, Kavita Makan, Colin Menezes, Merika Tsitsi, Temnewo Habte, Jeremy Nel, William Malebati, Mervin Mer, Adam Mohamed, Rajen Morar, Palesa Motshabi Chakane, Thendo Mpapele, Feroza Motara, Dalubuhle Ndiweni, Debbie White, Farzanah Laher, Shakeel McKenzie, and Sihle Mtshali.
Conflicts of Interest
M.C.N. reports grants from the Bill & Melinda Gates Foundation, European & Developing Countries Clinical Trials Partnership, Pfizer, AstraZeneca, and Sanofi-Pasteur; and personal fees from Pfizer and Sanofi-Pasteur. S.A.M. reports grants and personal fees from the Bill & Melinda Gates Foundation, and grants from the South African Medical Research Council, Novavax, Pfizer, Minervax, and European & Developing Countries Clinical Trials Partnership. G.K. reports grants from the Bill & Melinda Gates Foundation.
References
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Tracking SARS-COV-2 Variants. Available online: https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/sars-cov-2-genomic-surveillance-update/ (accessed on 19 January 2022).
- Gray, G.E.; Collie, S.; Garrett, N.; Goga, A.; Champion, J.; Zylstra, M.; Reddy, T.; Yende, N.; Seocharan, I.; Takalani, A.; et al. Vaccine effectiveness against hospital admission in South African health care workers who received a homologous booster of Ad26.COV2 during an Omicron COVID19 wave: Preliminary Results of the Sisonke 2 Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Reddy, D.L.; Dangor, Z.; Lala, N.; Johnstone, J.; Maswabi, L.; Tsitsi, J.M.L. COVID-19 mass vaccination campaign for healthcare workers in a low-resource setting: A clinician-driven initiative. S. Afr. Med. J. 2021, 111, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Baillie, V.L.; Kwatra, G.; Bhikha, S.; Verwey, C.; Menezes, C.; Cutland, C.L.; Moore, D.P.; Dangor, Z.; Adam, Y.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infection Among Healthcare Workers in South Africa: A Longitudinal Cohort Study. Clin. Infect. Dis. 2021, 73, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Kwatra, G.; Myers, J.E.; Jassat, W.; Dhar, N.; Mukendi, C.K.; Nana, A.J.; Blumberg, L.; Welch, R.; Ngorima-Mabhena, N.; et al. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, G.; Nunes, M.; Dhar, N.; Baillie, V.; Serafin, N.; Jones, S.; Madhi, S.A. Correlation of dried blood spots and plasma for quantification of Immunoglobulin (IgG) against Receptor binding domain and full length spike protein of SARS-CoV-2. J. Virol. Methods 2022, 300, 114394. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradnik, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- Bartsch, Y.; Tong, X.; Kang, J.; Avendano, M.J.; Serrano, E.F.; Garcia-Salum, T.; Pardo-Roa, C.; Riquelme, A.; Medina, R.A.; Alter, G. Preserved Omicron Spike specific antibody binding and Fc-recognition across COVID-19 vaccine platforms. medRxiv 2021. [Google Scholar] [CrossRef]
- Milne, G.; Hames, T.; Scotton, C.; Gent, N.; Johnsen, A.; Anderson, R.M.; Ward, T. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir. Med. 2021, 9, 1450–1466. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).