Public Health Surveillance for Adverse Events Following COVID-19 Vaccination in Africa
Abstract
:1. Introduction
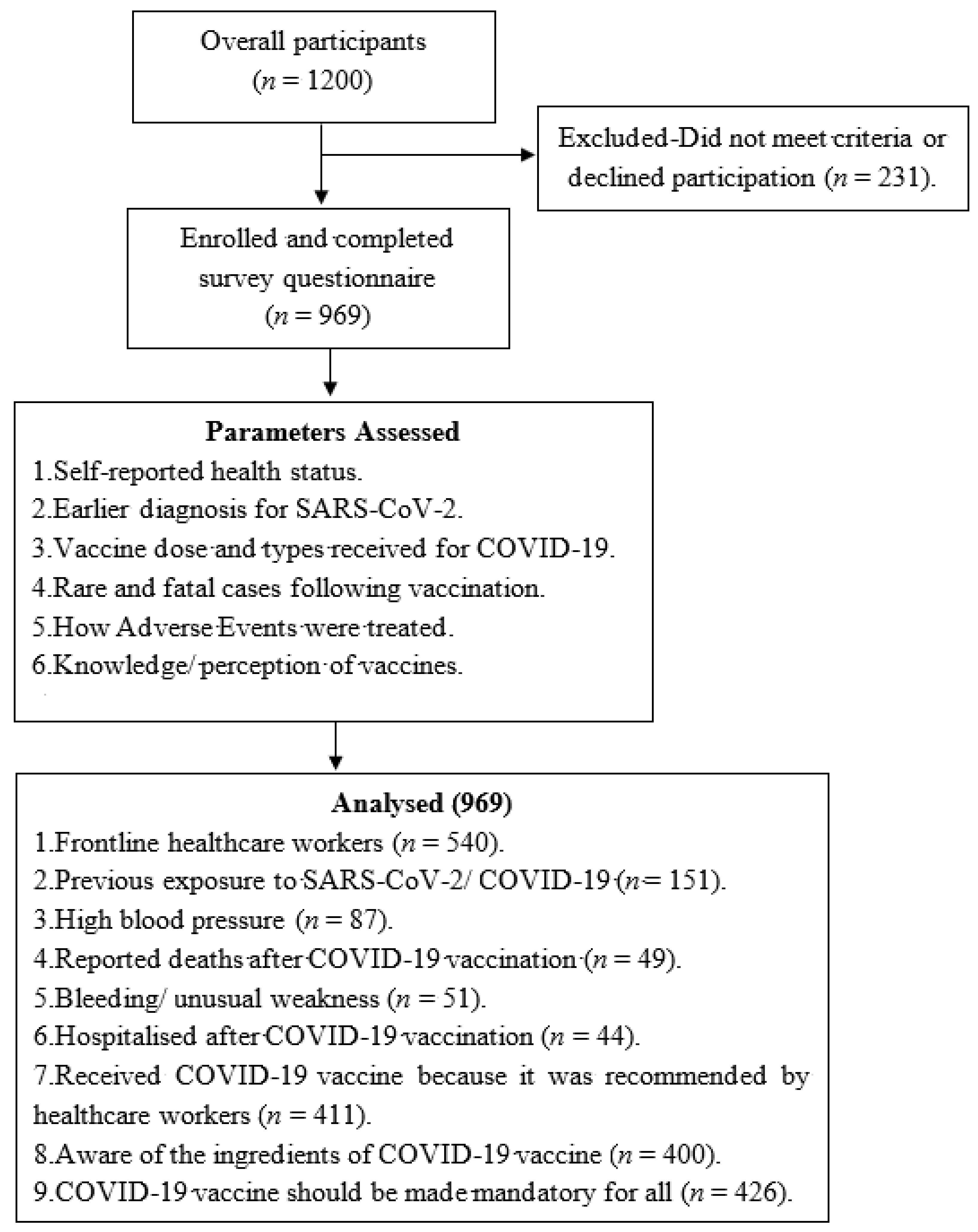
2. Materials and Methods
2.1. Study Design and Participants
2.2. Questionnaire
2.3. Ethics Approval and Consent
2.4. Data Analyses and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Study Questionnaire
- Consent Statement: Do you agree to participate in the study?I disagree with participating in the current study (0), end survey.I voluntarily agree to participate in the current study (1), Continue……..Adverse Events Following COVID-19 Vaccination in AfricaBiodata
- Have you been vaccinated against COVID-19?Yes (1), proceed, or No (0), end of survey.
- Age………………….18–24 (1); 25–34 (2); 35–44 (3); 45–54 (4); 55–64 (5); >65 (6)
- Gender (M/F). Male (1) Female (2) Other.
- Highest Level of Education Completed: None (0) Primary (1) Secondary (2) Tertiary (3) Other (4).
- Country of residence…………… A drop down of all countries.…………….
- Community setting (Urban/semi-urban/rural) Rural (1) Semi-urban (2) Urban (3). Dropdown menu to choose state.
- Occupation…………………. Frontline medical/healthcare worker (1) Frontline non-healthcare worker (2) Other (3).
- I have been diagnosed positive to SARS-CoV-2/COVID-19 before? Yes/No
- I have received the COVID-19 vaccine………… First dose (0) Complete dose (1).
- Date of first dose……………
- Date of Second dose…………………
- I know the brand name of the COVID-19 vaccine I was administered. Dropdown menu to choose vaccine brand name
- I experienced very common signs including:Tenderness, pain, warmth, itching or bruising where the injection was given.generally feeling unwell.feeling tired/fatigue.chills or feeling feverish.Headache.feeling sick/nausea.joint pain/muscle ache.Dropdown menu to choose multiple common signs
- I experienced common signs including:swelling, redness or a lump at the injection sitefeverbeing sick (vomiting) or diarrhoea.flu-like symptoms, such as high temperature, sore throat, runny nose, cough and chills.Dropdown menu to choose multiple common signs.
- I experienced uncommon signs including:Feeling dizzydecreased appetiteabdominal painenlarged lymph nodesexcessive sweating, itchy skin or rash.Dropdown menu to choose multiple common signs.Multiple choice questions (Yes/No):
- I experienced unknown severe allergic reaction (anaphylaxis).
- I experienced fever in 5–7 days following vaccination. Yes (1) No (0).
- I had pain at the site of COVID-19 vaccine injection. Yes (1) No (0).
- I experienced joint or muscle pain. Yes (1) No (0).
- I experienced general rash 7 and 10 days after vaccination Yes (1) No (0).
- I had seizure (black-out or convulsions) or high fever (after few hours or a few days) Yes (1) No (0).
- I had problems with hearing or vision, Hives (other itching or irritation) Yes (1) No (0).
- I experienced extreme drowsiness, and fainting. Yes (1) No (0).
- I experienced bleeding, or unusual weakness. Yes (1) No (0).
- I experienced difficulty in breathing or swallowing. Yes (1) No (0).
- Please state any other adverse event/sign or symptom you experienced following COVID-19 vaccination (if not already captured above). Yes (1) No (0).
- How was the adverse event treated? ……………….. At home (1) Hospital (2) Traditional remedy (3) I did not treat it, it resolved spontaneously (4) I did not have any adverse effect (5). Other (6).
- Have you ever had severe allergic reaction (anaphylaxis) following any other vaccination Yes (1) No (0).
- I know somebody who died following COVID-19 vaccination complication? Yes (1) No (0).
- Do you have any underlying disease(s)/health challenge(s)? Yes (1) No (0).
- Please state the underlying disease(s)/health challenge(s) you had before taking the COVID-19 vaccine (Select all that apply).
- Please state any other underlying disease(s)/health challenge(s) you had before taking the COVID-19 vaccine (if not captured above).
- I understand how vaccines work. No (0), Yes (1).
- I would be willing to participate in a clinical trial for a coronavirus vaccine. No (0), Yes (1).
- Are you aware of the ingredients of COVID-19 vaccine? No (0), Yes (1).
- Are you aware of the side effects of the COVID-19 vaccine? No (0), Yes (1).
- I received the COVID-19 vaccine because it was recommended by religious leaders (1), community elders (2), NGO (3) Government (4) Healthcare workers (5), scientists (6), news media (7), social media (8), celebrities (9), schools (10), public health organizations (NCDC) (11), my own research (12), friends (13), family (14), Not applicable (15).
- I got the COVID-19 vaccine jab because of the ease of my house/office to the vaccination center. No (0), Yes (1).
- To reach my nearest vaccination center, it took <15 min (1), <30 min (2), 1 h (3), <2 h (4), >2 h (5).
- The COVID-19 vaccine should be made mandatory for all. No (0), Yes (1), Maybe (2).
- For future cohort study, can we contact you through your email?Yes (1), email: ……………………., No (0), end survey.
References
- WHO. Vaccines and Immunization. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on 28 November 2021).
- WHO. First COVID-19 COVAX Vaccine Doses Administered in Africa. Available online: https://www.who.int/news/item/01-03-2021-first-covid-19-covax-vaccine-doses-administered-in-africa (accessed on 28 November 2021).
- CDC. Understanding Adverse Events and Side Effects; CDC: Atlanta, GA, USA, 2021. [Google Scholar]
- Konu, Y.R.; Gbeasor-Komlanvi, F.A.; Yerima, M.; Sadio, A.J.; Tchankoni, M.K.; Zida-Compaore, W.I.C.; Nayo-Apetsianyi, J.; Afanvi, K.A.; Agoro, S.; Salou, M. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021. Arch. Public Health 2021, 79, 207. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ostropolets, A.; Makadia, R.; Shoaibi, A.; Rao, G.; Sena, A.G.; Martinez-Hernandez, E.; Delmestri, A.; Verhamme, K.; Rijnbeek, P.R.; et al. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: Multinational network cohort study. BMJ 2021, 373, n1435. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Public-Health-Ontario. Adverse Events Following Immunization (AEFIs) for COVID-19 in Ontario. Available online: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-aefi-report.pdf?sc_lang=en (accessed on 23 January 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, C.E.; Zuckerman, S.P.; Conant, E.F. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. Am. J. Roentgenol. 2021, 217, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Novak, N.; Hamelmann, E.; Werfel, T.; Wagenmann, M.; Taube, C.; Bauer, A.; Merk, H.; Rabe, U.; Jung, K.; et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA. Allergo J. Int. 2021, 30, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Renoud, L.; Khouri, C.; Revol, B.; Lepelley, M.; Perez, J.; Roustit, M.; Cracowski, J.-L. Association of Facial Paralysis With mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern. Med. 2021, 181, 1243–1245. [Google Scholar] [CrossRef]
- CDC. Selected Adverse Events Reported after COVID-19 Vaccination. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html (accessed on 28 November 2021).
- ECDC. Suspected Adverse Reactions to COVID19 Vaccination and the Safety of Substances of Human Origin—3 June 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Suspected-adverse-reactions-to-COVID-19-vaccination-and-safety-of-SoHO.pdf (accessed on 13 January 2022).
- MHRA. Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting; Medicines & Healthcare Products Regulatory Agency: London, UK, 2022. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 23 March 2022).
- Anjorin, A.; Odetokun, I.; Abioye, A.; Elnadi, H.; Umoren, M.; Damaris, B.; Eyedo, J.; Umar, H.; Nyandwi, J.; Abdalla, M.; et al. Will Africans Take COVID-19 Vaccination? PLoS ONE 2021, 16, e0260575. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Tafuri, S. A public health perspective on the responsibility of mass media for the outcome of the anti-COVID-19 vaccination campaign: The AstraZeneca case. Ann. Ig. 2022. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Esba, L.C.A.; Al Jeraisy, M. Reported Adverse Effects following COVID-19 Vaccination at a Tertiary Care Hospital, Focus on Cerebral Venous Sinus Thrombosis (CVST). Expert Rev. Vaccines 2021, 20, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Kim, J.; Oh, C.E.; Lee, J.-Y. Adverse events following immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J. Korean Med. Sci. 2021, 36, e114. [Google Scholar] [CrossRef] [PubMed]
- Sakinah, E.N.; Nugraha, M.Y.; Qodar, T.S.; Mulyono, B.W.; Tohari, A.I. COVID-19 Vaccines Programs: Adverse events following immunization (AEFI) among medical Clerkship Student in Jember, Indonesia. BMC Pharmacol. Toxicol. 2021, 22, 58. [Google Scholar]
- EMA, European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Assessment Report—Comirnaty (Pfizer/BioNTech). EMA/707383/2020. 2021. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf (accessed on 19 February 2021).
- Hernández, A.F.; Calina, D.; Poulas, K.; Docea, A.O.; Tsatsakis, A.M. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol. Rep. 2021, 8, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Putcha, V.R.; Dwivedi, L.K.; Singh, D. Serious adverse events and fatal outcomes following COVID-19 vaccination in the UK: Lessons for other countries. Int. J. Community Soc. Dev. 2021, 3, 396–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Anjorin, A.; Abioye, A.; Asowata, O.; Soipe, A.; Kazeem, M.; Adesanya, I.; Raji, M.; Adesanya, M.; Oke, F.; Lawal, F. Comorbidities and the COVID-19 pandemic dynamics in Africa. Trop. Med. Int. Health 2021, 26, 2–13. [Google Scholar] [CrossRef]
- WHO. Causality Assessment of an Adverse Event Following Immunization (AEFI). User Manual for the Revised WHO Classification, January 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/259959/9789241513654-eng.pdf?sequence=1&isAllowed=y (accessed on 23 March 2022).
- Stefanizzi, P.; Stella, P.; Ancona, D.; Malcangi, K.N.; Bianchi, F.P.; De Nitto, S.; Ferorelli, D.; Germinario, C.A.; Tafuri, S. Adverse Events Following Measles-Mumps-Rubella-Varicella Vaccination and the Case of Seizures: A Post Marketing Active Surveillance in Puglia Italian Region, 2017–2018. Vaccines 2019, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- CDC. What to Do if You Had an Allergic Reaction after Getting a COVID-19 Vaccine. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html (accessed on 26 January 2022).
- Taber, J.M.; Leyva, B.; Persoskie, A. Why do people avoid medical care? A qualitative study using national data. J. Gen. Intern. Med. 2015, 30, 290–297. [Google Scholar] [CrossRef] [Green Version]
- CDC. Vaccine Adverse Event Reporting System (VAERS). Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html (accessed on 28 November 2021).
- Shaker, M.; Abrams, E.M.; Greenhawt, M. A Cost-Effectiveness Evaluation of Hospitalizations, Fatalities, and Economic Outcomes Associated with Universal Versus Anaphylaxis Risk-Stratified COVID-19 Vaccination Strategies. J. Allergy Clin. Immunol. Pract. 2021, 9, 2658–2668.e3. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.K.; Soveri, A.; Lewandowsky, S.; Karlsson, L.; Karlsson, H.; Nolvi, S.; Karukivi, M.; Lindfelt, M.; Antfolk, J. Fearing the disease or the vaccine: The case of COVID-19. Personal. Individ. Differ. 2021, 172, 110590. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, G.F.; Weber, E.U.; Hsee, C.K.; Welch, N. Risk as feelings. Psychol. Bull. 2001, 127, 267–286. [Google Scholar] [CrossRef] [PubMed]
- López, F.; Català, M.; Prats, C.; Estrada, O.; Oliva, I.; Prat, N.; Isnard, M.; Vallès, R.; Vilar, M.; Clotet, B.; et al. A Cost–Benefit Analysis of COVID-19 Vaccination in Catalonia. Vaccines 2022, 10, 59. [Google Scholar] [CrossRef]
- Azzarone, B.; Veneziani, I.; Moretta, L.; Maggi, E. Pathogenic Mechanisms of Vaccine-Induced Immune Thrombotic Thrombocytopenia in People Receiving Anti-COVID-19 Adenoviral-Based Vaccines: A Proposal. Front. Immunol. 2021, 12, 728513. [Google Scholar] [CrossRef]
- Edwards, K.M.; Orenstein, W.A. COVID-19: Vaccines. Available online: https://www.uptodate.com/contents/covid-19-vaccines (accessed on 5 March 2022).




| Country | Frequency | Proportion (%) |
|---|---|---|
| Nigeria | 327 | 33.7 |
| Ghana | 226 | 23.3 |
| Kenya | 94 | 9.7 |
| Diaspora | 83 | 8.6 |
| Morocco | 52 | 5.4 |
| Egypt | 50 | 5.2 |
| Somalia | 49 | 5.1 |
| Sudan | 40 | 4.1 |
| Rwanda | 27 | 2.8 |
| Category | Frequency | Percentage |
|---|---|---|
| Age | ||
| 18–24 | 84 | 8.7 |
| 25–34 | 394 | 40.7 |
| 35–44 | 268 | 27.7 |
| 45–54 | 123 | 12.7 |
| 55–64 | 69 | 7.1 |
| >65 | 31 | 3.2 |
| Gender | ||
| Male | 514 | 53.0 |
| Female | 455 | 47.0 |
| Education | ||
| Tertiary | 804 | 83.0 |
| Secondary | 49 | 5.1 |
| Primary | 10 | 1.0 |
| Others | 89 | 9.2 |
| None | 17 | 1.8 |
| Occupation | ||
| Frontline Healthcare Workers | 540 | 55.7 |
| Frontline Non-healthcare Workers | 127 | 13.1 |
| Others | 302 | 31.2 |
| Community | ||
| Urban | 727 | 75.0 |
| Semi-urban | 149 | 15.4 |
| Rural | 93 | 9.6 |
| Frequency (n = 969) | Proportion (%) | |
|---|---|---|
| Earlier diagnosed positive | ||
| No | 799 | 82.5 |
| Yes | 151 | 15.6 |
| Missing | 19 | 2.0 |
| Underlying conditions | ||
| No | 729 | 75.2 |
| Yes | 240 | 24.8 |
| CVD | 93 | 38.8 |
| Asthma | 43 | 17.9 |
| Diabetes | 31 | 12.9 |
| Obesity | 26 | 10.8 |
| Infectious diseases | 14 | 5.8 |
| GIT Diseases | 10 | 4.2 |
| Haematological disorder | 9 | 3.8 |
| Cancer | 5 | 2.1 |
| Arthritis | 2 | 0.8 |
| Glaucoma | 2 | 0.8 |
| Others | 5 | 2.1 |
| Frequency n = 969 | Proportion % | |
|---|---|---|
| Vaccine dose | ||
| First dose | 811 | 83.7 |
| Complete dose | 158 | 16.3 |
| Vaccine type | ||
| Adenovector | 766 | 79 |
| mRNA | 105 | 10.8 |
| Inactivated whole virus | 64 | 6.6 |
| Live attenuated vaccine | 2 | 0.2 |
| Others | 32 | 3.3 |
| Vaccine Brand | ||
| Oxford–AstraZeneca | 754 | 77.8 |
| Johnson & Johnson | 5 | 0.5 |
| Covaxin | 9 | 0.9 |
| Sinopharm-BBIBP | 44 | 4.5 |
| Moderna | 17 | 1.8 |
| Pfizer–BioNTech | 88 | 9.1 |
| CoronaVac | 6 | 0.6 |
| Sputnik V | 7 | 0.7 |
| Sinopharm-WIBP | 5 | 0.5 |
| Covi Vac | 2 | 0.2 |
| Others | 32 | 3.3 |
| Bleeding | n = 918 | AEFV | % | p-Value |
|---|---|---|---|---|
| Adenovector | 733 | 37 | 5 | 0.33 |
| mRNA | 98 | 5 | 5.1 | |
| Inactivated vaccine | 57 | 6 | 10.5 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 3 | 10.7 | |
| Seizure | ||||
| Adenovector | 728 | 31 | 4.3 | 0.10 |
| mRNA | 102 | 3 | 2.9 | |
| Inactivated vaccine | 60 | 4 | 6.7 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 4 | 14.3 | |
| Breathing difficulty | ||||
| Adenovector | 750 | 19 | 2.5 | 0.90 |
| mRNA | 100 | 3 | 3 | |
| Inactivated vaccine | 61 | 2 | 3.3 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 30 | 5 | 16.7 | |
| Hearing/Vision | ||||
| Adenovector | 757 | 11 | 1.5 | 0.09 |
| mRNA | 101 | 3 | 2.9 | |
| Inactivated vaccine | 60 | 3 | 5 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 4 | 14.3 | |
| Severe allergic reaction | ||||
| Adenovector | 752 | 16 | 2.1 | 0.35 |
| mRNA | 102 | 3 | 2.9 | |
| Inactivated vaccine | 60 | 3 | 5 | |
| Live attenuated | 2 | 0 | 0 | |
| Others | 28 | 4 | 14.3 | |
| Frequency (n = 969) | Percent (%) | p-Value | |
|---|---|---|---|
| Bleeding/Unusual weakness | |||
| No | 918 | 94.7 | <0.0001 |
| Yes | 51 | 5.3 | |
| Died after vaccination | |||
| No | 920 | 94.9 | <0.0001 |
| * Yes | 49 | 5.1 | |
| Seizure (convulsion) or high fever after hours or a few days | |||
| No | 927 | 95.7 | <0.0001 |
| Yes | 42 | 4.3 | |
| Breathing difficulty | |||
| No | 943 | 97.3 | <0.0001 |
| Yes | 26 | 2.7 | |
| Hearing/Vision problem | |||
| No | 948 | 97.8 | <0.0001 |
| Yes | 21 | 2.2 | |
| Clinical trial | |||
| No | 622 | 64.2 | <0.0001 |
| Yes | 347 | 35.8 |
| Frequency (n = 969) | Percentage | p-Value | |
|---|---|---|---|
| I experienced uncommon signs including: | <0.0001 | ||
| None | 651 | 67.1 | |
| Feeling dizzy | 116 | 11.9 | |
| Decreased appetite | 62 | 6.4 | |
| Excessive sweating | 41 | 4.2 | |
| Abdominal pain | 32 | 3.3 | |
| Itchy skin or rash | 28 | 2.9 | |
| Enlarged lymph nodes | 23 | 2.4 | |
| Menstrual disorder | 5 | 0.5 | |
| Hunger | 4 | 0.4 | |
| Increased libido | 2 | 0.2 | |
| I experienced common signs including: | |||
| None | 508 | 52.4 | <0.0001 |
| Fever | 320 | 33.0 | |
| Swelling, redness or a lump at the injection site | 176 | 18.2 | |
| Flu-like symptoms such as high temperature, sore throat, runny nose, cough and chills | 117 | 12.1 | |
| Being sick (vomiting) | 44 | 4.5 | |
| Diarrhoea | 20 | 2.1 | |
| Heaviness of the head | 2 | 0.2 | |
| Bone ache | 1 | 0.1 | |
| Lymph node enlargement | 1 | 0.1 | |
| I experienced very common signs including: | <0.0001 | ||
| None | 220 | 22.7 | |
| Feeling tired/fatigued | 388 | 40.0 | |
| Tenderness, pain, warmth, itching or bruising where the injection was given | 380 | 39.2 | |
| Headache | 363 | 37.5 | |
| Generally feeling unwell | 339 | 34.9 | |
| Chills or feeling feverish | 293 | 30.2 | |
| Joint pain/muscle ache | 269 | 27.8 | |
| Feeling sick/nausea | 115 | 11.9 | |
| Deep sleep | 5 | 0.5 | |
| Lymph in armpits | 3 | 0.3 | |
| Mouth sores | 1 | 0.1 | |
| Boil | 1 | 0.1 | |
| Experienced lower sex drive | 1 | 0.1 | |
| Diarrhoea | 1 | 0.1 | |
| Ear pain | 1 | 0.1 | |
| Chest pain | 1 | 0.1 | |
| Vomiting | 1 | 0.1 | |
| Blood (red) spot on left eye | 1 | 0.1 | |
| Tender swollen tongue, loss of taste and appetite | 1 | 0.1 | |
| Insomnia | 1 | 0.1 | |
| Dry cough | 1 | 0.1 | |
| Rhinitis | 1 | 0.1 | |
| Numbness at neck and hand after 2nd dose for one night | 1 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjorin, A.A.; Odetokun, I.A.; Nyandwi, J.B.; Elnadi, H.; Awiagah, K.S.; Eyedo, J.; Abioye, A.I.; Gachara, G.; Maisara, A.M.; Razouqi, Y.; et al. Public Health Surveillance for Adverse Events Following COVID-19 Vaccination in Africa. Vaccines 2022, 10, 546. https://doi.org/10.3390/vaccines10040546
Anjorin AA, Odetokun IA, Nyandwi JB, Elnadi H, Awiagah KS, Eyedo J, Abioye AI, Gachara G, Maisara AM, Razouqi Y, et al. Public Health Surveillance for Adverse Events Following COVID-19 Vaccination in Africa. Vaccines. 2022; 10(4):546. https://doi.org/10.3390/vaccines10040546
Chicago/Turabian StyleAnjorin, AbdulAzeez Adeyemi, Ismail A. Odetokun, Jean Baptiste Nyandwi, Hager Elnadi, Kwame Sherrif Awiagah, Joseph Eyedo, Ajibola Ibraheem Abioye, George Gachara, Aala MohmedOsman Maisara, Youssef Razouqi, and et al. 2022. "Public Health Surveillance for Adverse Events Following COVID-19 Vaccination in Africa" Vaccines 10, no. 4: 546. https://doi.org/10.3390/vaccines10040546
APA StyleAnjorin, A. A., Odetokun, I. A., Nyandwi, J. B., Elnadi, H., Awiagah, K. S., Eyedo, J., Abioye, A. I., Gachara, G., Maisara, A. M., Razouqi, Y., Yusuf Mohamud, M. F., Mhgoob, Z. E., Ajayi, T., Ntirenganya, L., Saibu, M., Salako, B. L., Elelu, N., Wright, K. O., Fasina, F. O., & Mosbah, R. (2022). Public Health Surveillance for Adverse Events Following COVID-19 Vaccination in Africa. Vaccines, 10(4), 546. https://doi.org/10.3390/vaccines10040546








.jpg)



