Drop the Needle; A Temperature Stable Oral Tablet Vaccine Is Protective against Respiratory Viral Pathogens
Abstract
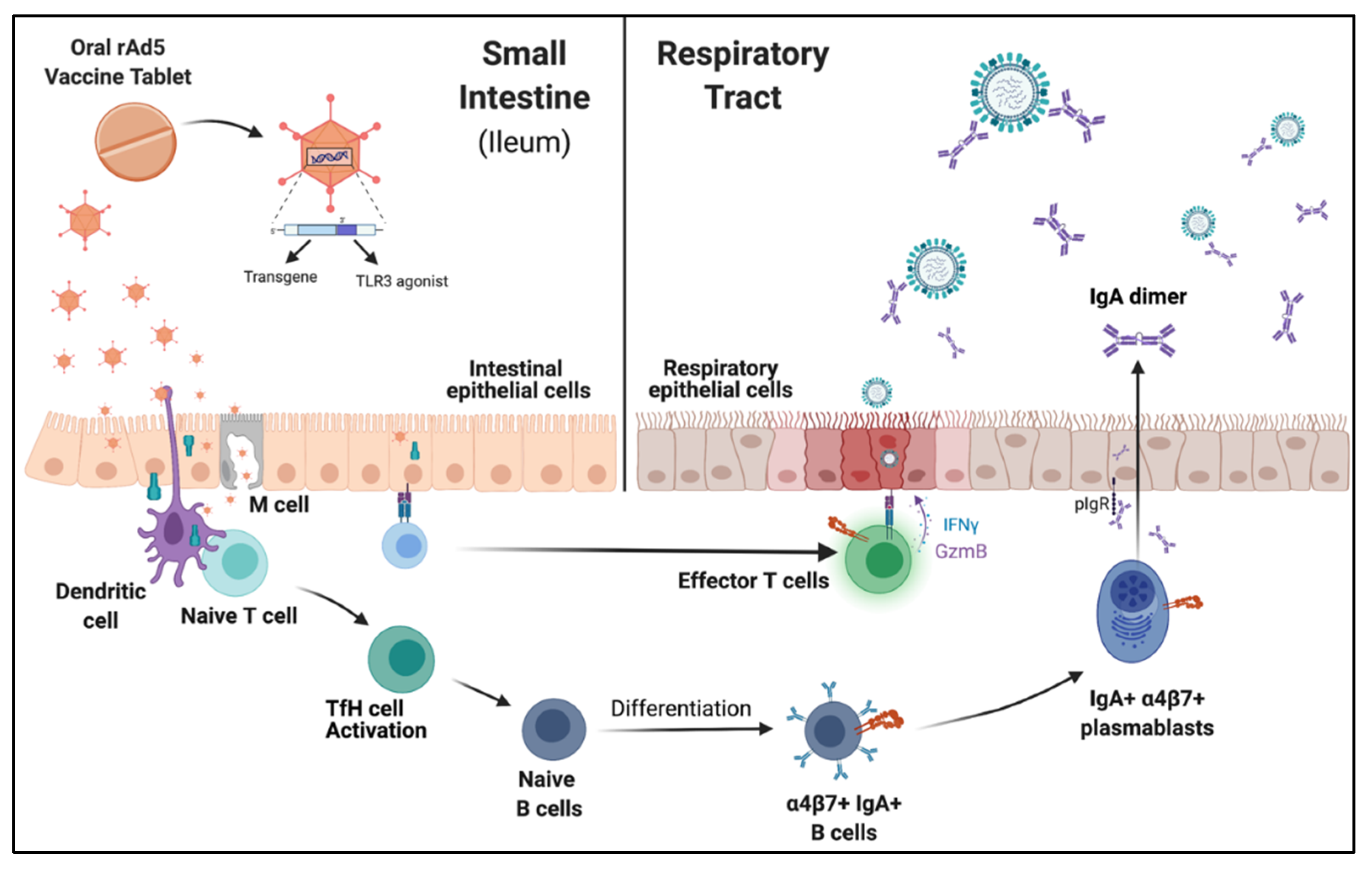
:1. Introduction
2. Developing Next Generation Vaccines
3. Advantages of Orally Delivered rAd Vaccines
4. Mucosal Vaccination Elicits Cellular and Humoral Responses
5. Mucosal IgA and Influenza Vaccine Efficacy
6. Humoral Reponses in the Mucosa Are Protective against Challenge and Reduce Transmission
7. Evaluating Novel Immune Correlates for Mucosal Vaccines
8. Anti-Vector Responses
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lowe, D. Cold Chain (and Colder Chain) Distribution. Science. 2020. Available online: https://blogs.sciencemag.org/pipeline/archives/2020/08/31/cold-chain-and-colder-chain-distribution (accessed on 16 October 2021).
- Jakab, Z.; Selbie, D.; Squires, N.; Mustafa, S.; Saikat, S. Building the evidence base for global health policy: The need to strengthen institutional networks, geographical representation and global collaboration. BMJ Glob. Health 2021, 6, e006852. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, G.C. Ensuring health equity during the COVID-19 pandemic: The role of public health infrastructure. Rev. Panam. Salud Pública 2020, 44, e70. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Briggs, K.T.; Taraban, M.B.; Brinson, R.G.; Marino, J.P. Grand Challenges in Pharmaceutical Research Series: Ridding the Cold Chain for Biologics. Pharm. Res. 2021, 38, 3–7. [Google Scholar] [CrossRef]
- QuadrantSolutions. 2021. Available online: https://investors.vaxart.com/static-files/cbf26eda-2ffa-4cb2-b031-0cb9b02fedac (accessed on 11 November 2021).
- Kim, L.; Martinez, C.J.; Hodgson, K.A.; Trager, G.R.; Brandl, J.R.; Sandefer, E.P.; Doll, W.J.; Liebowitz, D.; Tucker, S.N. Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule. Sci. Rep. 2016, 6, 37295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebowitz, D.; Gottlieb, K.; Kolhatkar, N.S.; Garg, S.J.; Asher, J.M.; Nazareno, J.; Kim, K.; McIlwain, D.R.; Tucker, S.N. Efficacy, immunogenicity, and safety of an oral influenza vaccine: A placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis. 2020, 20, 435–444. [Google Scholar] [CrossRef]
- Liebowitz, D.; Lindbloom, J.D.; Brandl, J.R.; Garg, S.J.; Tucker, S.N. High titre neutralising antibodies to influenza after oral tablet immunisation: A phase 1, randomised, placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 1041–1048. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Chen, H.; Rahil, Z.; Bidoki, N.H.; Jiang, S.; Bjornson, Z.; Kolhatkar, N.S.; Martinez, C.J.; Gaudilliere, B.; Hedou, J.; et al. Human influenza virus challenge identifies cellular correlates of protection for oral vaccination. Cell Host Microbe 2021, 29, 1828–1837.e1825. [Google Scholar] [CrossRef]
- Langel, S.N.; Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Kuehal, P.J.; Irshad, H.; Barrett, E.G.; Werts, A.; et al. Oral and intranasal Ad5 SARS-CoV-2 vaccines decrease disease and viral transmission in a golden hamster model. bioRxiv 2021. [Google Scholar] [CrossRef]
- WHO. Pandemic Influenza Vaccine Manufacturing Process and Timeline. 2009. Available online: https://www.who.int/news/item/06-08-2009-pandemic-influenza-vaccine-manufacturing-process-and-timeline (accessed on 23 October 2021).
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1. [Google Scholar] [CrossRef]
- GISRS. Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed on 20 February 2022).
- Appiah, G.D. Influenza Activity—United States, 2014–2015 Season and Composition of the 2015–16 Influenza Vaccine. Morb. Mortal. Wkly. Rep. 2015, 64, 583–590. [Google Scholar]
- Harding, A.T.; Heaton, N.S. Efforts to Improve the Seasonal Influenza Vaccine. Vaccines 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, K.M.; Lauring, A.S. Complexities of Viral Mutation Rates. J. Virol. 2018, 92, e01031-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Rappuoli, R.; De Gregorio, E.; Del Giudice, G.; Phogat, S.; Pecetta, S.; Pizza, M.; Hanon, E. Vaccinology in the post-COVID-19 era. Proc. Natl. Acad. Sci. USA 2021, 118, e2020368118. [Google Scholar] [CrossRef]
- Bazan, I.S.; Akgun, K.M. COVID-19 Healthcare Inequity: Lessons Learned from Annual Influenza Vaccination Rates to Mitigate COVID-19 Vaccine Disparities. Yale J. Biol. Med. 2021, 94, 509–515. [Google Scholar]
- Sen-Crowe, B.; McKenney, M.; Elkbuli, A. Disparities in global COVID-19 vaccination rates & allocation of resources to countries in need. Ann. Med. Surg. 2021, 68, 102620. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Levin, D. The U.S. Is Wasting Vaccine Doses, Even as Cases Rise and Other Countries Suffer Shortages. The New York Times, 2 August 2021. [Google Scholar]
- Klemes, J.J.; Jiang, P.; Fan, Y.V.; Bokhari, A.; Wang, X.C. COVID-19 pandemics Stage II—Energy and environmental impacts of vaccination. Renew. Sustain. Energy Rev. 2021, 150, 111400. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Shiver, J.W.; Fu, T.M.; Chen, L.; Casimiro, D.R.; Davies, M.E.; Evans, R.K.; Zhang, Z.Q.; Simon, A.J.; Trigona, W.L.; Dubey, S.A.; et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002, 415, 331–335. [Google Scholar] [CrossRef]
- Gutierrez-Martinez, E.; Planes, R.; Anselmi, G.; Reynolds, M.; Menezes, S.; Adiko, A.C.; Saveanu, L.; Guermonprez, P. Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets. Front. Immunol. 2015, 6, 363. [Google Scholar] [CrossRef] [Green Version]
- Wosen, J.E.; Mukhopadhyay, D.; Macaubas, C.; Mellins, E.D. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front. Immunol. 2018, 9, 2144. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.; van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef] [Green Version]
- Weldon, W.C.; Wang, B.Z.; Martin, M.P.; Koutsonanos, D.G.; Skountzou, I.; Compans, R.W. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS ONE 2010, 5, e12466. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.E.; Keefer, M.C.; Bunce, C.A.; Frances, D.; Abbink, P.; Maxfield, L.F.; Neubauer, G.H.; Nkolola, J.; Peter, L.; Lane, C.; et al. First-in-human randomized controlled trial of an oral, replicating adenovirus 26 vector vaccine for HIV-1. PLoS ONE 2018, 13, e0205139. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2021, 22, 236–250. [Google Scholar] [CrossRef]
- Poonam, P. The biology of oral tolerance and issues related to oral vaccine design. Curr. Pharm. Des. 2007, 13, 2001–2007. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Huhta, H.; Helminen, O.; Kauppila, J.H.; Salo, T.; Porvari, K.; Saarnio, J.; Lehenkari, P.P.; Karttunen, T.J. The Expression of Toll-like Receptors in Normal Human and Murine Gastrointestinal Organs and the Effect of Microbiome and Cancer. J. Histochem. Cytochem. 2016, 64, 470–482. [Google Scholar] [CrossRef] [Green Version]
- Cario, E.; Podolsky, D.K. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 2000, 68, 7010–7017. [Google Scholar] [CrossRef] [Green Version]
- Gourbeyre, P.; Berri, M.; Lippi, Y.; Meurens, F.; Vincent-Naulleau, S.; Laffitte, J.; Rogel-Gaillard, C.; Pinton, P.; Oswald, I.P. Pattern recognition receptors in the gut: Analysis of their expression along the intestinal tract and the crypt/villus axis. Physiol Rep. 2015, 3, e12225. [Google Scholar] [CrossRef]
- Akita, K.; Yasaka, K.; Shirai, T.; Ishii, T.; Harigae, H.; Fujii, H. Interferon alpha Enhances B Cell Activation Associated With FOXM1 Induction: Potential Novel Therapeutic Strategy for Targeting the Plasmablasts of Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 498703. [Google Scholar] [CrossRef]
- Syedbasha, M.; Bonfiglio, F.; Linnik, J.; Stuehler, C.; Wuthrich, D.; Egli, A. Interferon-lambda Enhances the Differentiation of Naive B Cells into Plasmablasts via the mTORC1 Pathway. Cell Rep. 2020, 33, 108211. [Google Scholar] [CrossRef] [PubMed]
- Hemann, E.A.; Green, R.; Turnbull, J.B.; Langlois, R.A.; Savan, R.; Gale, M., Jr. Interferon-lambda modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat. Immunol. 2019, 20, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Watanabe, I.; Ito, S.; Fujii, H.; Moriyama, M.; Tamura, S.; Takahashi, H.; Sawa, H.; Chiba, J.; Kurata, T.; et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 2005, 79, 2910–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaki, H.; Kure, S.; Oshiumi, H.; Sakoda, Y.; Suzuki, T.; Ainai, A.; Hasegawa, H.; Matsumoto, M.; Seya, T. Toll-like receptor 3 in nasal CD103(+) dendritic cells is involved in immunoglobulin A production. Mucosal Immunol. 2018, 11, 82–96. [Google Scholar] [CrossRef]
- Muramatsu, M.; Yoshida, R.; Yokoyama, A.; Miyamoto, H.; Kajihara, M.; Maruyama, J.; Nao, N.; Manzoor, R.; Takada, A. Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: Increased potential of IgA for heterosubtypic immunity. PLoS ONE 2014, 9, e85582. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Sano, K.; Suzuki, T.; Ainai, A.; Taga, Y.; Ueno, T.; Tabata, K.; Saito, K.; Wada, Y.; Ohara, Y.; et al. IgA tetramerization improves target breadth but not peak potency of functionality of anti-influenza virus broadly neutralizing antibody. PLoS Pathog. 2019, 15, e1007427. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Andrew, D.P.; Rott, L.S.; Kilshaw, P.J.; Butcher, E.C. Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur. J. Immunol. 1996, 26, 897–905. [Google Scholar] [CrossRef]
- Quiding-Jarbrink, M.; Nordstrom, I.; Granstrom, G.; Kilander, A.; Jertborn, M.; Butcher, E.C.; Lazarovits, A.I.; Holmgren, J.; Czerkinsky, C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J. Clin. Invest. 1997, 99, 1281–1286. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [Green Version]
- Scallan, C.D.; Tingley, D.W.; Lindbloom, J.D.; Toomey, J.S.; Tucker, S.N. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin. Vaccine Immunol. 2013, 20, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Effros, R.B. Immunosenescence: What does it mean to health outcomes in older adults? Curr. Opin. Immunol. 2009, 21, 418–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElhaney, J.E.; Ewen, C.; Zhou, X.; Kane, K.P.; Xie, D.; Hager, W.D.; Barry, M.B.; Kleppinger, A.; Wang, Y.; Bleackley, R.C. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009, 27, 2418–2425. [Google Scholar] [CrossRef] [Green Version]
- Tucker, S.N. Vaxart’s Oral Vaccine Candidate for Prevention of COVID-19: Hold the Needes and the Ice. 2021. Available online: https://www.humanvaccinesproject.org/event/march-11th-speaker-sean-tucker/ (accessed on 19 November 2021).
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [Green Version]
- Tamura, S.; Tanimoto, T.; Kurata, T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn. J. Infect. Dis. 2005, 58, 195–207. [Google Scholar]
- Taylor, H.P.; Dimmock, N.J. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J. Exp. Med. 1985, 161, 198–209. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawaguchi, A.; Ainai, A.; Tamura, S.; Ito, R.; Multihartina, P.; Setiawaty, V.; Pangesti, K.N.; Odagiri, T.; Tashiro, M.; et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc. Natl. Acad. Sci. USA 2015, 112, 7809–7814. [Google Scholar] [CrossRef] [Green Version]
- Johansen, F.E.; Braathen, R.; Brandtzaeg, P. Role of J chain in secretory immunoglobulin formation. Scand. J. Immunol. 2000, 52, 240–248. [Google Scholar] [CrossRef]
- Kok, T.W.; Costabile, M.; Tannock, G.A.; Li, P. Colocalization of intracellular specific IgA (icIgA) with influenza virus in patients' nasopharyngeal aspirate cells. J. Virol. Methods 2018, 252, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Bidgood, S.R.; Tam, J.C.; McEwan, W.A.; Mallery, D.L.; James, L.C. Translocalized IgA mediates neutralization and stimulates innate immunity inside infected cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13463–13468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazanec, M.B.; Kaetzel, C.S.; Lamm, M.E.; Fletcher, D.; Nedrud, J.G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 1992, 89, 6901–6905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrose, C.S.; Wu, X.; Jones, T.; Mallory, R.M. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine 2012, 30, 6794–6801. [Google Scholar] [CrossRef] [Green Version]
- Gould, V.M.W.; Francis, J.N.; Anderson, K.J.; Georges, B.; Cope, A.V.; Tregoning, J.S. Nasal IgA Provides Protection against Human Influenza Challenge in Volunteers with Low Serum Influenza Antibody Titre. Front. Microbiol. 2017, 8, 900. [Google Scholar] [CrossRef] [Green Version]
- Lambkin-Williams, R.; Gelder, C.; Broughton, R.; Mallett, C.P.; Gilbert, A.S.; Mann, A.; He, D.; Oxford, J.S.; Burt, D. An Intranasal Proteosome-Adjuvanted Trivalent Influenza Vaccine Is Safe, Immunogenic & Efficacious in the Human Viral Influenza Challenge Model. Serum IgG & Mucosal IgA Are Important Correlates of Protection against Illness Associated with Infection. PLoS ONE 2016, 11, e0163089. [Google Scholar] [CrossRef]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. 1972, 70, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: Development and application of a bayesian random-effects model. BMC Med. Res. Methodol. 2010, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Ohmit, S.E.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Monto, A.S. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis. 2011, 204, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Wan, X.F.; Ye, Z.; Plant, E.P.; Zhao, Y.; Xu, Y.; Li, X.; Finch, C.; Zhao, N.; Kawano, T.; et al. H3N2 Mismatch of 2014-15 Northern Hemisphere Influenza Vaccines and Head-to-head Comparison between Human and Ferret Antisera derived Antigenic Maps. Sci. Rep. 2015, 5, 15279. [Google Scholar] [CrossRef] [Green Version]
- Mohn, K.G.; Smith, I.; Sjursen, H.; Cox, R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccines Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.D.; Nieuwkoop, N.J.; Pronk, M.; de Bruin, E.; Leroux-Roels, G.; Huijskens, E.G.W.; van Binnendijk, R.S.; Krammer, F.; Koopmans, M.P.G.; Rimmelzwaan, G.F. Influenza virus-specific antibody dependent cellular cytoxicity induced by vaccination or natural infection. Vaccine 2017, 35, 238–247. [Google Scholar] [CrossRef]
- Sicca, F.; Neppelenbroek, S.; Huckriede, A. Effector mechanisms of influenza-specific antibodies: Neutralization and beyond. Expert Rev. Vaccines 2018, 17, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Seibert, C.W.; Rahmat, S.; Krause, J.C.; Eggink, D.; Albrecht, R.A.; Goff, P.H.; Krammer, F.; Duty, J.A.; Bouvier, N.M.; Garcia-Sastre, A.; et al. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J. Virol. 2013, 87, 7793–7804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, W.; Brandl, J.R.; Lindbloom, J.D.; Martinez, C.J.; Scallan, C.D.; Trager, G.R.; Tingley, D.W.; Kabongo, M.L.; Tucker, S.N. Oral administration of an adenovirus vector encoding both an avian influenza A hemagglutinin and a TLR3 ligand induces antigen specific granzyme B and IFN-gamma T cell responses in humans. Vaccine 2013, 31, 1752–1758. [Google Scholar] [CrossRef]

| Indication | Vaccine | Storage Temperature (°C) | Drug Product Within Specification |
|---|---|---|---|
| Influenza 1 | VXA.A1.1 | +25 | 426 days |
| Norovirus 2 | VXA-G2.4-NS | +30 | 246 days |
| Norovirus 2 | VXA-G2.4-NS | +40 | 34 days |
| Study Arm | Number of Subjects | Viral Shedding 1 | Illness 2 | Most Important Correlate of Protection |
|---|---|---|---|---|
| VXA-A1.1 | 58 | 21 (36%) | 17 (29%) | ASC IgA |
| IIV | 54 | 24 (44%) | 19 (35%) | Serum HAI |
| Placebo | 31 | 22 (71%) | 15 (48%) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flitter, B.A.; Braun, M.R.; Tucker, S.N. Drop the Needle; A Temperature Stable Oral Tablet Vaccine Is Protective against Respiratory Viral Pathogens. Vaccines 2022, 10, 593. https://doi.org/10.3390/vaccines10040593
Flitter BA, Braun MR, Tucker SN. Drop the Needle; A Temperature Stable Oral Tablet Vaccine Is Protective against Respiratory Viral Pathogens. Vaccines. 2022; 10(4):593. https://doi.org/10.3390/vaccines10040593
Chicago/Turabian StyleFlitter, Becca A., Molly R. Braun, and Sean N. Tucker. 2022. "Drop the Needle; A Temperature Stable Oral Tablet Vaccine Is Protective against Respiratory Viral Pathogens" Vaccines 10, no. 4: 593. https://doi.org/10.3390/vaccines10040593
APA StyleFlitter, B. A., Braun, M. R., & Tucker, S. N. (2022). Drop the Needle; A Temperature Stable Oral Tablet Vaccine Is Protective against Respiratory Viral Pathogens. Vaccines, 10(4), 593. https://doi.org/10.3390/vaccines10040593






