Adjuvant Curdlan Contributes to Immunization against Cryptococcus gattii Infection in a Mouse Strain-Specific Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Curdlan and BGP as Adjuvant
2.3. Administration of a Dectin-1 Ligand before Immunization with Heat-Killed C. gattii
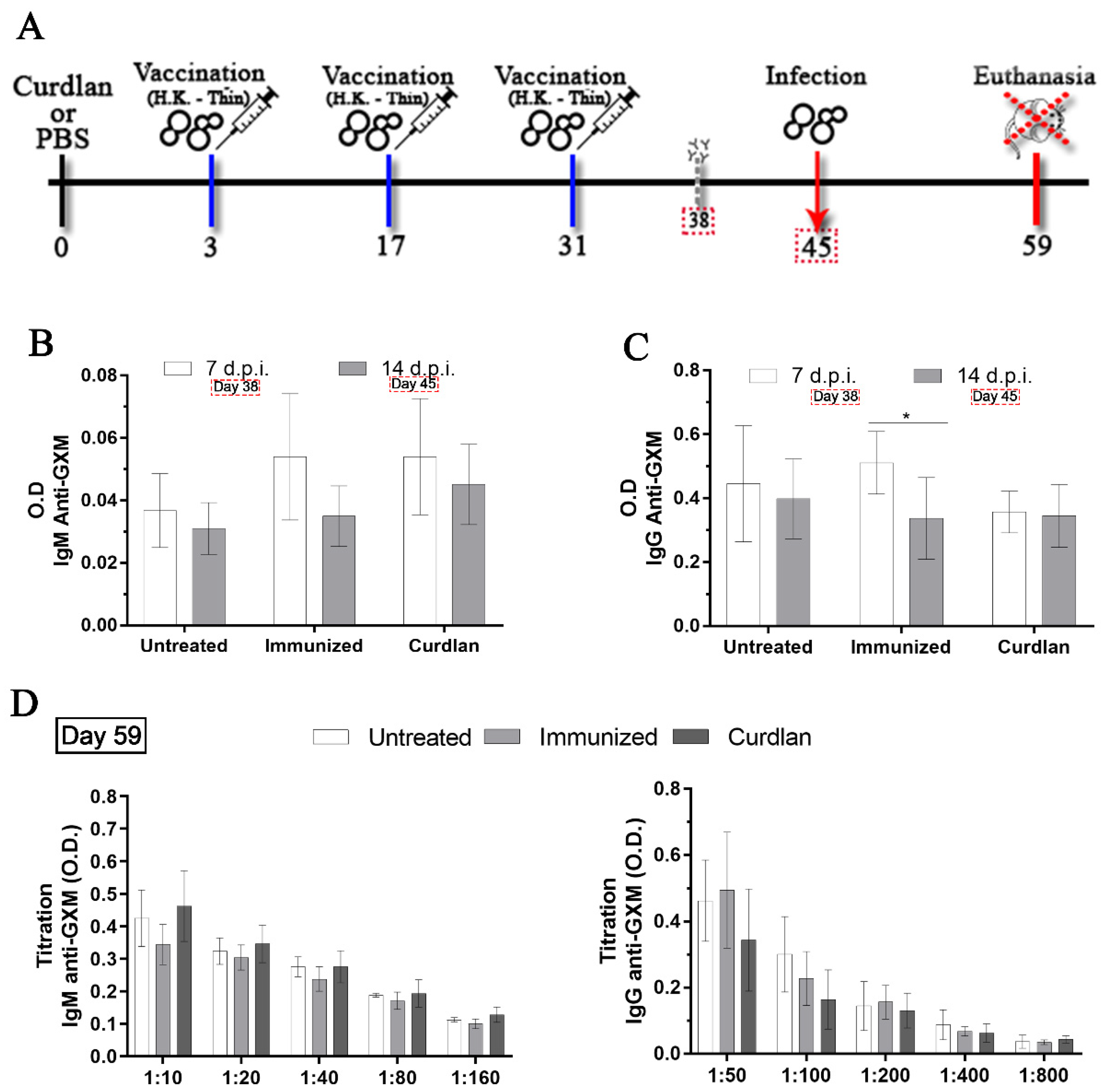
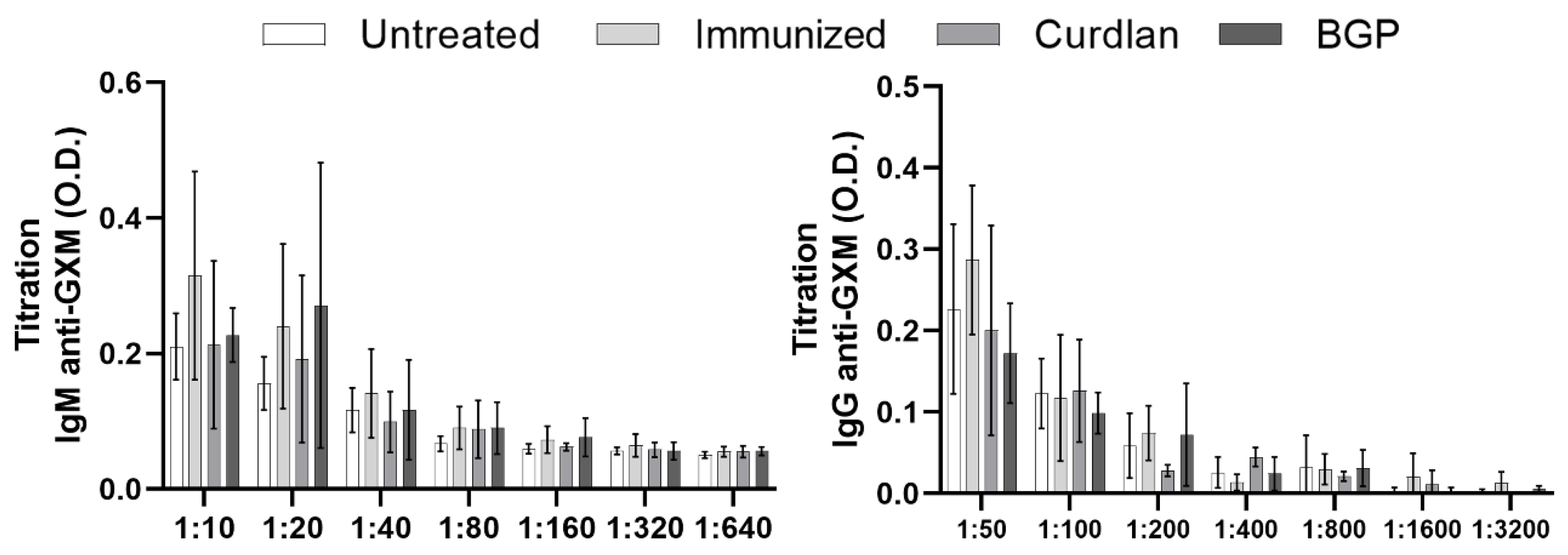
2.4. Measurement of Serum IgM and IgG anti-GXM Titers
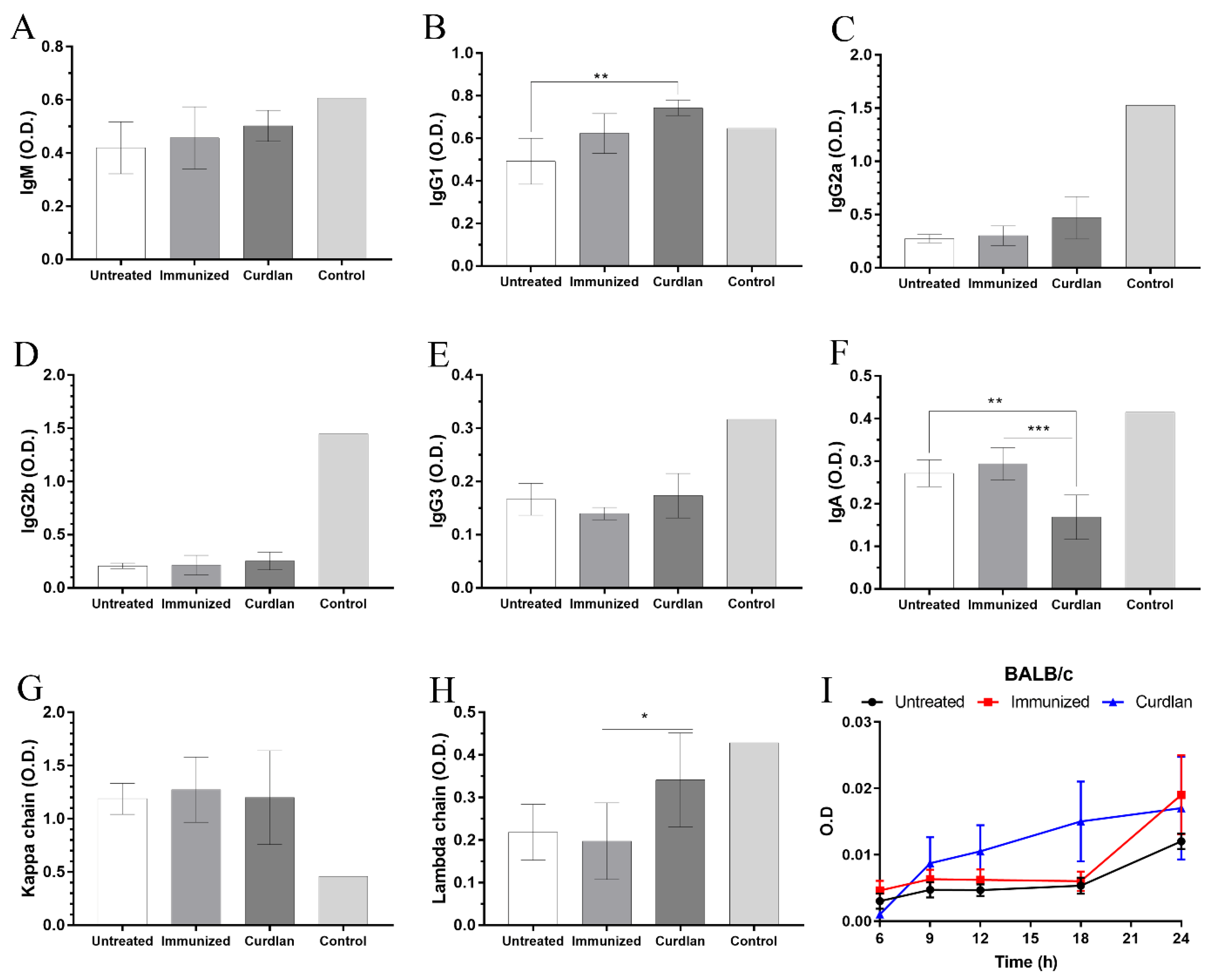
2.5. Isotyping Serum Immunoglobulins
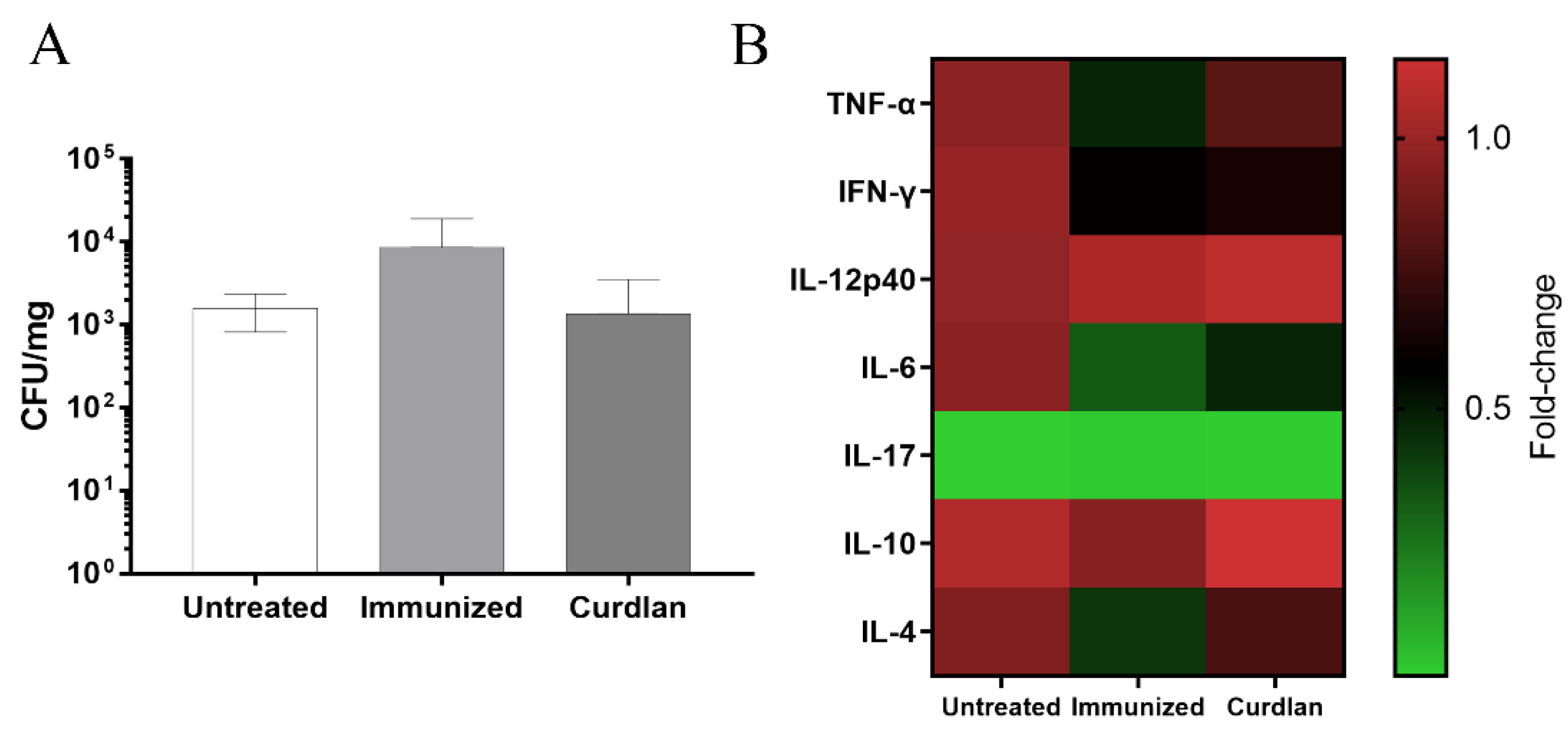
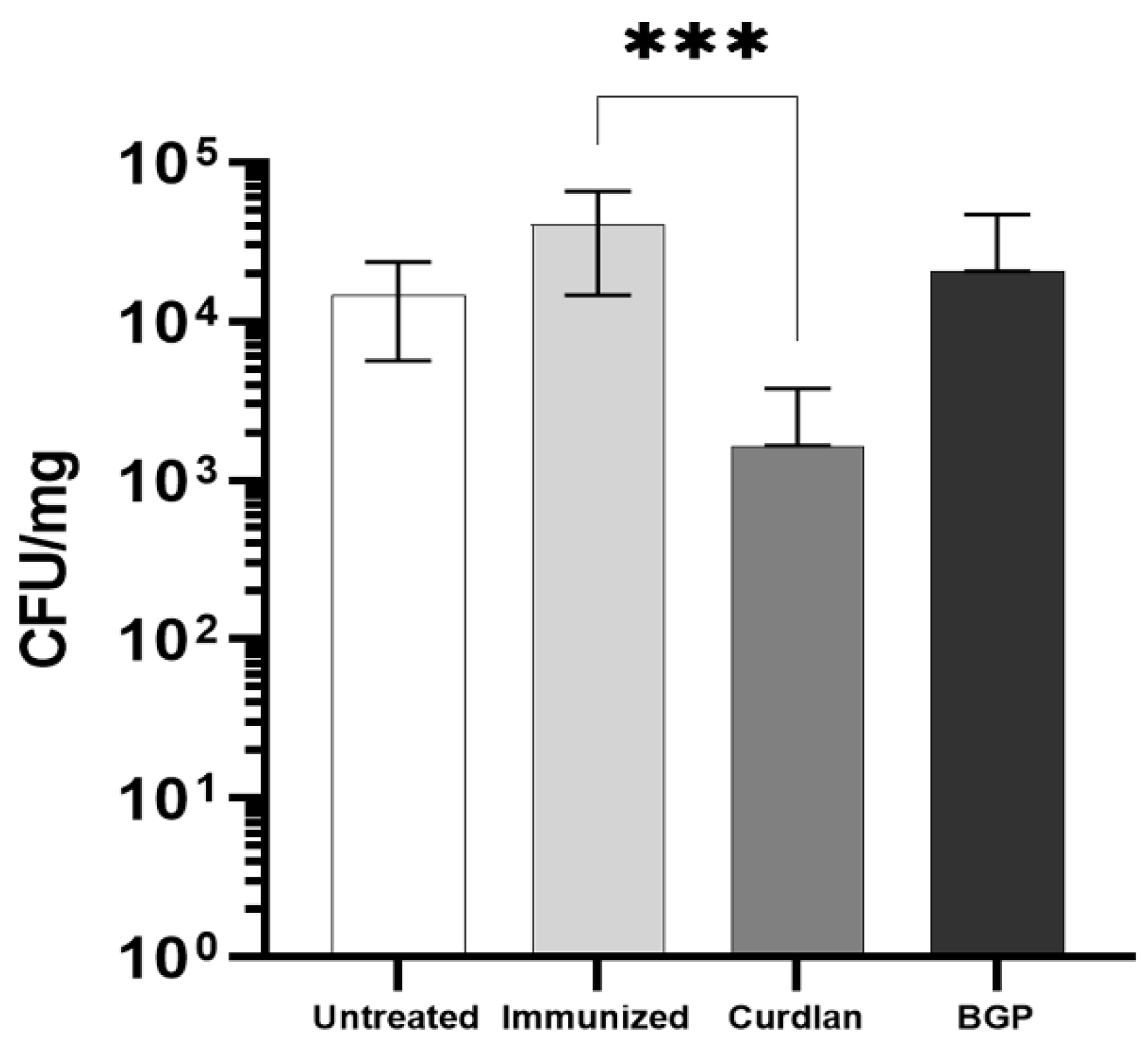
2.6. Quantification of Pulmonary Fungal Burden
2.7. Measurement of Pro- and Anti-Inflammatory Cytokines in the Lungs
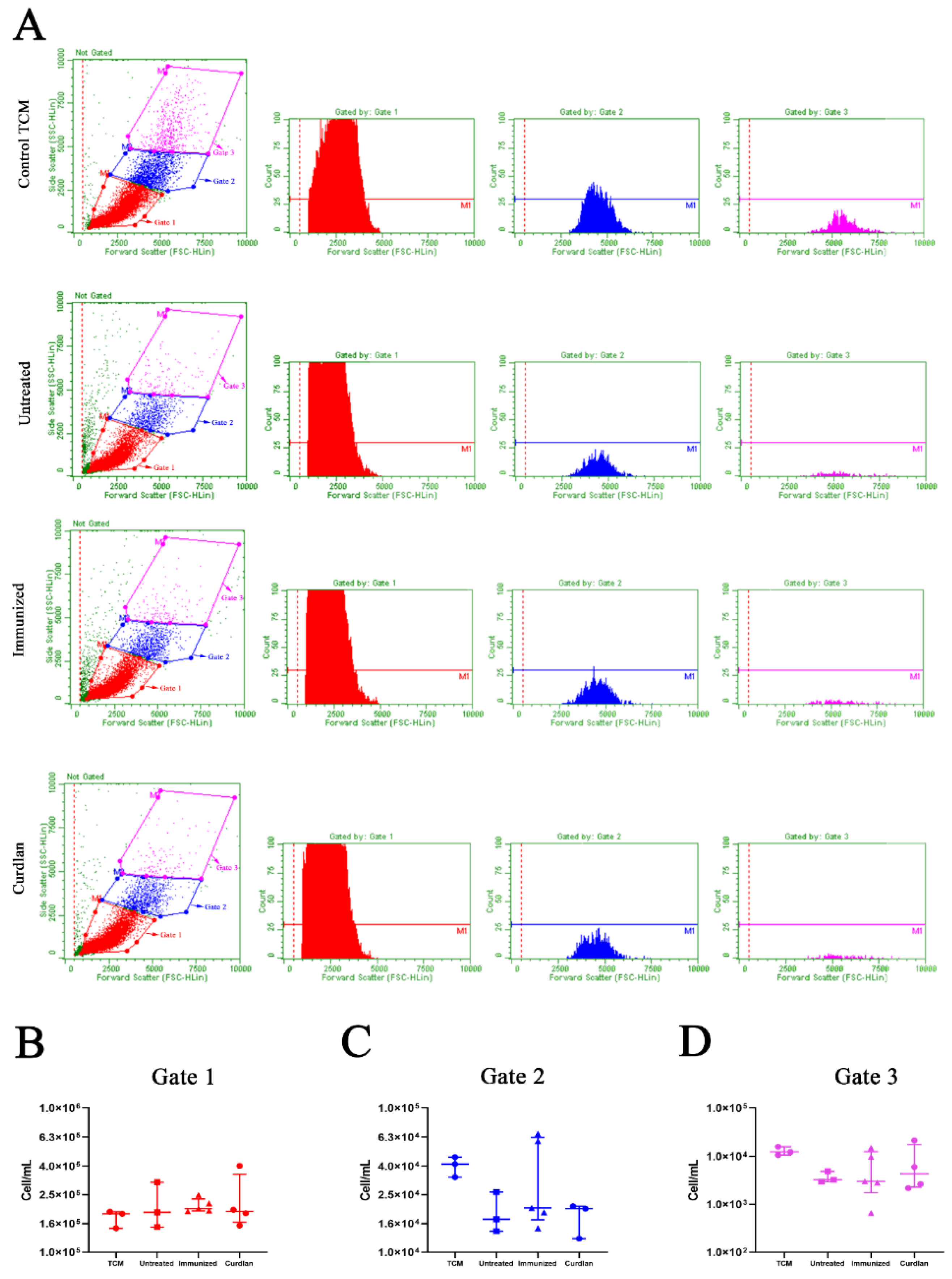
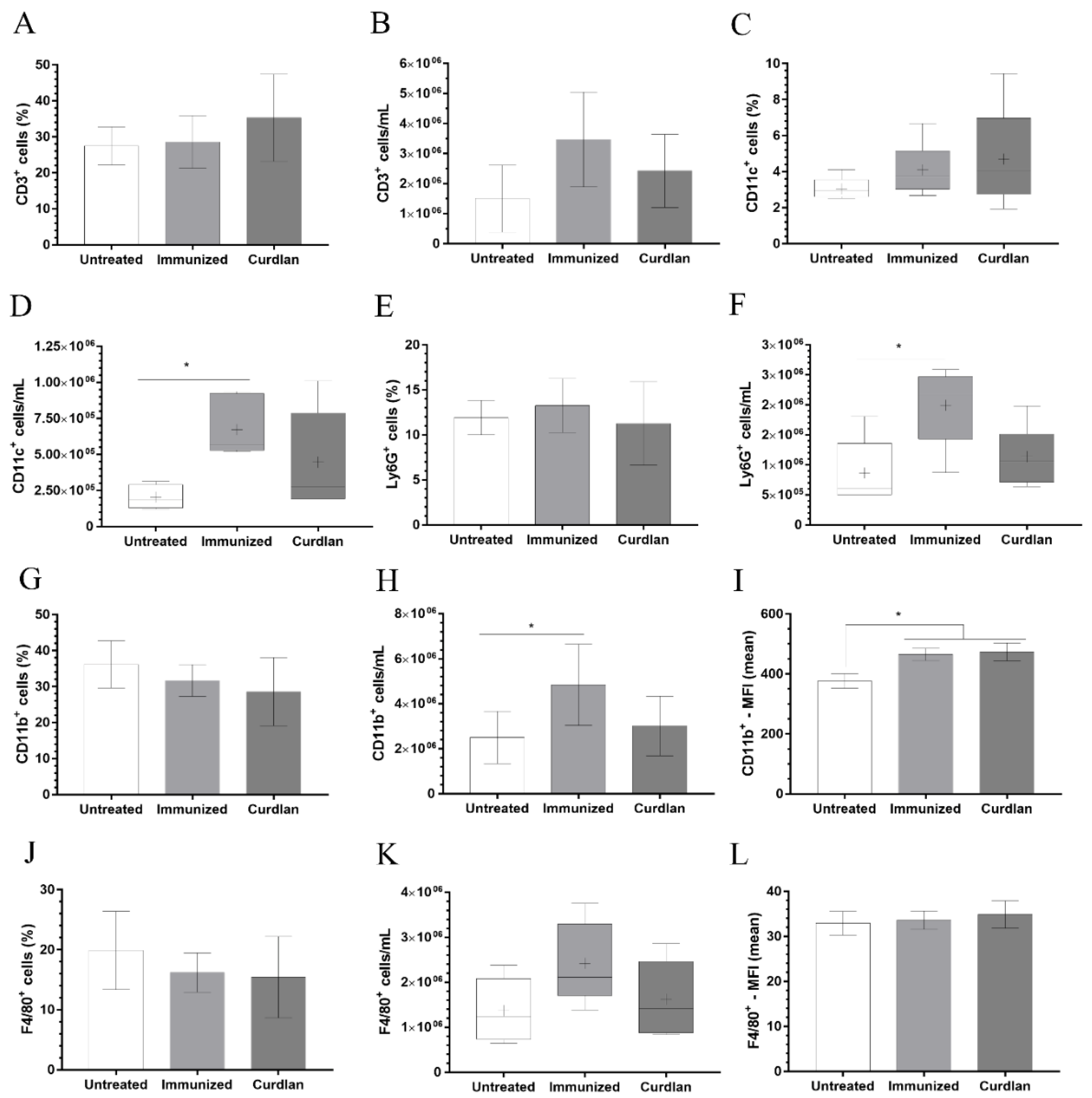
2.8. Phenotyping of Innate and Adaptive Immune Cells in the Lungs Using Flow Cytometry
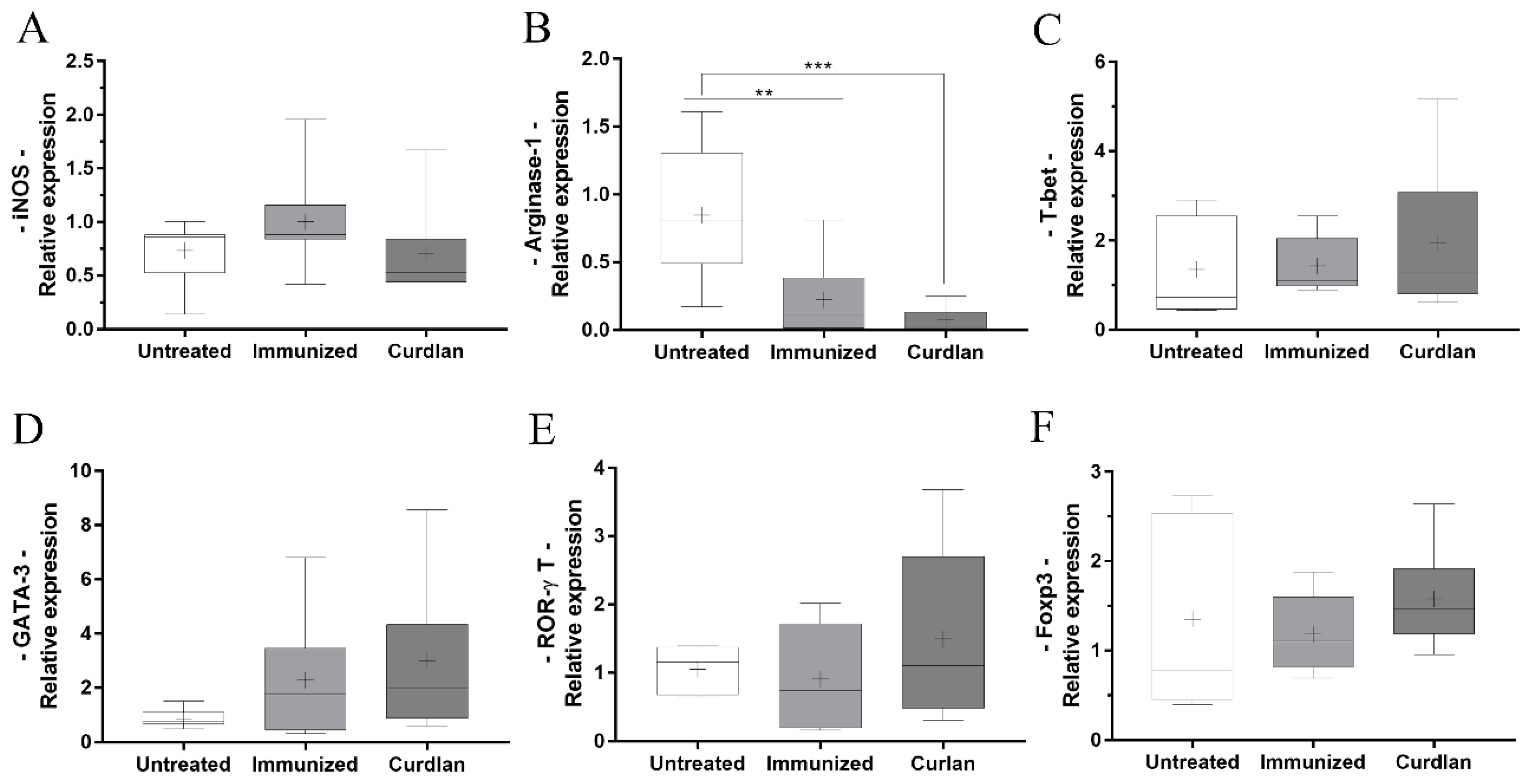
2.9. Measurement of Relative Gene Expression of Innate and Adaptive Immune Cell Differentiation Markers
2.10. Histopathological and Morphometric Analysis of the Pulmonary Tissue and Its C. gattii Burden
2.11. Evaluation of the Titanization Process of C. gattii in the Presence of Serum from Immunized Mice
2.12. Statistical Analyses
3. Results
3.1. Adjuvant Curdlan Slightly Improves the Switch of Immunoglobulin Isotypes in Immunized BALB/c Mice Challenged with C. gattii
3.2. In Vitro Formation of Titan Cells Is Not Affected by Serum from BALB/c Mice That Received Adjuvant Curdlan
3.3. Adjuvant Curdlan Altered the Cytokine Microenvironment in the Lungs of BALB/c Mice but Did Not Reduce the C. gattii Burden
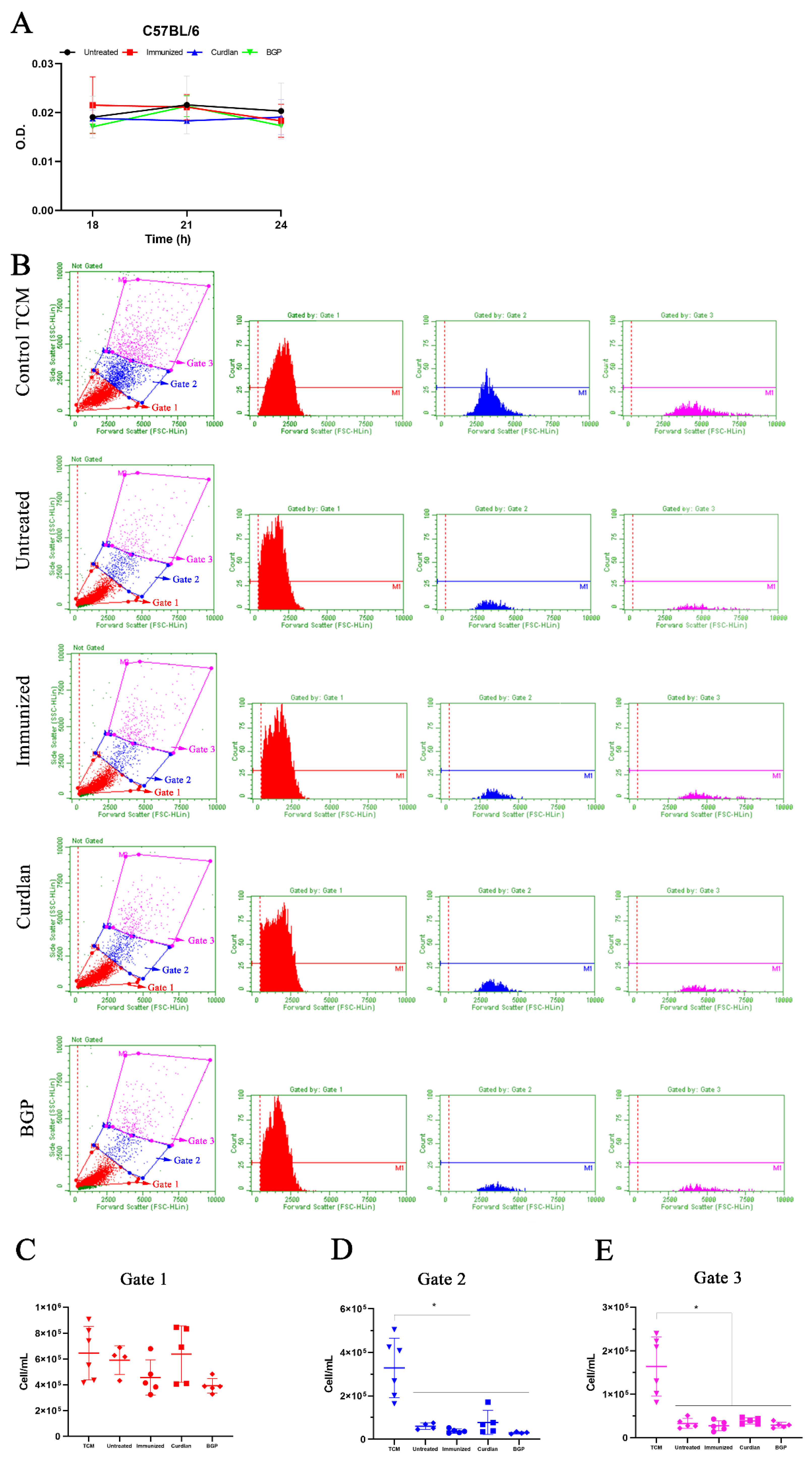
3.4. Immunization with Heat-Killed C. gattii Increases the Cellularity of the Innate immunity Cells in the Lungs That Is Restored to Basal Levels by the Adjuvant Curdlan
3.5. Immunized BALB/c Mice That Received Adjuvant Curdlan Had a Reduction in the Relative Expression of Arginase-1 in the Lung after the C. gattii Challenge
3.6. Curdlan Reduces the Average Area and Diameter of C. gattii Yeasts in the Lungs of BALB/c Mice
3.7. Immunized C57BL/6 Mice Treated with a Dectin-1 Ligand Had Higher Levels of IFN-γ and IL-10 in the Lung Tissue
3.8. Adjuvant Curdlan Facilitates the Reduction in the C. gattii Burden in the Lungs of C57BL/6 Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jermy, A. Stop neglecting fungi. Nat. Microbiol. 2017, 2, 17120. [Google Scholar] [CrossRef]
- Hadrich, I.; Ayadi, A. Epidemiology of antifungal susceptibility: Review of literature. J. Mycol. Med. 2018, 28, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Calling upon all public health mycologists. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and multi-national prevalence of fungal diseases—estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Dixit, A.; Carroll, S.F.; Qureshi, S.T. Cryptococcus gattii: An emerging cause of fungal disease in North America. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 840452. [Google Scholar] [CrossRef]
- Cano, E.J.; Yetmar, Z.A.; Razonable, R.R. Cryptococcus species other than Cryptococcus neoformans and Cryptococcus gattii: Are they clinically significant? Open Forum Infect. Dis. 2020, 7, ofaa527. [Google Scholar] [CrossRef]
- Basnayake, T.L.; Lim, A.; Currie, B.J. Pulmonary cryptococcal infection presenting with multiple lung nodules. Respir. Med. Case Rep. 2018, 23, 122–124. [Google Scholar] [CrossRef]
- Esher, S.K.; Zaragoza, O.; Alspaugh, J.A. Cryptococcal pathogenic mechanisms: A dangerous trip from the environment to the brain. Mem. Inst. Oswaldo Cruz. 2018, 113, e180057. [Google Scholar] [CrossRef]
- Ngamskulrungroj, P.; Chang, Y.; Sionov, E.; Kwon-Chung, K.J. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 2012, 3, e00103-12. [Google Scholar] [CrossRef]
- Campuzano, A.; Wormley, F.L. Innate immunity against cryptococcus, from recognition to elimination. J. Fungi 2018, 4, 33. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Perfect, J.R. Cryptococcosis. Diagn. Treat. Hum. Mycoses 2006, 20, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Coates, C.J.; Seoane, P.I.; Garelnabi, M.; Taylor-Smith, L.M.; Monteith, P.; Macleod, C.L.; Escaron, C.J.; Brown, G.D.; Hall, R.A.; et al. Characterizing the mechanisms of nonopsonic uptake of cryptococci by macrophages. J. Immunol. 2018, 200, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyagi, K.; Koguchi, Y.; Kinjo, Y.; Uezu, K.; Kinjo, T.; Akamine, M.; Fujita, J.; Kawamura, I.; Mitsuyama, M.; et al. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol. Med. Microbiol. 2006, 47, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K. Recognition mechanism of pathogen-associated molecular patterns and role of innate immune lymphocytes in fungal infection. Rinsho Byori 2009, 57, 779–785. [Google Scholar]
- Decote-Ricardo, D.; LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nascimento, D.O.; Nunes, M.P.; Morrot, A.; Freire-de-Lima, L.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Immunomodulatory role of capsular polysaccharides constituents of Cryptococcus neoformans. Front. Med. 2019, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Syme, R.M.; Spurrell, J.C.L.; Amankwah, E.K.; Green, F.H.Y.; Mody, C.H. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcγ receptor II for presentation to T lymphocytes. Infect. Immun. 2002, 70, 5972–5981. [Google Scholar] [CrossRef]
- Monari, C.; Pericolini, E.; Bistoni, G.; Casadevall, A.; Kozel, T.R.; Vecchiarelli, A. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of Fas ligand in macrophages. J. Immunol. 2005, 174, 3461–3468. [Google Scholar] [CrossRef]
- Coelho, C.; Bocca, A.L.; Casadevall, A. Chapter One-The Tools for Virulence of Cryptococcus neoformans. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 87, pp. 1–41. [Google Scholar]
- Ben-Abdallah, M.; Sturny-Leclère, A.; Avé, P.; Louise, A.; Moyrand, F.; Weih, F.; Janbon, G.; Mémet, S. Fungal-Induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-κB. PLoS Pathog. 2012, 8, e1002555. [Google Scholar] [CrossRef]
- Eva, P.; Elio, C.; Claudia, M.; Magdia, D.J.; Francesco, B.; Arturo, C.; Anna, V. Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell. Microbiol. 2006, 8, 267–275. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Chow, S.-K.; Bistoni, F.; Cenci, E.; Casadevall, A. Cryptococcus neoformans galactoxylomannan is a potent negative immunomodulator, inspiring new approaches in anti-inflammatory immunotherapy. Immunotherapy 2011, 3, 997–1005. [Google Scholar] [CrossRef]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; DosReis, G.A.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell. Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Mansour, M.K.; Levitz, S.M. Receptor-mediated clearance of Cryptococcus neoformans capsular polysaccharide in vivo. Infect. Immun. 2005, 73, 8429–8432. [Google Scholar] [CrossRef]
- Nakamura, K.; Kinjo, T.; Saijo, S.; Miyazato, A.; Adachi, Y.; Ohno, N.; Fujita, J.; Kaku, M.; Iwakura, Y.; Kawakami, K. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol. Immunol. 2007, 51, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Mansour, M.K.; Shoham, S.; Rottman, J.B.; Levitz, S.M. Involvement of CD14, toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 2004, 72, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Midiri, A.; Messina, L.; Tomasello, F.; Garufi, G.; Catania, M.R.; Bombaci, M.; Beninati, C.; Teti, G.; Mancuso, G. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 2005, 35, 870–878. [Google Scholar] [CrossRef]
- Walsh, N.M.; Wuthrich, M.; Wang, H.; Klein, B.; Hull, C.M. Characterization of C-type lectins reveals an unexpectedly limited interaction between Cryptococcus neoformans spores and Dectin-1. PLoS ONE 2017, 12, e0173866. [Google Scholar] [CrossRef]
- Monari, C.; Bistoni, F.; Casadevall, A.; Pericolini, E.; Pietrella, D.; Kozel, T.R.; Vecchiarelli, A. Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J. Infect. Dis. 2005, 191, 127–137. [Google Scholar] [CrossRef]
- Monari, C.; Bistoni, F.; Vecchiarelli, A. Glucuronoxylomannan exhibits potent immunosuppressive properties. FEMS Yeast Res. 2006, 6, 537–542. [Google Scholar] [CrossRef]
- Beenhouwer, D.O.; Shapiro, S.; Feldmesser, M.; Casadevall, A.; Scharff, M.D. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infect. Immun. 2001, 69, 6445–6455. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Wormley, F.L. Cryptococcus antigens and immune responses: Implications for a vaccine. Expert Rev. Vaccines 2013, 12, 1261–1272. [Google Scholar] [CrossRef]
- Caballero Van Dyke, M.C.; Wormley, F.L. A Call to arms: Quest for a cryptococcal vaccine. Trends Microbiol. 2018, 26, 436–446. [Google Scholar] [CrossRef]
- Herring, A.C.; Lee, J.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Induction of Interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-Cell-Mediated immunity to pulmonary Cryptococcus neoformans infection. Infect. Immun. 2002, 70, 2959. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Tompkins, K.C.; McNamara, A.; Jain, A.V.; Moore, B.B.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am. J. Pathol. 2009, 175, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Angkasekwinai, P.; Sringkarin, N.; Supasorn, O.; Fungkrajai, M.; Wang, Y.H.; Chayakulkeeree, M.; Ngamskulrungroj, P.; Angkasekwinai, N.; Pattanapanyasat, K. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect. Immun. 2014, 82, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Huston, S.M.; Li, S.S.; Stack, D.; Timm-McCann, M.; Jones, G.J.; Islam, A.; Berenger, B.M.; Xiang, R.F.; Colarusso, P.; Mody, C.H. Cryptococcus gattii is killed by dendritic cells, but evades adaptive immunity by failing to induce dendritic cell maturation. J. Immunol. 2013, 191, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Jamil, K.; Polyak, M.J.; Feehan, D.D.; Surmanowicz, P.; Stack, D.; Li, S.S.; Ogbomo, H.; Olszewski, M.; Ganguly, A.; Mody, C.H. Phagosomal F-Actin Retention by Cryptococcus gattii induces dendritic cell immunoparalysis. mBio 2020, 11, e01821-20. [Google Scholar] [CrossRef]
- Zhai, B.; Wozniak, K.L.; Masso-Silva, J.; Upadhyay, S.; Hole, C.; Rivera, A.; Wormley, F.L.; Lin, X. Development of Protective Inflammation and Cell-Mediated Immunity against Cryptococcus neoformans after Exposure to Hyphal Mutants. mBio 2015, 6, e01433-15. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Hameed, R.S.; Wozniak, K.L.; Hole, C.R.; Leopold Wager, C.M.; Weintraub, S.T.; Lopez-Ribot, J.L.; Wormley, F.L., Jr. Vaccine-mediated immune responses to experimental pulmonary Cryptococcus gattii infection in mice. PLoS ONE 2014, 9, e104316. [Google Scholar] [CrossRef]
- Specht, C.A.; Lee, C.K.; Huang, H.; Hester, M.M.; Liu, J.; Luckie, B.A.; Torres Santana, M.A.; Mirza, Z.; Khoshkenar, P.; Abraham, A.; et al. Vaccination with recombinant cryptococcus proteins in glucan particles protects mice against cryptococcosis in a manner dependent upon mouse strain and cryptococcal species. mBio 2017, 8, e01872-17. [Google Scholar] [CrossRef]
- Murphy, J.W.; Schafer, F.; Casadevall, A.; Adesina, A. Antigen-induced protective and nonprotective cell-mediated immune components against Cryptococcus neoformans. Infect. Immun. 1998, 66, 2632–2639. [Google Scholar] [CrossRef]
- Coltri, K.C.; Oliveira, L.L.; Ruas, L.P.; Vendruscolo, P.E.; Goldman, M.H.; Panunto-Castelo, A.; Roque-Barreira, M.C. Protection against Paracoccidioides brasiliensis infection conferred by the prophylactic administration of native and recombinant ArtinM. Med. Mycol. 2010, 48, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Loyola, W.; Custodio, L.A.; Felipe, I.; Conchon-Costa, I.; Carvalho, P.G.d.; da Silva Quirino, G.F.; da Rosa Santos Silva, L.F.; Gaziri, L.C. Artin M enhances TNF-α production and phagocytosis of Candida albicans mediated by dectin-1 and mannose receptors. Int. Immunopharmacol. 2012, 12, 378–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alegre, A.C.; Oliveira, A.F.; Dos Reis Almeida, F.B.; Roque-Barreira, M.C.; Hanna, E.S. Recombinant paracoccin reproduces the biological properties of the native protein and induces protective Th1 immunity against Paracoccidioides brasiliensis infection. PLoS Negl. Trop. Dis. 2014, 8, e2788. [Google Scholar] [CrossRef]
- Alegre-Maller, A.C.P.; Mendonça, F.C.; da Silva, T.A.; Oliveira, A.F.; Freitas, M.S.; Hanna, E.S.; Almeida, I.C.; Gay, N.J.; Roque-Barreira, M.C. Therapeutic administration of recombinant paracoccin confers protection against Paracoccidioides brasiliensis Infection: Involvement of TLRs. PLoS Negl. Trop. Dis. 2014, 8, e3317. [Google Scholar] [CrossRef]
- Magalhães, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin. Vaccine Immunol. CVI 2012, 19, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P. β-glucan as a new tool in vaccine development. Scand. J. Immunol. 2020, 91, e12833. [Google Scholar] [CrossRef]
- Li, P.; Wang, F. Polysaccharides: Candidates of promising vaccine adjuvants. Drug Discov. Ther. 2015, 9, 88–93. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, K.H.; Lee, H.K.; Kim, J.S.; Kim, Y.G.; Lee, J.H.; Kim, K.H.; Yun, J.; Hwang, B.Y.; Hong, J.T.; et al. Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int. Immunopharmacol. 2016, 39, 71–78. [Google Scholar] [CrossRef]
- LeibundGut-Landmann, S.; Gross, O.; Robinson, M.J.; Osorio, F.; Slack, E.C.; Tsoni, S.V.; Schweighoffer, E.; Tybulewicz, V.; Brown, G.D.; Ruland, J.; et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef]
- Underhill, D.M.; Rossnagle, E.; Lowell, C.A.; Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. J. Blood 2005, 106, 2543–2550. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by beta-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory properties and antitumor activities of glucans (Review). Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Hester, M.M.; Lee, C.K.; Abraham, A.; Khoshkenar, P.; Ostroff, G.R.; Levitz, S.M.; Specht, C.A. Protection of mice against experimental cryptococcosis using glucan particle-based vaccines containing novel recombinant antigens. Vaccine 2020, 38, 620–626. [Google Scholar] [CrossRef]
- Ghosh, K.; Sharma, G.; Saha, A.; Kar, S.; Das, P.K.; Ukil, A. Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. J. Infect. Dis. 2013, 207, 1016–1025. [Google Scholar] [CrossRef]
- Boehm, D.T.; Wolf, M.A.; Hall, J.M.; Wong, T.Y.; Sen-Kilic, E.; Basinger, H.D.; Dziadowicz, S.A.; Gutierrez, M.P.; Blackwood, C.B.; Bradford, S.D.; et al. Intranasal acellular pertussis vaccine provides mucosal immunity and protects mice from Bordetella pertussis. NPJ Vaccines 2019, 4, 40. [Google Scholar] [CrossRef]
- Negi, S.; Pahari, S.; Das, D.K.; Khan, N.; Agrewala, J.N. Curdlan limits mycobacterium tuberculosis survival through STAT-1 regulated nitric oxide production. Front. Microbiol. 2019, 10, 1173. [Google Scholar] [CrossRef]
- Higashi, T.; Hashimoto, K.; Takagi, R.; Mizuno, Y.; Okazaki, Y.; Tanaka, Y.; Matsushita, S. Curdlan induces DC-Mediated Th17 Polarization via Jagged1 activation in human dendritic cells. Allergol. Int. 2010, 59, 161–166. [Google Scholar] [CrossRef]
- Chaffey, A.; Hamonic, G.; Chand, D.; Mutwiri, G.K.; Wilson, H.L. The adjuvants Polyphosphazene (PCEP) and a combination of curdlan plus leptin promote a Th17-Type immune response to an intramuscular vaccine in mice. Vaccines 2021, 9, 507. [Google Scholar] [CrossRef]
- Carroll, S.F.; Lafferty, E.I.; Flaczyk, A.; Fujiwara, T.M.; Homer, R.; Morgan, K.; Loredo-Osti, J.C.; Qureshi, S.T. Susceptibility to progressive Cryptococcus neoformans pulmonary infection is regulated by loci on mouse chromosomes 1 and 9. Infect. Immun. 2012, 80, 4167–4176. [Google Scholar] [CrossRef]
- Zaragoza, O.; Alvarez, M.; Telzak, A.; Rivera, J.; Casadevall, A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect. Immun. 2007, 75, 2729–2739. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; McNamara, D.A.; Hernandez, Y.; Huffnagle, G.B.; Toews, G.B.; Olszewski, M.A. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect. Immun. 2008, 76, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Carroll, S.F.; Homer, R.; Qureshi, S.T. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect. Immun. 2008, 76, 4745. [Google Scholar] [CrossRef] [PubMed]
- Ikeda-Dantsuji, Y.; Ohno, H.; Tanabe, K.; Umeyama, T.; Ueno, K.; Nagi, M.; Yamagoe, S.; Kinjo, Y.; Miyazaki, Y. Interferon-γ promotes phagocytosis of Cryptococcus neoformans but not Cryptococcus gattii by murine macrophages. J. Infect. Chemother. 2015, 21, 831–836. [Google Scholar] [CrossRef]
- Casadevall, A.; Mukherjee, J.; Scharff, M.D. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 1992, 154, 27–35. [Google Scholar] [CrossRef]
- Fonseca, F.L.; Nohara, L.L.; Cordero, R.J.B.; Frases, S.; Casadevall, A.; Almeida, I.C.; Nimrichter, L.; Rodrigues, M.L. Immunomodulatory Effects of Serotype B Glucuronoxylomannan from Cryptococcus gattii Correlate with Polysaccharide Diameter. Infect. Immun. 2010, 78, 3861–3870. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Levitz, S.M. Isolation and purification of antigenic components of Cryptococcus. Methods Mol. Biol. 2009, 470, 71–83. [Google Scholar] [CrossRef]
- Oliveira-Brito, P.K.M.; Rezende, C.P.; Almeida, F.; Roque-Barreira, M.C.; da Silva, T.A. iNOS/Arginase-1 expression in the pulmonary tissue over time during Cryptococcus gattii infection. Innate Immun. 2019, 26, 117–129. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Pianalto, K.M.; Nichols, C.B.; Zaragoza, O.; Alspaugh, J.A.; Pirofski, L.A. Human IgM inhibits the formation of titan-like cells in Cryptococcus neoformans. Infect. Immun. 2020, 88, e00046-20. [Google Scholar] [CrossRef]
- Wormley, F.L.; Perfect, J.R.; Steele, C.; Cox, G.M. Protection against Cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans Strain. Infect. Immun. 2007, 75, 1453. [Google Scholar] [CrossRef]
- Kawakami, K.; Tohyama, M.; Xie, Q.; Saito, A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin. Exp. Immunol. 1996, 104, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Specht, C.A.; Lee, C.K.; Huang, H.; Tipper, D.J.; Shen, Z.T.; Lodge, J.K.; Leszyk, J.; Ostroff, G.R.; Levitz, S.M. Protection against experimental cryptococcosis following vaccination with glucan particles containing cryptococcus alkaline extracts. mBio 2015, 6, e01905-15. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.J.; Oyston, P.C.F.; Williamson, D.E. Potential of the β-glucans to enhance innate resistance to biological agents. Expert Rev. Anti-Infect. Ther. 2010, 8, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.F.; Johnston, S.A. Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis. Fungal Genet. Biol. 2015, 78, 76–86. [Google Scholar] [CrossRef]
- Voelz, K.; May, R.C. Cryptococcal interactions with the host immune system. Eukaryot. Cell 2010, 9, 835–846. [Google Scholar] [CrossRef]
- Devi, S.J. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 1996, 14, 841–844. [Google Scholar] [CrossRef]
- Chow, S.K.; Casadevall, A. Evaluation of Cryptococcus neoformans galactoxylomannan-protein conjugate as vaccine candidate against murine cryptococcosis. Vaccine 2011, 29, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C.; Lester, S.; Black, W.; Fyfe, M.; Raverty, S. British Columbia: Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can. Vet. J. 2002, 43, 792–794. [Google Scholar]
- MacDougall, L.; Kidd, S.E.; Galanis, E.; Mak, S.; Leslie, M.J.; Cieslak, P.R.; Kronstad, J.W.; Morshed, M.G.; Bartlett, K.H. Spread of Cryptococcus gattii in British Columbia, Canada, and Detection in the Pacific Northwest, USA. Emerg. Infect. Dis. 2007, 13, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Upton, A.; Fraser, J.A.; Kidd, S.E.; Bretz, C.; Bartlett, K.H.; Heitman, J.; Marr, K.A. First contemporary case of human infection with Cryptococcus gattii in Puget Sound: Evidence for spread of the Vancouver Island Outbreak. J. Clin. Microbiol. 2007, 45, 3086–3088. [Google Scholar] [CrossRef][Green Version]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.A.; Janbon, G.; Idnurm, A.; Bahn, Y.-S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Goncer, I.; Bongomin, F.; Doran, H.M.; Novak-Frazer, L.; Masania, R.; Moore, C.B.; Richardson, M.D. A case of pulmonary cryptococcoma due to Cryptococcus gattii in the United Kingdom. Med. Mycol. Case Rep. 2018, 21, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Enoch, D.A.; Ludlam, H.A.; Brown, N.M. Invasive fungal infections: A review of epidemiology and management options. J. Med. Microbiol. 2006, 55, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New facets of antifungal therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef]
- Azevedo, R.V.D.M.; Rizzo, J.; Rodrigues, M.L. Virulence factors as targets for anticryptococcal therapy. J. Fungi 2016, 2, 29. [Google Scholar] [CrossRef]
- Custodio, L.A.; Loyola, W.; Conchon-Costa, I.; da Silva Quirino, G.F.; Felipe, I. Protective effect of Artin M from extract of Artocarpus integrifolia seeds by Th1 and Th17 immune response on the course of infection by Candida albicans. Int. Immunopharmacol. 2011, 11, 1510–1515. [Google Scholar] [CrossRef]
- Feldmesser, M.; Casadevall, A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 1997, 158, 790. [Google Scholar]
- Zhao, Y.; Wang, Y.; Upadhyay, S.; Xue, C.; Lin, X. Activation of meiotic genes mediates ploidy reduction during cryptococcal infection. Curr. Biol. 2020, 30, 1387–1396.e1385. [Google Scholar] [CrossRef]
- Hommel, B.; Mukaremera, L.; Cordero, R.J.B.; Coelho, C.; Desjardins, C.A.; Sturny-Leclère, A.; Janbon, G.; Perfect, J.R.; Fraser, J.A.; Casadevall, A.; et al. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog. 2018, 14, e1006982. [Google Scholar] [CrossRef]
- Zhou, X.; Ballou, E.R. The Cryptococcus neoformans titan cell: From in vivo phenomenon to in vitro model. Curr. Clin. Microbiol. Rep. 2018, 5, 252–260. [Google Scholar] [CrossRef]
- Cannon, G.J.; Swanson, J.A. The macrophage capacity for phagocytosis. J. Cell Sci. 1992, 101, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Erwig, L.P.; Gow, N.A. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chrétien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Voelz, K.; Johnston, S.A.; Smith, L.M.; Hall, R.A.; Idnurm, A.; May, R.C. ‘Division of labour’ in response to host oxidative burst drives a fatal Cryptococcus gattii outbreak. Nat. Commun. 2014, 5, 5194. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; May, R.C. Mitochondria and the regulation of hypervirulence in the fatal fungal outbreak on Vancouver Island. Virulence 2010, 1, 197–201. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, P.; Berrevoets, C.; Rösch, C.; Schols, H.A.; Verhoef, K.; Wichers, H.J.; Debets, R.; Govers, C. Curdlan, zymosan and a yeast-derived β-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunol. Immunother. 2021, 70, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Mambula, S.S.; Simons, E.R.; Hastey, R.; Selsted, M.E.; Levitz, S.M. Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect. Immun. 2000, 68, 6257–6264. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Wong, B.; Newman, S.L. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J. Immunol. 1996, 156, 3836–3840. [Google Scholar]
- Mednick, A.J.; Feldmesser, M.; Rivera, J.; Casadevall, A. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 2003, 33, 1744–1753. [Google Scholar] [CrossRef]
- Milam, J.E.; Herring-Palmer, A.C.; Pandrangi, R.; McDonald, R.A.; Huffnagle, G.B.; Toews, G.B. Modulation of the pulmonary type 2 T-cell response to Cryptococcus neoformans by intratracheal delivery of a tumor necrosis factor alpha-expressing adenoviral vector. Infect. Immun. 2007, 75, 4951–4958. [Google Scholar] [CrossRef]
- Hernandez, Y.; Arora, S.; Erb-Downward, J.R.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 2005, 174, 1027–1036. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Brito, P.K.M.; de Campos, G.Y.; Guimarães, J.G.; Serafim da Costa, L.; Silva de Moura, E.; Lazo-Chica, J.E.; Roque-Barreira, M.C.; da Silva, T.A. Adjuvant Curdlan Contributes to Immunization against Cryptococcus gattii Infection in a Mouse Strain-Specific Manner. Vaccines 2022, 10, 620. https://doi.org/10.3390/vaccines10040620
Oliveira-Brito PKM, de Campos GY, Guimarães JG, Serafim da Costa L, Silva de Moura E, Lazo-Chica JE, Roque-Barreira MC, da Silva TA. Adjuvant Curdlan Contributes to Immunization against Cryptococcus gattii Infection in a Mouse Strain-Specific Manner. Vaccines. 2022; 10(4):620. https://doi.org/10.3390/vaccines10040620
Chicago/Turabian StyleOliveira-Brito, Patrícia Kellen Martins, Gabriela Yamazaki de Campos, Júlia Garcia Guimarães, Letícia Serafim da Costa, Edanielle Silva de Moura, Javier Emílio Lazo-Chica, Maria Cristina Roque-Barreira, and Thiago Aparecido da Silva. 2022. "Adjuvant Curdlan Contributes to Immunization against Cryptococcus gattii Infection in a Mouse Strain-Specific Manner" Vaccines 10, no. 4: 620. https://doi.org/10.3390/vaccines10040620
APA StyleOliveira-Brito, P. K. M., de Campos, G. Y., Guimarães, J. G., Serafim da Costa, L., Silva de Moura, E., Lazo-Chica, J. E., Roque-Barreira, M. C., & da Silva, T. A. (2022). Adjuvant Curdlan Contributes to Immunization against Cryptococcus gattii Infection in a Mouse Strain-Specific Manner. Vaccines, 10(4), 620. https://doi.org/10.3390/vaccines10040620





