B-Cell-Based Immunotherapy: A Promising New Alternative
Abstract
:1. Introduction
2. Dual Role of B Cells
3. Anti-Tumor Activity of B Cells
4. The Pro-Tumor Activity of B Cells
4.1. Immune Cells
4.2. Cytokines and Metabolites
4.3. Expression of Immune Checkpoint on B Cells
4.4. Hypoxia
5. B-Cell-Based Immunotherapy and Their Clinical Applications
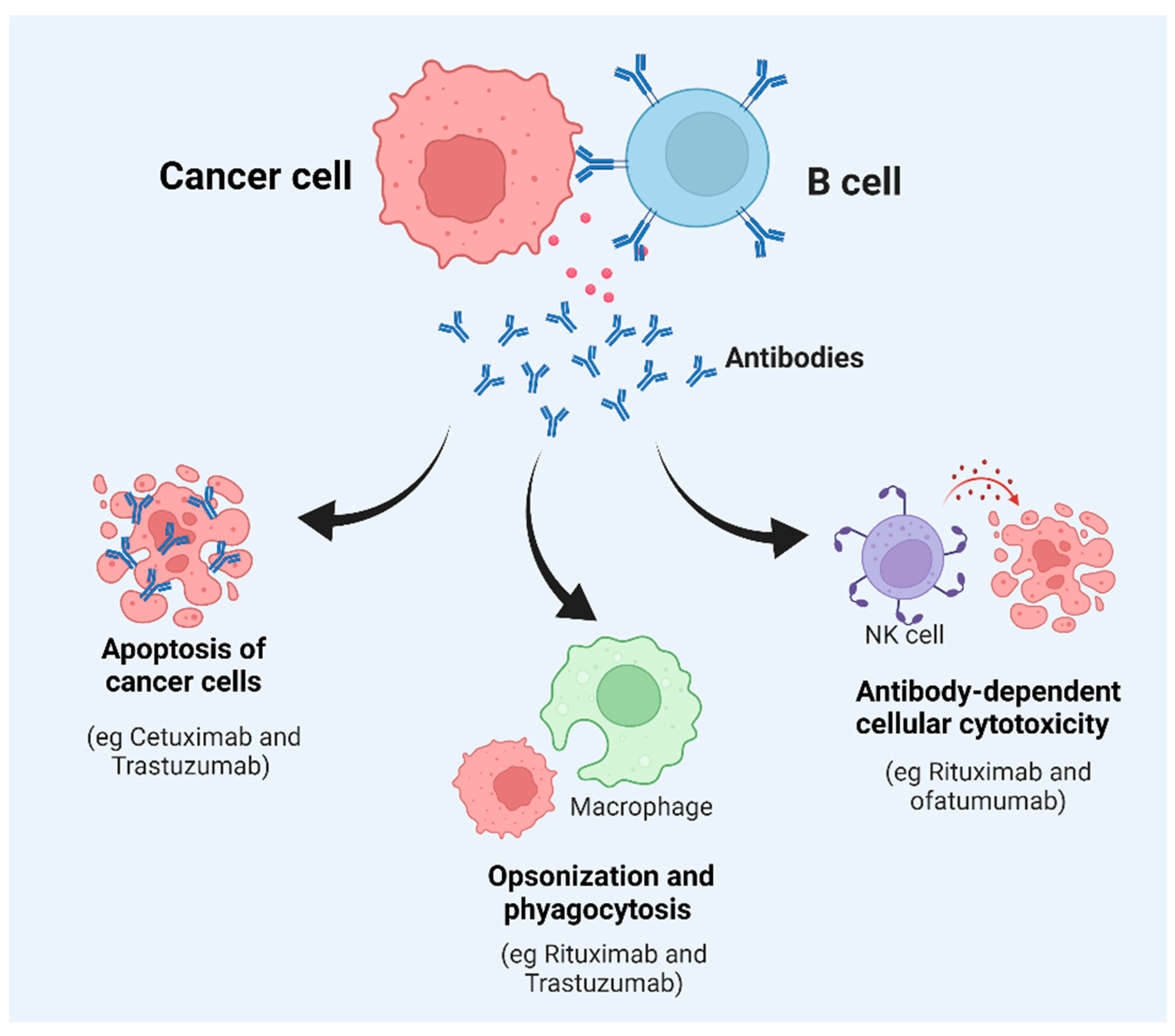
5.1. Monoclonal Antibody (mAb)
5.2. Inhibiting or Depleting B Cells
5.3. Activated B Cells to Suppress Tumor Growth
5.4. Tertiary Lymphoid Structure (TLS)
5.5. Immunotherapy Based on Tumor-Associated Autoantibodies
5.6. B-Cell-Epitope-Based Vaccine
5.7. Role of Immunoglobulin in Tumor Therapy
5.8. Role of Cytokines and Their Association with Tumorigenesis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Naseem, M.; Lo, J.H.; Battaglin, F.; Soni, S.; Puccini, A.; Berger, M.D.; Zhang, W.; Baba, H.; Lenz, H.-J. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat. Rev. 2019, 73, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Maggs, L.; Cattaneo, G.; Dal, A.E.; Moghaddam, A.S.; Ferrone, S. CAR T Cell-Based Immunotherapy for the Treatment of Glioblastoma. Front. Neurosci. 2021, 15, 535. [Google Scholar] [CrossRef]
- Kriegsmann, K.; Kriegsmann, M.; Cremer, M.; Schmitt, M.; Dreger, P.; Goldschmidt, H.; Müller-Tidow, C.; Hundemer, M. Cell-based immunotherapy approaches for multiple myeloma. Br. J. Cancer 2019, 120, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Appelgren, D.; Eriksson, P.; Ernerudh, J.; Segelmark, M. Marginal-Zone B-Cells Are Main Producers of IgM in Humans, and Are Reduced in Patients With Autoimmune Vasculitis. Front. Immunol. 2018, 9, 2242. [Google Scholar] [CrossRef] [Green Version]
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef] [Green Version]
- Suan, D.; Sundling, C.; Brink, R. Plasma cell and memory B cell differentiation from the germinal center. Curr. Opin. Immunol. 2017, 45, 97–102. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307. [Google Scholar] [CrossRef]
- Martin, F.; Chan, A.C. B cell immunobiology in disease: Evolving concepts from the clinic. Annu. Rev. Immunol. 2006, 24, 467–496. [Google Scholar] [CrossRef] [PubMed]
- Fremd, C.; Schuetz, F.; Sohn, C.; Beckhove, P.; Domschke, C. B cell-regulated immune responses in tumor models and cancer patients. OncoImmunology 2013, 2, e25443. [Google Scholar] [CrossRef] [PubMed]
- Coronella-Wood, J.A.; Hersh, E.M. Naturally occurring B-cell responses to breast cancer. Cancer Immunol. Immunother. 2003, 52, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 2017, 8, 15081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36. [Google Scholar] [CrossRef] [Green Version]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [Green Version]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., II; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17. [Google Scholar] [CrossRef] [Green Version]
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017, 169, 736–749.e18. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.C.A.; Nelson, B.H. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, J.D.G.; Peyper, J.M.; Blackburn, J.M. B cells and antibody production in melanoma. Mamm. Genome 2018, 29, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Kläsener, K.; Geoffroy Andrieux, J.J.; Reth, M. CD20 as a gatekeeper of the resting state of human B cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2021342118. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Demissie, E.; Pillai, S. B Lymphocytes and Cancer: A Love–Hate Relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Teitz-Tennenbaum, S.; Donald, E.J.; Li, M.; Chang, A.E. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J. Immunol. 2009, 183, 3195–3203. [Google Scholar] [CrossRef]
- Carmi, Y.; Spitzer, M.; Linde, I.; Burt, B.M.; Prestwood, T.R.; Perlman, N.; Davidson, M.G.; Kenkel, J.A.; Segal, E.; Pusapati, G.; et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 2015, 521, 99–104. [Google Scholar] [CrossRef]
- Fridman, W.H.; Petitprez, F.; Meylan, M.; Chen, T.W.-W.; Sun, C.-M.; Roumenina, L.T.; Sautes-Fridman, C. B cells and cancer: To B or not to B? J. Exp. Med. 2021, 218, e20200851. [Google Scholar] [CrossRef]
- Tao, H.; Lu, L.; Xia, Y.; Dai, F.; Wang, Y.; Bao, Y.; Lundy, S.; Ito, F.; Pan, Q.; Zhang, X.; et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur. J. Immunol. 2015, 45, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Kemp, T.J.; Moore, J.M.; Griffith, T.S. Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeox-ynucleotide stimulation. J. Immunol. 2004, 173, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Jahrsdörfer, B.; Blackwell, S.E.; Wooldridge, J.E.; Huang, J.; Andreski, M.W.; Jacobus, L.S.; Taylor, C.M.; Weiner, G.J. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood 2006, 108, 2712–2719. [Google Scholar] [CrossRef] [Green Version]
- Rubtsov, A.V.; Rubtsova, K.; Kappler, J.W.; Jacobelli, J.; Friedman, R.S.; Marrack, P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J. Immunol. 2015, 195, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, T.C.; Ebner, P.J.; Moore, B.L.; Squalls, O.G.; Waugh, K.A.; Eruslanov, E.B.; Singhal, S.; Mitchell, J.D.; Franklin, W.A.; Merrick, D.T.; et al. Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non–Small Cell Lung Cancer Patients. Cancer Immunol. Res. 2017, 5, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Tan, T.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; DeNardo, D.G.; et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 2010, 17, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, F.; Garris, C.; Lai, C.P.; Newton, A.; Pfirschke, C.; Engblom, C.; Alvarez, D.; Sprachman, M.; Evavold, C.; Magnuson, A.; et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016, 352, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Iwata, Y.; Matsushita, T.; Horikawa, M.; DiLillo, D.J.; Yanaba, K.; Venturi, G.M.; Szabolcs, P.M.; Bernstein, S.H.; Magro, C.M.; Williams, A.D.; et al. Characterization of a rare IL-10–competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011, 117, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Wang, H.; Liu, Z. The Role of Regulatory B Cells in Patients with Acute Myeloid Leukemia. Med. Sci. Monit. 2019, 25, 3026–3031. [Google Scholar] [CrossRef]
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345. [Google Scholar] [CrossRef]
- de Visser, K.E.; Korets, L.V.; Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte de-pendent. Cancer Cell 2005, 7, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Morgan, R.; Podack, E.R.; Rosenblatt, J. B cell regulation of anti-tumor immune response. Immunol. Res. 2013, 57, 115–124. [Google Scholar] [CrossRef]
- Perez-Chacon, G.; Vincent-Fabert, C.; Zapata, J.M. Editorial: Mouse Models of B Cell Malignancies. Front. Immunol. 2021, 12, 4546. [Google Scholar] [CrossRef] [PubMed]
- Largeot, A.; Pagano, G.; Gonder, S.; Moussay, E.; Paggetti, J. The B-Side of Cancer Immunity: The Underrated Tune. Cells 2019, 8, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Zhang, J.; Wang, J.; Fu, J.; Li, T.; Zheng, X.; Wang, B.; Gu, S.; Jiang, P.; Fan, J.; et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat. Genet. 2019, 51, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Sumner, W.A.; Miyauchi, S.; Cohen, E.E.; Califano, J.A.; Sharabi, A.B. Role of B Cells in Responses to Checkpoint Blockade Immunotherapy and Overall Survival of Cancer Patients. Clin. Cancer Res. 2021, 27, 6075–6082. [Google Scholar] [CrossRef]
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21–Induced Granzyme B–Expressing B Cells Infiltrate Tumors and Regulate T Cells. Cancer Res. 2013, 73, 2468–2479. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.-M.; Thornton, A.M.; DiPaolo, R.J.; Shevach, E.M. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 2006, 107, 3925–3932. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Schafer, C.C.; Hough, K.; Tousif, S.; Duncan, S.R.; Kearney, J.F.; Ponnazhagan, S.; Hsu, H.-C.; Deshane, J.S. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT. J. Immunol. 2018, 201, 278–295. [Google Scholar] [CrossRef] [Green Version]
- Wejksza, K.; Lee-Chang, C.; Bodogai, M.; Bonzo, J.; Gonzalez, F.J.; Lehrmann, E.; Becker, K.; Biragyn, A. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome prolifera-tor-activated receptor alpha. J. Immunol. 2013, 190, 2575–2584. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, E.M.; De, S.; Weiss, R.; Feng, D.; Hall, K.; Kilic, S.; Bhanot, G.; Ganesan, S.; Ran, S.; Barnes, B.J. IRF5 is a novel regulator of CXCL13 expression in breast cancer that regulates CXCR5(+) B- and T-cell trafficking to tumor-conditioned media. Immunol. Cell Biol. 2015, 93, 486–499. [Google Scholar] [CrossRef]
- Willsmore, Z.N.; Harris, R.J.; Crescioli, S.; Hussein, K.; Kakkassery, H.; Thapa, D.; Cheung, A.; Chauhan, J.; Bax, H.J.; Chenoweth, A.; et al. B Cells in Patients With Melanoma: Implications for Treatment With Checkpoint Inhibitor Antibodies. Front. Immunol. 2021, 11, 622442. [Google Scholar] [CrossRef]
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. USA 2001, 98, 13866–13871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caro-Maldonado, A.; Wang, R.; Nichols, A.G.; Kuraoka, M.; Milasta, S.; Sun, L.D.; Gavin, A.L.; Abel, E.D.; Kelsoe, G.; Green, D.R.; et al. Metabolic Reprogramming Is Required for Antibody Production That Is Suppressed in Anergic but Exaggerated in Chronically BAFF-Exposed B Cells. J. Immunol. 2014, 192, 3626–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Lao, X.-M.; Pan, Q.; Ning, N.; Yet, J.; Xu, Y.; Li, S.; Chang, A.E. Adoptive Transfer of Tumor Reactive B Cells Confers Host T-Cell Immunity and Tumor Regression. Clin. Cancer Res. 2011, 17, 4987–4995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, A.W.; Wickenhauser, C.; Salins, P.C.; Kappler, M.; Bukur, J.; Seliger, B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. J. Transl. Med. 2016, 14, 85. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Rousseau, R.; Yvon, E.; Schwartz, M.; Dotti, G.; Foster, A.; Havlik-Cooper, D.; Grilley, B.; Gee, A.; Baker, K.; et al. Responses to Human CD40 Ligand/Human Interleukin-2 Autologous Cell Vaccine in Patients with B-Cell Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2005, 11, 6916–6923. [Google Scholar] [CrossRef] [Green Version]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Brand, T.M.; Iida, M.; Wheeler, D.L. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol. Ther. 2011, 11, 777–792. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, E.; De Palma, R.; Orditura, M.; De Vita, F.; Ciardiello, F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin. Exp. Immunol. 2009, 158, 1–9. [Google Scholar] [CrossRef]
- Gajria, D.; Chandarlapaty, S. HER2-amplified breast cancer: Mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev. Anticancer Ther. 2011, 11, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Valabrega, G.; Milani, A.; Montemurro, F.; Gioeni, L.; Aglietta, M. Role of trastuzumab in the management of HER2-positive metastatic breast cancer. Breast Cancer Targets Ther. 2010, 2, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Jo, M.; Jung, S.T. Recent Achievements and Challenges in Prolonging the Serum Half-Lives of Therapeutic IgG Antibodies Through Fc Engineering. BioDrugs 2021, 35, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Reslan, L.; Dalle, S.; Dumontet, C. Understanding and circumventing resistance to anticancer monoclonal antibodies. mAbs 2009, 1, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarvaria, A.; Madrigal, A.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell. Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [Green Version]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Larsen, M.S.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Munoz-Erazo, L.; Rhodes, J.L.; Marion, V.C.; Kemp, R.A. Tertiary lymphoid structures in cancer—considerations for patient prognosis. Cell. Mol. Immunol. 2020, 17, 570–575. [Google Scholar] [CrossRef]
- Madrid, F.F.; Maroun, M.-C.; Olivero, O.A.; Long, M.; Stark, A.; Grossman, L.I.; Binder, W.; Dong, J.; Burke, M.; Nathanson, S.D.; et al. Autoantibodies in breast cancer sera are not epiphenomena and may participate in carcinogenesis. BMC Cancer 2015, 15, 407. [Google Scholar] [CrossRef] [Green Version]
- Heo, C.-K.; Bahk, Y.Y.; Cho, E.-W. Tumor-associated autoantibodies as diagnostic and prognostic biomarkers. BMB Rep. 2012, 45, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.E.; Chan, G.; Liu, Y.; Hegan, D.C.; Dalal, S.; Dray, E.; Kwon, Y.; Xu, Y.; Xu, X.; Peterson-Roth, E.; et al. Targeting Cancer with a Lupus Autoantibody. Sci. Transl. Med. 2012, 4, 157ra142. [Google Scholar] [CrossRef] [Green Version]
- Disis, M.L.; Disis, M.L.; Goodell, V.; Schiffman, K.; Knutson, K.L. Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J. Clin. Immunol. 2004, 24, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennhold, K.; Thelen, M.; Schlößer, H.A.; Haustein, N.; Reuter, S.; Garcia-Marquez, M.; Lechner, A.; Kobold, S.; Rataj, F.; Utermöhlen, O.; et al. Using Antigen-Specific B Cells to Combine Antibody and T Cell–Based Cancer Immunotherapy. Cancer Immunol. Res. 2017, 5, 730–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Huang, J.; Zhang, S.; Liu, Q.; Liao, Q.; Qiu, X. Immunoglobulin Expression in Cancer Cells and Its Critical Roles in Tumorigenesis. Front. Immunol. 2021, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Peng, H.; Gao, J.; Nong, A.; Hua, H.; Yang, S.; Chen, L.; Wu, X.; Zhang, H.; Wang, J. Current insights into the expression and functions of tumor-derived immunoglobulins. Cell Death Discov. 2021, 7, 148. [Google Scholar] [CrossRef]
- Ou, Z.; Wang, Y.; Liu, L.; Li, L.; Yeh, S.; Qi, L.; Chang, C. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget 2015, 6, 26065–26078. [Google Scholar] [CrossRef] [Green Version]

| S. No. | Drug | Cancer | Intervention | NCT Number | Phase Trial |
|---|---|---|---|---|---|
| 1. | Tumor-derived immunoglobulin idiotype antigen vaccines | B-cell lymphoma Follicular lymphoma Lymphoma | Id-KLH vaccine GM-CSF | NCT00001512 | Phase 1 (National Cancer Institute) |
| 2. | Idelalisib in combination with chemotherapeutic agents, immunomodulatory agents, and anti-CD20 mAb | Indolent non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and mantle cell lymphoma | Idelalisib, Rituximab, Bendamustine, Ofatumumab, Fludarabine, Everolimus, Bortezomib, Chlorambucil, and Lenalidomide | NCT01088048 | Phase 1 (Gilead Sciences) |
| 3. | Atezolizumab + immunomodulatory agents | Acute myeloid leukemia | Atezolizumab and Guadecitabine | NCT02892318 | Phase 1 (Hoffmann-La Roche) |
| 4. | TF2 + radio immunotherapy | Small-cell lung cancer CEA-expressing non-small-cell lung carcinoma (NSCLC) | Antibody TF2 radiation: IMP-288-Lutetium Radiation: IMP-288-Indium | NCT01221675 | Phase 1 Phase 2 (Centre René Gauducheau) |
| 5. | Oregovomab (antibody) + chemotherapy | Ovarian neoplasms | Carboplatin and Paclitaxel Biological: Oregovomab | NCT01616303 | Phase 2 (Quest PharmaTech Inc.) |
| 6. | CD40 agonistic mAbs APX005M | NSCLC, melanoma, urothelial carcinoma, MSI-H, and head and neck cancer | APX005M | NCT02482168 | Phase 1 (Apexigen, Inc.) |
| 7. | BMS-986156 +/− Nivolumab | Solid tumors | BMS-986156 and Nivolumab | NCT02598960 | Phase 1 Phase 2 (Bristol-Myers Squibb) |
| 8. | Intramuscular administration of autologous total IgG | Human cancers | Advanced solid tumor | NCT03695757 | Phase 1 Phase 2 (Ajou University School of Medicine) |
| 9. | Ipilimumab | High-risk stage III melanoma | Ipilimumab and placebo | NCT00636168 | Phase 3 (Bristol-Myers Squibb) |
| 10. | Carbo/Caelyx or Carbo/Doxorubicin with Tocilizumab (mAb IL-6R) and Peg-Intron | Recurrent ovarian cancer | Tocilizumab and interferon alpha 2-b, and Carboplatin with Caelyx or Doxorubicin | NCT01637532 | Phase 1 Phase 2 (Leiden University Medical Center) |
| 11. | Immunostimulant antibody in combination with chemotherapy | Pancreatic neoplasm | mAb chemotherapy | NCT00711191 | Phase 1 (Hoffmann-La Roche) |
| 12. | Edrecolomab | Mucinous adenocarcinoma of the colon, signet ring adenocarcinoma of the colon, stage IIA colon cancer, stage IIB colon cancer, and stage IIC colon cancer | Edrecolomab laboratory biomarker analysis | NCT00002968 | Phase 3 (National Cancer Institute) |
| 13. | Rituximab | Lymphoma | Autologous immunoglobulin idiotype-KLH conjugate vaccine Sargramostim | NCT00071955 | Phase 2 (Genitope Corporation) |
| 14. | Combination of Bevacizumab and Allogeneic NK immunotherapy | Malignant solid tumor | Bevacizumab NK immunotherapy | NCT02857920 | Phase 1 Phase 2 (Fuda Cancer Hospital, Guangzhou) |
| 15. | Belantamab mafodotin | Multiple myeloma | Belantamab mafodotin | NCT04177823 | Phase 1 (GlaxoSmithKline) |
| 16. | MOv18 IgE, chimeric IgE | Human cancers | MOv18 IgE | NCT02546921 | Phase 1 (Cancer Research UK) |
| 17. | CD40 agonistic antibody APX005M + Nivolumab | Metastatic non-small-cell lung cancer, metastatic melanoma, and neoplasm of lung melanoma | APX005M Nivolumab | NCT03123783 | Phase 1 Phase 2 (Apexigen, Inc.) |
| 18. | Galunisertib (LY2157299) and Durvalumab (MEDI4736) | Metastatic pancreatic cancer | Galunisertib Durvalumab | NCT02734160 | Phase 1 (Eli Lilly and Company) |
| 19. | Chemoembolization or ablation | Hepatocellular cancer, biliary tract neoplasms, liver cancer, hepatocellular carcinoma, and biliary cancer | Tremelimumab RFA TACE Cryoablation | NCT01853618 | Phase 1 Phase 2 (National Cancer Institute) |
| 20. | CT-011 in combination with Rituximab | Lymphoma | CT-011 Rituximab | NCT00904722 | Phase 2 (M.D. Anderson Cancer Center) |
| 21. | ²¹²Pb-TCMC-Trastuzumab radio immunotherapy | Breast neoplasms, peritoneal neoplasms, ovarian neoplasms, pancreatic neoplasms, and stomach neoplasms | ²¹²Pb-TCMC-Trastuzumab Biological: Trastuzumab | NCT01384253 | Phase 1 (Orano Med LLC) |
| 22. | Vaccine and antibody treatment | Prostatic neoplasms | PROSTVAC-V/TRICOM PROSTVAC-F/TRICOM MDX-010 Sargramostim | NCT00113984 | Phase 1 (National Cancer Institute) |
| 23. | FATE-NK100 as monotherapy and in combination with mAbs | HER2-positive gastric cancer, colorectal cancer, head and neck squamous cell carcinoma, EGFR-positive solid tumor, advanced solid tumors, HER2-positive breast cancer, hepatocellular carcinoma, non-small-cell lung cancer, renal cell carcinoma, pancreatic cancer, and melanoma | FATE-NK100 Cetuximab Trastuzumab | NCT03319459 | Phase 1 (Fate Therapeutics) |
| 24. | Radiation and mAbs to OX40 (MEDI6469) | Metastatic breast cancer Lung metastases Liver metastases | MEDI6469 | NCT01862900 | Phase 1 (Providence Health & Services) |
| 25. | Toripalimab | Malignant lymphoma | Toripalimab | NCT03316144 | Phase 1 (Shanghai Junshi Bioscience Co., Ltd.) |
| 26. | Valproate prior to immunotherapy targeting CD20 | Chronic lymphocytic leukemia | Valproate | NCT02144623 | Early Phase 1 (Lund University Hospital) |
| 27. | Ublituximab in combination with Lenalidomide | Non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, small lymphocytic lymphoma, B-cell lymphomas, marginal zone lymphoma, mantle cell lymphoma, and Waldenstrom’s macroglobulinemia | Ublituximab Lenalidomide | NCT01744912 | Phase 1 Phase 2 (TG Therapeutics, Inc.) |
| 28. | JTX-2011 alone and in combination with anti-PD-1 or anti-CTLA-4 | Human cancers | JTX-2011 Nivolumab and Ipilimumab Pembrolizumab | NCT02904226 | Phase 1 Phase 2 (Jounce Therapeutics, Inc.) |
| 29. | Motolimod, Doxorubicin, and Durvalumab | Ovarian cancer | Durvalumab Pegylated Liposomal Doxorubicin Motolimod | NCT02431559 | Phase 1 Phase 2 (Ludwig Institute for Cancer Research) |
| Combination Peptide Antibodies | Cancer Treatment |
|---|---|
| αHER-2 + αIGF-1R | Breast cancer |
| HER-2 + HER-3 | Breast, pancreatic, and colon cancer |
| HER-3 + EGFR | Breast cancer |
| HER1 + HER2 | Colorectal cancer |
| HER1-418 + IGF-1R-56 | Pancreatic cancer |
| HER1-418 + HER-3-461 | Pancreatic cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.L.; Khan, N.; Basu, S.; Soni, V. B-Cell-Based Immunotherapy: A Promising New Alternative. Vaccines 2022, 10, 879. https://doi.org/10.3390/vaccines10060879
Gupta SL, Khan N, Basu S, Soni V. B-Cell-Based Immunotherapy: A Promising New Alternative. Vaccines. 2022; 10(6):879. https://doi.org/10.3390/vaccines10060879
Chicago/Turabian StyleGupta, Sneh Lata, Naeem Khan, Srijani Basu, and Vijay Soni. 2022. "B-Cell-Based Immunotherapy: A Promising New Alternative" Vaccines 10, no. 6: 879. https://doi.org/10.3390/vaccines10060879
APA StyleGupta, S. L., Khan, N., Basu, S., & Soni, V. (2022). B-Cell-Based Immunotherapy: A Promising New Alternative. Vaccines, 10(6), 879. https://doi.org/10.3390/vaccines10060879









