Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design and Ethical Issues
2.2. Sample Collection and RT-PCR
2.3. SARS-CoV-2 Sequencing
2.3.1. Library Preparation and Sequencing
2.3.2. NGS Data Analysis
2.4. Immunological Analysis
- –
- SDK−—mean squared deviation of the optical density of the negative control.
- X is the mean OD value of negative control samples 1 and 2.
- PC < 0.9—negative, PC > 1.1—positive.
2.5. Statistical Analysis
3. Results
3.1. Dominant Variants of the Virus and Their Features
3.2. Features of the Immune Response Depending on the Vaccination Status
3.3. Effectiveness of Vaccination with Sputnik Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and Efficacy of an rAd26 and rAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Butt, A.A.; Omer, S.B.; Yan, P.; Shaikh, O.S.; Mayr, F.B. SARS-CoV-2 vaccine effectiveness in a high-risk national population in a real-world setting. Ann. Intern. Med. 2021, 174, 1404–1408. [Google Scholar] [CrossRef]
- Pawlowski, C.; Lenehan, P.; Puranik, A.; Agarwal, V.; Venkatakrishnan, A.J.; Niesen, M.J.M.; Venkatakrishnan, A.J.; Niesen, M.J.M.; O’Horo, J.C.; Virk, A.; et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med 2021, 2, 979–992.E8. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA 2021, 326, e218565. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Moderna applies for US and EU approval as vaccine trial reports 94.1% efficacy. BMJ 2020, 371, m4709. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Kuppili, S.; Kumar Suvvari, T.; Kandi, V.; Behera, A.; Verma, S.; Kudrat-E-Zahan Biswal, S.K.; Al-Noor, T.H.; El-Ajaily, M.M.; Sarangi, A.K.; et al. SARS-CoV-2 and its variants of concern including Omicron: A never ending pandemic. Chem. Biol. Drug Des. 2022, 99, 769–788. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Kmiec, D.; Koepke, L.; Zech, F.; Jacob, T.; Sparrer, K.M.J.; Kirchhoff, F. Omicron: What makes the latest SARS-CoV-2 variant of concern so concerning? J. Virol. 2022, 96, jvi0207721. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 2020, 20, 533–534. Available online: https://coronavirus.jhu.edu/map.html (accessed on 24 April 2022). [CrossRef]
- Mahase, E. Covid-19: Booster dose reduces infections and severe illness in over 60s, Israeli study reports. BMJ 2021, 374, n2297. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: Antibody boost after third dose varies greatly by vaccine, study finds. BMJ 2021, 375, n3011. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- González, S.; Olszevicki, S.; Salazar, M.; Calabria, A.; Regairaz, L.; Marín, L.; Campos, P.; Varela, T.; Martínez, V.V.G.; Ceriani, L.; et al. Efficacy of the first component of Gam-COVID-Vac (Sputnik V) on reduction of SARS-CoV-2 confirmed infections, hospitalisations and mortality in patients aged 60-79: A retrospective cohort study in Argentina. EClinicalMedicine 2021, 40, 101126. [Google Scholar] [CrossRef]
- Dolzhikova, I.V.; Gushchin, V.A.; Shcheblyakov, D.V.; Tsybin, A.N.; Shchetinin, A.M.; Pochtovyi, A.A.; Komissarov, A.B.; Kleymenov, D.A.; Kuznetsova, N.A.; Tukhvatulin, A.I.; et al. One-shot immunization with Sputnik Light (the first component of Sputnik V vaccine) is effective against SARS-CoV-2 Delta variant: Efficacy data on the use of the vaccine in civil circulation in Moscow. medRxiv 2021. [Google Scholar] [CrossRef]
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Pályi, B.; Formanek-Balku, E.; Molnár, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary-the HUN-VE study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef]
- AlQahtani, M.; Bhattacharyya, S.; Alawadi, A.; Al Mahmeed, H.; Al Sayed, J.; Justman, J.; El-Sadr, W.M.; Hidary, J.; Mukherjee, S. Morbidity and mortality from COVID-19 post-vaccination breakthrough infections in association with vaccines and the emergence of variants in Bahrain. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Barchuk, A.; Cherkashin, M.; Bulina, A.; Berezina, N.; Rakova, T.; Kuplevatskaya, D.; Stanevich, O.; Skougarevskiy, D.; Okhotin, A. Vaccine Efficacy against Referral to Hospital and Severe Lung Injury Associated with COVID- 19: A Population-Based Case- Control Study in St. Petersburg, Russia. medRxiv 2022. [Google Scholar] [CrossRef]
- Gushchin, V.A.; Tsyganova, E.V.; Ogarkova, D.A.; Adgamov, R.R.; Shcheblyakov, D.V.; Glukhoedova, N.V.; Zhilenkova, A.S.; Kolotii, A.G.; Zaitsev, R.D.; Logunov, D.Y.; et al. Sputnik V protection from COVID-19 in people living with HIV under antiretroviral therapy. EClinicalMedicine 2022, 46, 101360. [Google Scholar] [CrossRef] [PubMed]
- Klink, G.V.; Safina, K.; Nabieva, E.; Shvyrev, N.; Garushyants, S.; Alekseeva, E.; Komissarov, A.B.; Danilenko, D.M.; Pochtovyi, A.A.; Divisenko, E.V.; et al. The rise and spread of the SARS-CoV-2 AY.122 lineage in Russia. Virus Evol. 2022, 8, veac017. [Google Scholar] [CrossRef] [PubMed]
- FastQC: A Quality Control Analysis Tool for High throughput Sequencing Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 7 April 2022).
- Martin, M. Cutadapt Removes Adapter Sequences from High-throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 2016, 1–22. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrison, E.; Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- The Number of People Vaccinated against COVID-19 in Moscow. Available online: https://gogov.ru/covid-v-stats/msk#data (accessed on 24 April 2022).
- Coronavirus Statistics in Moscow. Available online: https://gogov.ru/covid-19/msk#data (accessed on 24 April 2022).
- Hightower, A.; Orenstein, W.; Martin, S. Recommendations for the use of Taylor series confidence intervals for estimates of vaccine efficacy. Bull. World Health Organ. 1988, 66, 99–105. [Google Scholar]
- Evaluation of COVID-19 Vaccine Effectiveness. WHO Interim Guidance. 17 March 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1 (accessed on 24 April 2022).
- Available online: https://sputnikvaccine.com/rus/about-vaccine (accessed on 24 April 2022).
- Gushchin, V.A.; Dolzhikova, I.V.; Shchetinin, A.M.; Odintsova, A.S.; Siniavin, A.E.; Nikiforova, M.A.; Pochtovyi, A.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Burgasova, O.A.; et al. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines 2021, 9, 779. Available online: https://github.com/artic-network/artic-ncov2019/tree/master/primer_schemes/nCoV-2019 (accessed on 24 April 2022). [CrossRef] [PubMed]
- Grousova, D.M.; Dolzhikova, I.V.; Zorkov, I.D.; Ilyukhina, A.A.; Kovyrshina, A.V.; Kuzina, A.V.; Botikov, A.G.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Izhaeva, F.M.; et al. Sputnik V vaccine protects human ACE2-transgenic mice from lethal challenge with SARS-CoV-2 Variants of Concern (VOC: B.1.1.7, B.1.351, B.1.1.28/P.1, B.1.617.2). 2022; in press. [Google Scholar]
- Lapa, D.; Grousova, D.M.; Matusali, G.; Meschi, S.; Colavita, F.; Bettini, A.; Gramigna, G.; Francalancia, M.; Garbuglia, A.R.; Girardi, E.; et al. Retention of Neutralizing Response against SARS-CoV-2 Omicron Variant in Sputnik V-Vaccinated Individuals. Vaccines 2022, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Jinal, N.B.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. medRxiv 2021. [Google Scholar] [CrossRef]
- Parra-Lucares, A.; Segura, P.; Rojas, V.; Pumarino, C.; Saint-Pierre, G.; Toro, L. Emergence of SARS-CoV-2 Variants in the World: How Could This Happen? Life 2022, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; ur Rahman, M.; Arfan, M.; Shah, Z.; Kumam, P.; Deebani, W. Investigation of time-fractional SIQR Covid-19 mathematical model with fractal-fractional Mittage-Leffler kernel. AEJ 2022, 61, 7771–7779. [Google Scholar] [CrossRef]
- Yakusheva, O.; van den Broek-Altenburg, E.; Brekke, G.; Atherly, A. Lives saved and lost in the first six month of the US COVID-19 pandemic: A retrospective cost-benefit analysis. PLoS ONE 2022, 17, e0261759. [Google Scholar] [CrossRef]
| Mild, n = 240 | Moderate, n = 420 | Severe, n = 60 | Critical, n = 78 | p Value | |
|---|---|---|---|---|---|
| Gender Women, n, % Men, n, % | 136 (56.7%) 104 (43.3%) | 247 (58.8%) 173 (41.2%) | 35 (58.3%) 25 (41.7%) | 42 (53.8%) 36 (46.2%) | 0.849 (Pearson’s chi-squared test) |
| Age Me(Q1–Q3) | 74 (64–83) | 69 (59–76) | 72 (63.5–82) | 73 (66–82) | <0.001 * p24 = 0.019 * p21 < 0.001 * |
| Vaccinated (starting with one component) | Taken into account 236 67 (28.4%) | Taken into account 401 119 (29.7%) | Taken into account 59 13 (22.0%) | Taken into account 75 11 (14.7%) | 0.043 * (Pearson’s chi-squared test) p24 = 0.044 * |
| Not considered due to not being vaccinated with Sputnik V | 4 | 19 | 1 | 3 |
| Mild, n = 142 | Moderate, n = 284 | Severe, n = 35 | Critical, n = 45 | p | |
|---|---|---|---|---|---|
| Delta variant | 7 (4.9%) | 20 (7.0%) | 4 (11.4%) | 10 (22.2%) | 0.004 * (Fisher’s exact test) p14 = 0.002 * p24 = 0.003 |
| Omicron variant | 135 (95.1%) | 264 (93.0%) | 31 (88.6%) | 35 (77.8%) |
| Mild, n = 142 | Moderate, n = 284 | Severe, n = 35 | Critical, n = 45 | p Value | |
|---|---|---|---|---|---|
| Viral load of the Omicron variant | |||||
| PCR + | 127 (94.1%) | 247 (93.6%) | 31 (100.0%) | 32 (91.4%) | |
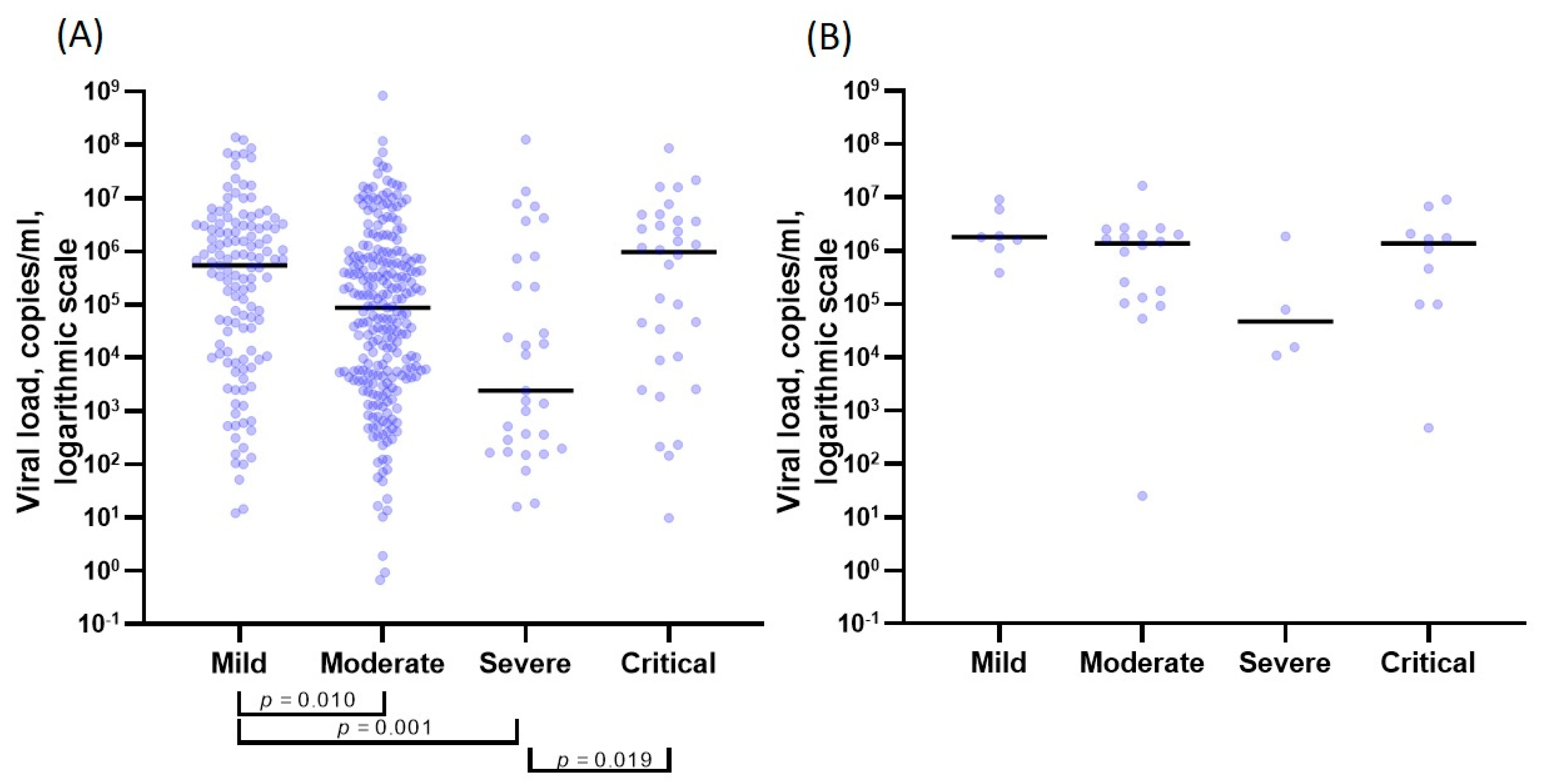
| Viral load, Ct Me(Q1–Q3) | 26.39 (24.23–31.42) | 29.23 (25.78–33.06) | 34.53 (27.57–36.76) | 26.66 (24.00–32.67) | <0.001 * (the Kruskal–Wallis test) p12 = 0.009 * p13 < 0.001 * p43 = 0.020 * |
| Viral load, copies/mL Me(Q1–Q3) | 5.51 × 105 (1.25 × 104–3.03 × 106) | 8.71 × 104 (4.64 × 103–7.43 × 105) | 2.42 × 103 (2.43 × 102–4.80 × 105) | 9.63 × 105 (9.78 × 103– 3.73 × 106 ) | <0.001 * (the Kruskal–Wallis test) p12 = 0.010 * p13 = 0.001 * p43 = 0.019 * |
| Viral load of the Delta variant | |||||
| PCR+ | 7 (100.0%) | 19 (95.0%) | 4 (100.0%) | 10 (100.0%) | |
| Viral load, Ct Me(Q1–Q3) | 25.16 (21.02–25.54) | 26.22 (24.70–28.63) | 30.22 (27.58–31.60) | 26.13 (25.23–27.85) | 0.063 (the Kruskal–Wallis test) |
| Viral load, copies/mL Me(Q1–Q3) | 1.79 × 106 (1.37 × 106–3.92 × 106) | 1.37 × 106 (1.31 × 105–2.00 × 106) | 4.71 × 104 (1.33 × 104–9.64 × 105) | 1.38 × 106 (9.93 × 104–2.09 × 106) | 0.184 (the Kruskal–Wallis test) |
| p (differences between various strains) | 0.094 (The Mann–Whitney test) | 0.010 * (The Mann–Whitney test) | 0.352 (The Mann–Whitney test) | 0.805 (The Mann–Whitney test) | |
| IgG NC | Mild | Moderate | Severe | Critical | p |
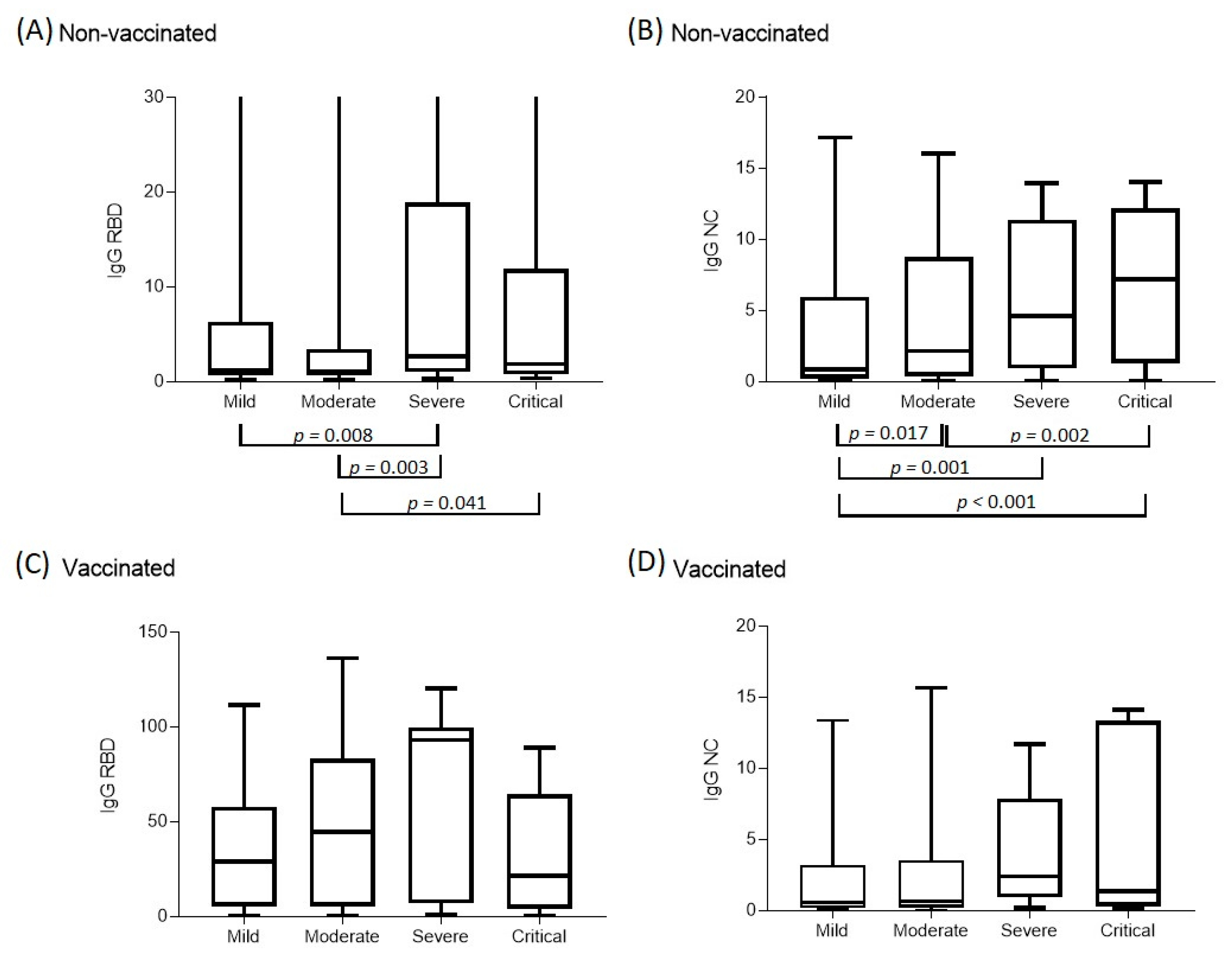
| Unvaccinated Me(Q1–Q3) | n = 168 0.86 (0.24–5.97) | n = 277 2.16 (0.38–8.77) | n = 46 4.61 (1.05–11.09) | n = 65 7.22 (1.33–12.18) | <0.001 * (the Kruskal–Wallis test) p12 = 0.017 * p13 = 0.001 * p14 < 0.001 * p24 = 0.002 * |
| Vaccinated with 1 component Me(Q1–Q3) | n = 5 0.63 (0.19–0.75) | n = 17 2.23 (0.20–12.57) | n = 1 -- | n = 2 5.03 (3.29–6.76) | 0.593 (the Kruskal–Wallis test) |
| Vaccinated with 2 components Me(Q1–Q3) | n = 40 0.59 (0.21–3.05) | n = 83 0.68 (0.22–3.51) | n = 9 2.42 (1.62–6.94) | n = 8 1.36 (0.46–13.11) | 0.306 (the Kruskal–Wallis test) |
| Vaccinated with 3–4 components Me(Q1–Q3) | n = 22 0.45 (0.23–4.72) | n = 19 0.37 (0.16–1.79) | n = 3 0.13 (0.11–0.98) | n = 1 | 0.357 (the Kruskal–Wallis test) |
| p | 0.682 | 0.008 * p02 = 0.044 * | 0.154 | 0.788 | |
| IgG RBD | Mild | Moderate | Severe | Critical | p |
| Unvaccinated Me(Q1–Q3) | n = 168 1.17 (0.64–5.9) | n = 277 1.08 (0.69–3.22) | n = 46 2.66 (1.03–17.79) | n = 65 1.89 (0.80–11.93) | 0.007 * (the Kruskal–Wallis test) p13 =0.008 * p23 = 0.003 * p24 = 0.041 * |
| Vaccinated with 1 component Me(Q1–Q3) | n = 5 32.19 (3.18–72.02) | n = 17 3.10 (1.68–24.90) | n = 1 -- | n = 2 69.49 (56.29–82.69) | 0.090 (the Kruskal–Wallis test) |
| Vaccinated with 2 components Me(Q1–Q3) | n = 40 29.07 (6.19–57.00) | n = 83 44.86 (5.78–83.11) | n = 9 93.00 (7.06–95.83) | n = 8 21.41 (4.44–57.70) | 0.265 (the Kruskal–Wallis test) |
| Vaccinated with 3–4 components Me(Q1–Q3) | n = 22 38.54 (14.56–59.07) | n = 19 53.01 (36.39–79.26) | n = 3 44.52 (28.66–45.49) | n = 1 | 0.357 (the Kruskal–Wallis test) |
| p | <0.001 * p01 = 0.046 * p02 < 0.001 * p03 < 0.001 * | <0.001 * p02 < 0.001 * p03 < 0.001 * | 0.003 * p02 = 0.012 * | 0.018 * posteriori comparisons revealed no differences |
| Quantity of 18+ People in Moscow 10,600,000 | Quantity of Vaccinated 6,000,000 | Quantity of Previously ill 2,232,836 | Quantity of Non-Immune 2,367,164 | |
|---|---|---|---|---|
| Patients with at least 1 component of vaccine, n VE % (95% CI) | Patients with at least 2 components of vaccine, n VE % (95% CI) | Patients with at least 3–4 components of vaccine, n VE % (95% CI) | Infected and not vaccinated, n | |
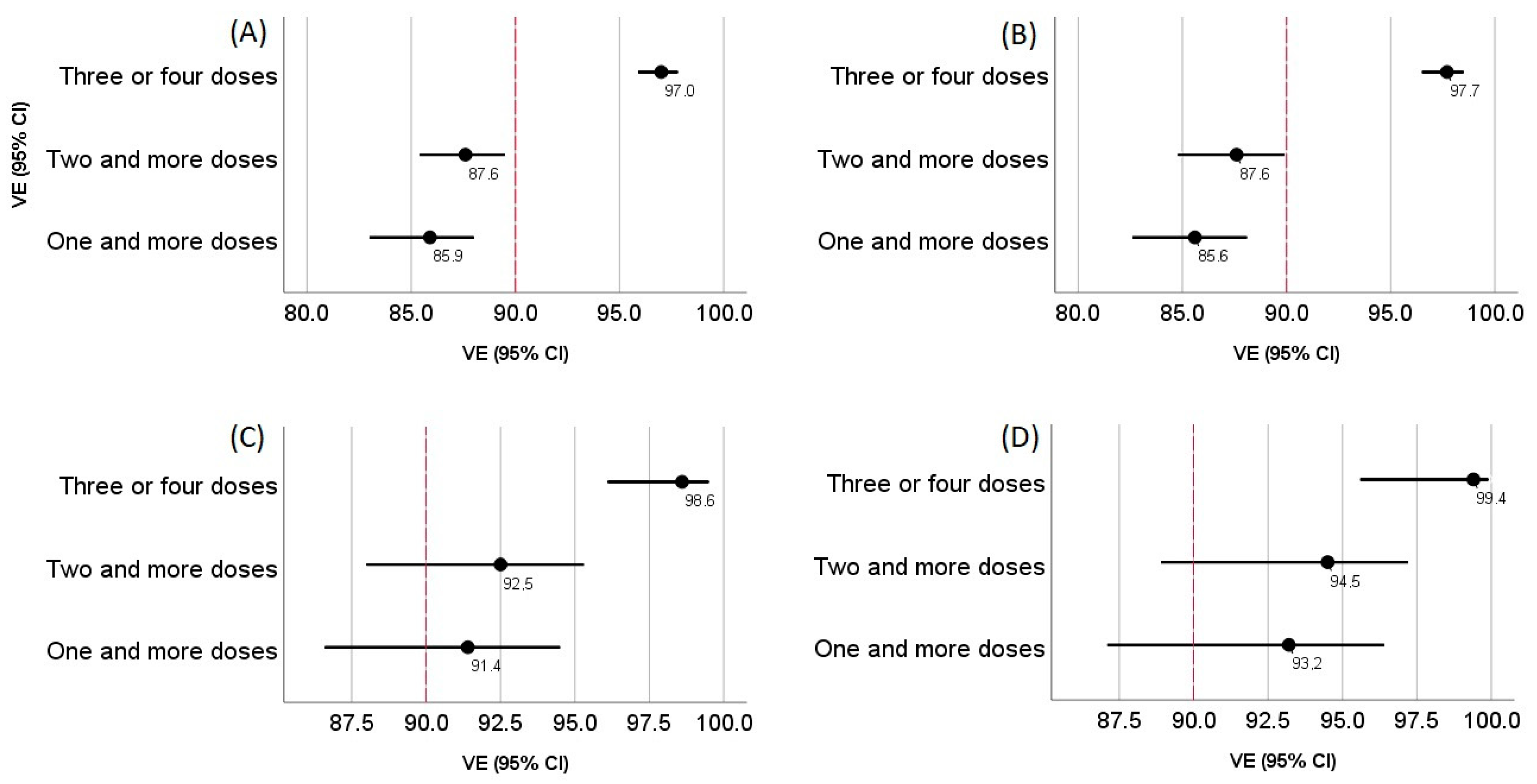
| Patients with mild and more severe forms of the disease | 210 85.9% (95% CI 83.0–88.0%) | 185 87.6% (95% CI 85.4–89.5%) | 45 97.0% (95% CI 95.9–97.8%) | 561 |
| Patients with moderate and more severe forms of the disease | 143 85.6% (95% CI 82.6–88.1%) | 123 87.6% (95% CI 84.8–89.9%) | 23 97.7% (95% CI 96.5–98.5%) | 392 |
| Patients with severe and critical forms of the disease | 24 91.4% (95% CI 86.6–94.5%) | 21 92.5% (95% CI 88.0–95.3%) | 4 98.6% (95% CI 96.1–99.5%) | 110 |
| Patients in critical condition | 11 93.2% (95% CI 87.1–96.4%) | 9 94.5% (95% CI 88.9–97.2%) | 1 99.4% (95% CI 95.6–99.9%) | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkoda, A.S.; Gushchin, V.A.; Ogarkova, D.A.; Stavitskaya, S.V.; Orlova, O.E.; Kuznetsova, N.A.; Keruntu, E.N.; Pochtovyi, A.A.; Pukhov, A.V.; Kleymenov, D.A.; et al. Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance. Vaccines 2022, 10, 938. https://doi.org/10.3390/vaccines10060938
Shkoda AS, Gushchin VA, Ogarkova DA, Stavitskaya SV, Orlova OE, Kuznetsova NA, Keruntu EN, Pochtovyi AA, Pukhov AV, Kleymenov DA, et al. Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance. Vaccines. 2022; 10(6):938. https://doi.org/10.3390/vaccines10060938
Chicago/Turabian StyleShkoda, Andrey S., Vladimir A. Gushchin, Darya A. Ogarkova, Svetlana V. Stavitskaya, Olga E. Orlova, Nadezhda A. Kuznetsova, Elena N. Keruntu, Andrei A. Pochtovyi, Alexander V. Pukhov, Denis A. Kleymenov, and et al. 2022. "Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance" Vaccines 10, no. 6: 938. https://doi.org/10.3390/vaccines10060938
APA StyleShkoda, A. S., Gushchin, V. A., Ogarkova, D. A., Stavitskaya, S. V., Orlova, O. E., Kuznetsova, N. A., Keruntu, E. N., Pochtovyi, A. A., Pukhov, A. V., Kleymenov, D. A., Krzhanovsky, V. G., Vasina, D. V., Shkuratova, N. V., Shidlovskaya, E. V., Gorbunov, A. L., Kustova, D. D., Mazurina, E. A., Kozlova, S. R., Soboleva, A. V., ... Gintsburg, A. L. (2022). Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance. Vaccines, 10(6), 938. https://doi.org/10.3390/vaccines10060938









