Rare Adverse Events Associated with BNT162b2 mRNA Vaccine (Pfizer-BioNTech): A Review of Large-Scale, Controlled Surveillance Studies
Abstract
:1. Introduction
2. Methods
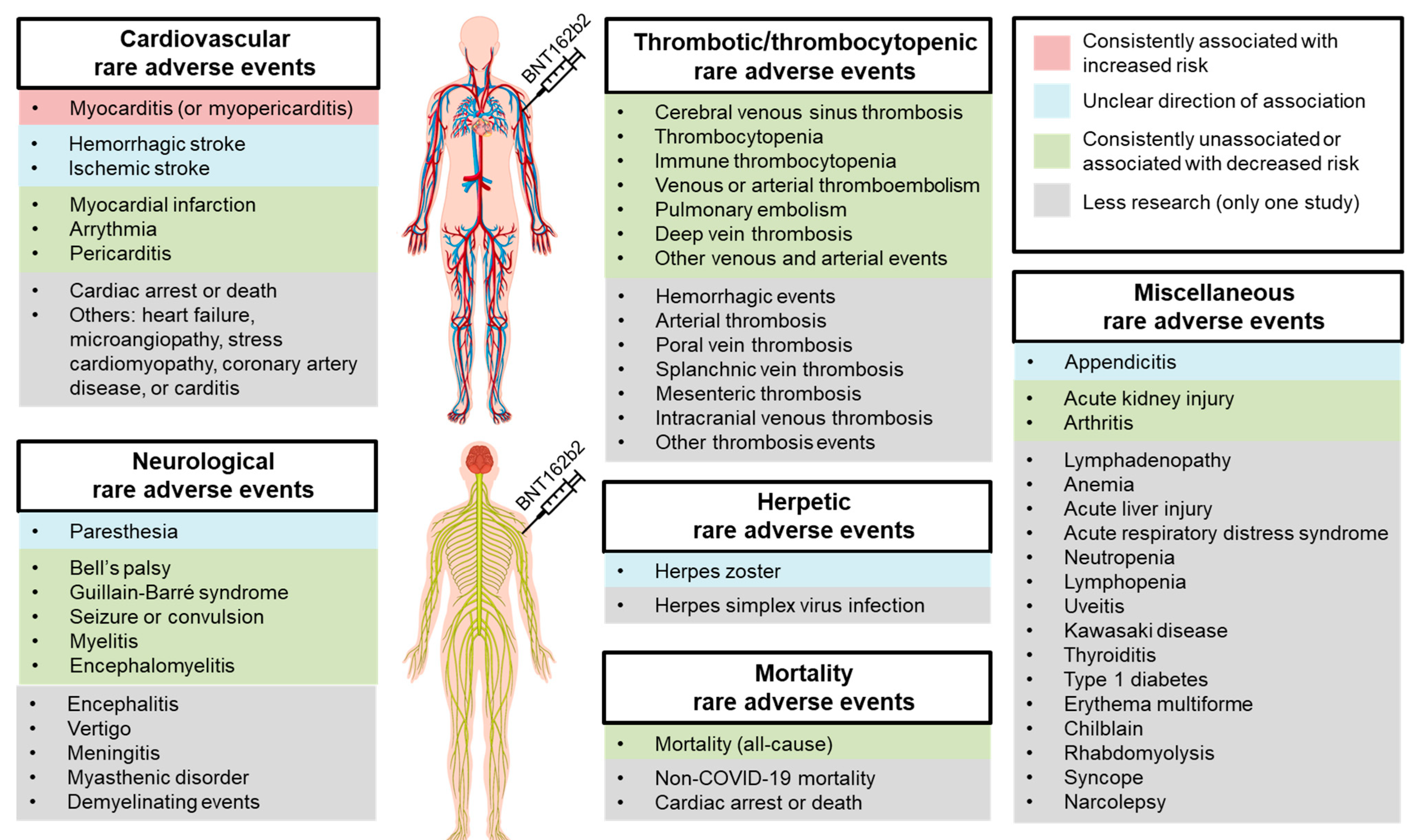
3. Results
3.1. Cardiovascular Adverse Events
3.2. Herpetic Adverse Events
3.3. Thrombotic or Thrombocytopenic Adverse Events
3.4. Neurological Adverse Events
3.5. Mortality Adverse Events
3.6. Other Miscellaneous Adverse Events
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 4 April 2022).
- Loomba, S.; de Figueiredo, A.; Piatek, S.J.; de Graaf, K.; Larson, H.J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 2021, 5, 337–348. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, A.; Simas, C.; Karafillakis, E.; Paterson, P.; Larson, H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: A large-scale retrospective temporal modelling study. Lancet 2020, 396, 898–908. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Gisondi, M.A.; Barber, R.; Faust, J.S.; Raja, A.; Strehlow, M.C.; Westafer, L.M.; Gottlieb, M. A Deadly Infodemic: Social Media and the Power of COVID-19 Misinformation. J. Med. Internet Res. 2022, 24, e35552. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dhawan, M.; Emran, T.B.; Rabaan, A.A.; Alhumaid, S. COVID-19 vaccine hesitancy—Reasons and solutions to achieve a successful global vaccination campaign to tackle the ongoing pandemic. Hum. Vaccin. Immunother. 2021, 17, 3495–3499. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Observational Studies Versus Randomized Controlled Trials: Avenues to Causal Inference in Nephrology. Adv. Chronic Kidney Dis. 2012, 19, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, C.M. Randomized controlled trials. Plast. Reconstr. Surg. 2011, 127, 1707–1712. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Locke, E.R.; O’Hare, A.M.; Bohnert, A.S.B.; Boyko, E.J.; Hynes, D.M.; Berry, K. COVID-19 Vaccination Effectiveness Against Infection or Death in a National U.S. Health Care System: A Target Trial Emulation Study. Ann. Intern. Med. 2022, 175, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccin. Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V. Phase IV of Drug Development. Perspect. Clin. Res. 2010, 1, 57–60. [Google Scholar]
- Lee, G.M.; Romero, J.R.; Bell, B.P. Postapproval Vaccine Safety Surveillance for COVID-19 Vaccines in the US. JAMA 2020, 324, 1937–1938. [Google Scholar] [CrossRef]
- Billingsley, A. FDA COVID-19 Vaccine Approval: Live Updates on Pfizer, Moderna, and J&J Vaccines. Available online: https://www.goodrx.com/conditions/COVID-19/fda-COVID-19-vaccine-approval-updates (accessed on 5 January 2022).
- Halim, M. COVID-19 Vaccination Efficacy and Safety Literature Review. J. Immunol. Allergy 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.J.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.A.; et al. Risk of anaphylaxis after vaccination in children and adults. J. Allergy Clin. Immunol. 2016, 137, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Rabaan, A.A.; Tirupathi, R.; Alomari, M.A.; Alshakhes, A.S.; Alshawi, A.M.; Ahmed, G.Y.; Almusabeh, H.M.; et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.J.; Stowe, J.; Ramsay, M.E.; Miller, E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: A national cohort study in England. Lancet Reg. Health Eur. 2022, 13, 100260. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernan, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Birabaharan, M.; Kaelber, D.C.; Karris, M.Y. Risk of herpes zoster reactivation after messenger RNA COVID-19 vaccination: A cohort study. J. Am. Acad Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef] [PubMed]
- Husby, A.; Hansen, J.V.; Fosbol, E.; Thiesson, E.M.; Madsen, M.; Thomsen, R.W.; Sorensen, H.T.; Andersen, M.; Wohlfahrt, J.; Gislason, G.; et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: Population based cohort study. BMJ 2021, 375, e068665. [Google Scholar] [CrossRef] [PubMed]
- Hviid, A.; Hansen, J.V.; Thiesson, E.M.; Wohlfahrt, J. Association of AZD1222 and BNT162b2 COVID-19 Vaccination With Thromboembolic and Thrombocytopenic Events in Frontline Personnel: A Retrospective Cohort Study. Ann. Intern. Med. 2022, 175, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef]
- Karlstad, O.; Hovi, P.; Husby, A.; Harkanen, T.; Selmer, R.M.; Pihlstrom, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundstrom, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600–612. [Google Scholar] [CrossRef]
- Kerr, S.; Joy, M.; Torabi, F.; Bedston, S.; Akbari, A.; Agrawal, U.; Beggs, J.; Bradley, D.; Chuter, A.; Docherty, A.B.; et al. First dose ChAdOx1 and BNT162b2 COVID-19 vaccinations and cerebral venous sinus thrombosis: A pooled self-controlled case series study of 11.6 million individuals in England, Scotland, and Wales. PLoS Med. 2022, 19, e1003927. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.T.T.; Huang, L.; Chui, C.S.L.; Wan, E.Y.F.; Li, X.; Wong, C.K.H.; Chan, E.W.W.; Ma, T.; Lum, D.H.; Leung, J.C.N.; et al. Multimorbidity and adverse events of special interest associated with COVID-19 vaccines in Hong Kong. Nat. Commun. 2022, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Raventos, B.; Roel, E.; Pistillo, A.; Martinez-Hernandez, E.; Delmestri, A.; Reyes, C.; Strauss, V.; Prieto-Alhambra, D.; Burn, E.; et al. Association between COVID-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: Population based cohort and self-controlled case series analysis. BMJ 2022, 376, e068373. [Google Scholar] [CrossRef] [PubMed]
- McKeigue, P.M.; Burgul, R.; Bishop, J.; Robertson, C.; McMenamin, J.; O’Leary, M.; McAllister, D.A.; Colhoun, H.M. Association of cerebral venous thrombosis with recent COVID-19 vaccination: Case-crossover study using ascertainment through neuroimaging in Scotland. BMC Infect. Dis. 2021, 21, 1275. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2021, 28, 410–422. [Google Scholar] [CrossRef]
- Shasha, D.; Bareket, R.; Sikron, F.H.; Gertel, O.; Tsamir, J.; Dvir, D.; Mossinson, D.; Heymann, A.D.; Zacay, G. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine: Historical cohort study. Clin. Microbiol. Infect. 2022, 28, 130–134. [Google Scholar] [CrossRef]
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Chui, C.S.L.; Lai, F.T.T.; Chan, E.W.Y.; Li, X.; Yan, V.K.C.; Gao, L.; Yu, Q.; Lam, I.C.H.; Chun, R.K.C.; et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: A case series and nested case-control study. Lancet Infect. Dis. 2022, 22, 64–72. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Chui, C.S.L.; Wang, Y.; Ng, V.W.S.; Yan, V.K.C.; Lai, F.T.T.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Wong, C.S.M.; et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: A self-controlled case series and nested case-control study. Lancet Reg. Health West Pac. 2022, 21, 100393. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, W.N.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Walker, V.; Denholm, R.; Akbari, A.; Omigie, E.; Hollings, S.; et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022, 19, e1003926. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Chevinsky, J.R.; Tao, G.; Lavery, A.M.; Kukielka, E.A.; Click, E.S.; Malec, D.; Kompaniyets, L.; Bruce, B.B.; Yusuf, H.; Goodman, A.B.; et al. Late Conditions Diagnosed 1–4 Months Following an Initial Coronavirus Disease 2019 (COVID-19) Encounter: A Matched-Cohort Study Using Inpatient and Outpatient Administrative Data-United States, 1 March-30 June 2020. Clin. Infect. Dis. 2021, 73, S5–S16. [Google Scholar] [CrossRef]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmarinas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with COVID-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Yong, S.J.; Liu, S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 2021, e2315. [Google Scholar] [CrossRef]
- Walter, R.; Hartmann, K.; Fleisch, F.; Reinhart, W.H.; Kuhn, M. Reactivation of herpesvirus infections after vaccinations? Lancet 1999, 353, 810. [Google Scholar] [CrossRef]
- Rothova, A.; de Groot, J.D.; Mudrikova, T. Reactivation of acute retinal necrosis after flu H1N1 vaccination. Br. J. Ophthalmol. 2011, 95, 291. [Google Scholar] [CrossRef]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. Reactivation of Varicella Zoster Virus after Vaccination for SARS-CoV-2. Vaccines 2021, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Katsikas Triantafyllidis, K.; Giannos, P.; Mian, I.T.; Kyrtsonis, G.; Kechagias, K.S. Varicella Zoster Virus Reactivation Following COVID-19 Vaccination: A Systematic Review of Case Reports. Vaccines 2021, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Bouhassira, D.; Kassianos, G.; Leplege, A.; Schmader, K.E.; Weinke, T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.H.; Ho, J.D.; Chen, Y.H.; Lin, H.C. Increased risk of stroke after a herpes zoster attack: A population-based follow-up study. Stroke 2009, 40, 3443–3448. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Warkentin, T.E. Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia. Crit. Care Med. 2022, 50, e80–e86. [Google Scholar] [CrossRef]
- Loo, J.; Spittle, D.A.; Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax 2021, 76, 412–420. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Diamond, A.; Kapoor, S.; Arafah, Y.; Nayak, L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020, 194, 101–115. [Google Scholar] [CrossRef]
- Xu, S.; Huang, R.; Sy, L.S.; Glenn, S.C.; Ryan, D.S.; Morrissette, K.; Shay, D.K.; Vazquez-Benitez, G.; Glanz, J.M.; Klein, N.P.; et al. COVID-19 Vaccination and Non-COVID-19 Mortality Risk—Seven Integrated Health Care Organizations, United States, December 14, 2020–July 31, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1520–1524. [Google Scholar] [CrossRef]
- Nelson, J.C.; Jackson, M.L.; Weiss, N.S.; Jackson, L.A. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J. Clin. Epidemiol. 2009, 62, 687–694. [Google Scholar] [CrossRef]
- Simonsen, L.; Taylor, R.J.; Viboud, C.; Miller, M.A.; Jackson, L.A. Mortality benefits of influenza vaccination in elderly people: An ongoing controversy. Lancet Infect. Dis. 2007, 7, 658–666. [Google Scholar] [CrossRef]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.; Lenehan, P.J.; Silvert, E.; Niesen, M.J.M.; Corchado-Garcia, J.; O’Horo, J.C.; Virk, A.; Swift, M.D.; Halamka, J.; Badley, A.D.; et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv 2021. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef]
- Bellos, I.; Karageorgiou, V.; Viskin, D. Myocarditis following mRNA COVID-19 vaccination: A pooled analysis. Vaccine 2022, 40, 1768–1774. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.F.; Cai, J.P.; Wong, W.M.; Yip, C.C.; et al. Intravenous injection of COVID-19 mRNA vaccine can induce acute myopericarditis in mouse model. Clin. Infect. Dis. 2021, 74, 1933–1950. [Google Scholar] [CrossRef]
- Knowlton, K.U. Insights from a murine model of COVID-19 mRNA vaccination-induced myopericarditis: Could accidental intravenous vaccine injection induce myopericarditis? Clin. Infect. Dis. 2021, 74, 1951–1952. [Google Scholar] [CrossRef]
- Remschmidt, C.; Wichmann, O.; Harder, T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: A systematic review. BMC Infect. Dis. 2015, 15, 429. [Google Scholar] [CrossRef] [Green Version]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
| Author | Database | Sample Size | Design | Follow-Up Time |
|---|---|---|---|---|
| Andrews, et al. [24] | National Immunization Management System, UK | 1.7 and 15.1 million person-time years in vaccinated and unvaccinated group, respectively | Controlled cohort | Within 28 days after receiving either first or second dose |
| Barda, et al. [25] | Calit Health Services, Israel | 0.88 million people in each vaccinated and unvaccinated group | Controlled cohort | Within 21 days after receiving either first or second dose |
| Birabaharan, et al. [26] | TriNetX Analytics Network, USA | 0.36 million people in each vaccinated and unvaccinated group | Controlled cohort | Within 28 days after receiving either first or second dose |
| Hippisley-Cox, et al. [27] | National Immunization Management System, UK | 9.5 million people | Self-controlled case series | Within 8–28 days of receiving the first dose |
| Husby, et al. [28] | Danish Vaccination Register and National Patient Register, Denmark | 3.5 million vaccinated and 0.21 million unvaccinated people | Self-controlled case series and controlled cohort | Within 28 days after receiving either first or second dose |
| Hviid, et al. [29] | Danish Vaccination Register and National Patient Register, Denmark | 0.10 million vaccinated and 0.13 million unvaccinated people | Controlled cohort | Within 28 days after receiving either first or second dose |
| Jabagi, et al. [30] | National Health Data System, France | 3.9 million people (aged ≥75 years only) | Self-controlled case series | Within 14 days after receiving either first or second dose |
| Karlstad, et al. [31] | Nationwide Health Registers from Denmark, Finland, Norway, and Sweden | 15 million vaccinated and 4.3 million unvaccinated people | Controlled cohort | Within 28 days after receiving either first or second dose |
| Kerr, et al. [32] | National Health Service, UK | 12.6 million people | Self-controlled case series | Within 28 days after receiving the first dose |
| Klein, et al. [33] | Vaccine Safety Database, USA | 6.2 million people | Self-controlled case series | Within 21 days after receiving either first or second dose |
| Lai, et al. [34] | Hospital Authority, Hong Kong | 0.15 million vaccinated and 0.55 million unvaccinated people | Controlled cohort | Within 28 days after receiving the first dose |
| Li, et al. [35] | Clinical Practice Research Datalink Aurum, UK, and Information System for Research in Primary Care, Spain | 3.6 million people | Self-controlled case series | Within 21 days of the first dose |
| McKeigue, et al. [36] | National Health Service, Scotland | 2.7 million doses | Case-crossover | Within 14 days after receiving either first or second dose |
| Mevorach, et al. [37] | Ministry of Health, Israel | 5 million vaccinated and 9.9 million unvaccinated people | Controlled cohort | Within 30 days after receiving the second dose |
| Patone, et al. [38] | National Immunization Management System, UK | 12.1 million people | Self-controlled case series | Within 28 days after receiving the first dose |
| National Health Service, Scotland | 1.1 million people | Self-controlled case series | Within 28 days after receiving the first dose | |
| Patone, et al. [39] | National Immunization Management System, UK | 17 million people | Self-controlled case series | Within 28 days after receiving the first dose |
| Shasha, et al. [40] | Meuhedet Health Maintenance Organization, Israel | 0.23 million in each vaccinated and unvaccinated group | Controlled cohort | Within an average of 22 and 32 days after receiving the first and second dose, respectively |
| Simpson, et al. [41] | National Health Service, Scotland | 0.82 million | Self-controlled case series | Within 28 days after receiving the first dose |
| Wan, et al. [42] | Hospital Authority, Hong Kong | 0.54 million | Nested case-control | Within 42 days after receiving either first or second dose |
| Wan, et al. [43] | Hospital Authority, Hong Kong | 1.96 million | Nested case-control and self-controlled case series | Within 28 days after receiving either first or second dose |
| Whiteley, et al. [44] | National Health Service, UK | 8.7 million vaccinated and 25 million unvaccinated people | Controlled cohort and self-controlled case series | Within 28 days after receiving the first dose |
| Mevorach et al. [37] | Patone et al. [39] | Karlstad et al. [31] | Husby et al. [28] | Barda et al. [25] | Klein et al. [33] | Jabagi et al. [30] | Whiteleyet al. [44] | Simpson et al. [41] | Patone et al. [38] | Hippisley-Cox et al. [27] | Lai et al. [34] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myocarditis | RR: 2.35 EC: 1.35 per 0.1 million people⸸ | RR: 3.4 EC: 3 per 1 million people ⸶ | RR: 2.04 EC: 0.67 per 0.1 million people ⸿ | HR: 3.73 EC: 1.3 per 0.1 million people ‡ ⸹ | RR: 3.24 EC: 2.7 per 0.1 million people | RR: 3.75 EC: 6.2 per 1 million doses † ⸹ | - | - | - | - | - | - |
| Hemorrhagic stroke | - | - | - | - | - | Non-sig. | Non-sig. | HR: 0.77 ⸘ | Non-sig. | RR: 1.24 EC: 60 per 10 million people ⁋ | - | - |
| Ischemic stroke | - | - | - | - | - | Non-sig. | Non-sig. | HR: 0.90 ⸘ | - | - | RR: 1.06 EC: 143 per 10 million people | - |
| Myocardial infarction | - | - | - | - | Non-sig. | Non-sig. | Non-sig. | - | - | - | Non-sig. | - |
| Arrhythmia | - | - | - | - | Non-sig. | Non-sig. | - | - | - | - | - | - |
| Pericarditis | - | - | - | - | Non-sig. | Non-sig. | - | - | - | - | - | - |
| Cardiac arrest or death | - | - | - | HR: 0.51 | - | - | - | - | - | - | - | - |
| Others ** | - | - | - | - | - | - | - | - | - | - | - | Non-sig. |
| Barda et al. [25] | Wan et al. [43] | Shasha et al. [40] | Birabaharan et al. [26] | |
|---|---|---|---|---|
| Herpes zoster | RR: 1.43 EC: 15.8 per 100,000 people | iRR: 5.23 ‡ EC: 7 per 1 million doses | Non-sig. | Non-sig. |
| Herpes simplex virus infection | Non-sig. | - | - | - |
| McKeigue et al. [36] | Kerr et al. [32] | Andrews et al. [24] | Hippisley-Cox et al. [27] | Hviid et al. [29] | Simpson et al. [41] | Klein et al. [33] | Whiteley et al. [44] | Barda et al. [25] | Jabagi et al. [30] | Patone et al. [38] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebral venous sinus thrombosis | Non-sig. | Non-sig. | Non-sig. | Non-sig. | Non-sig. | Non-sig. | Non-sig. | - | - | - | - |
| Thrombocytopenia | - | - | Non-sig. | Non-sig. | Non-sig. | Non-sig. | - | Non-sig. | Non-sig. | - | - |
| Venous thromboembolism | - | - | - | Non-sig. | - | - | Non-sig. | - | - | - | - |
| Arterial thromboembolism | - | - | - | Non-sig. | - | Non-sig. | - | - | - | - | - |
| Pulmonary embolism | - | - | - | - | Non-sig. | - | Non-sig. | HR: 0.78 ‡ | Non-sig. | Non-sig. | - |
| Deep vein thrombosis | - | - | - | - | Non-sig. | - | - | HR: 0.82 ⸸ | - | - | - |
| Intracranial hemorrhage | - | - | - | - | - | - | - | - | RR: 0.48 EC: −2.9 per 100,000 people | - | - |
| Subarachnoid hemorrhage | - | - | - | - | - | - | - | - | - | - | Non-sig. |
| Arterial thrombosis | - | - | - | - | Non-sig. | - | - | - | - | - | - |
| Immune thrombocytopenia | - | - | - | - | - | Non-sig. | Non-sig. | - | - | - | - |
| Disseminated intravascular coagulation | - | - | - | - | - | - | Non-sig. | - | - | - | - |
| Portal vein thrombosis | - | - | - | - | - | - | - | Non-sig. | - | - | - |
| Splanchnic vein thrombosis | - | - | - | - | Non-sig. | - | - | - | - | - | - |
| Mesenteric thrombosis | - | - | - | - | - | - | - | HR: 0.65 ‡ | - | - | - |
| Intracranial venous thrombosis | - | - | - | - | - | - | - | Non-sig. | - | - | - |
| Hemorrhagic events | - | - | - | - | - | Non-sig. | - | - | - | - | - |
| Other venous events | - | - | Non-sig. | - | - | - | - | Non-sig. | - | - | - |
| Other arterial events | - | - | - | Non-sig. | - | - | - | HR: 0.69 ‡ | - | - | - |
| Other thrombosis events | - | - | - | - | - | - | - | - | RR: 0.46 EC: −2.2 per 100,000 people | - | - |
| Wan et al. [42] | Li et al. [35] | Shasha et al. [40] | Barda et al. [25] | Klein et al. [33] | Patone et al. [38] | Lai et al. [34] | |
|---|---|---|---|---|---|---|---|
| Bell’s palsy | Non-sig. | Non-sig. | Non-sig. | Non-sig. | Non-sig. | Non-sig. | Non-sig. |
| Paraesthesia | - | - | RR: 1.21 EC: 39.5 per 10,000 person-years | Non-sig. | - | - | - |
| Guillain-Barré syndrome | - | - | Non-sig. | - | Non-sig | Non-sig. | - |
| Seizure or convulsion | - | - | - | Non-sig. | Non-sig. | - | Non-sig. |
| Vertigo | - | - | - | Non-sig. | - | - | - |
| Myelitis | - | - | - | - | Non-sig. | Non-sig. | Non-sig. |
| Encephalomyelitis | - | - | - | - | Non-sig. | - | Non-sig. |
| Encephalitis | - | - | - | - | - | Non-sig. | - |
| Meningitis | - | - | - | - | - | Non-sig. | - |
| Myasthenic disorder | - | - | - | - | - | Non-sig. | - |
| Demyelinating events | - | - | - | - | - | Non-sig. | - |
| Hviid et al. [29] | Whiteley et al. [44] | Xu et al. [61] | Husby et al. [28] | |
|---|---|---|---|---|
| Mortality | Non-sig. | HR: 0.24 ‡ | - | - |
| Non-COVID-19 mortality | - | - | RR: 0.41 ⸸ | - |
| Cardiac arrest or death | - | - | - | HR: 0.51 |
| Barda et al. [25] | Klein et al. [33] | Lai et al. [34] | |
|---|---|---|---|
| Appendicitis | RR: 1.4 EC: 5 per 100,000 people | Non-sig. | - |
| Lymphadenopathy | RR: 2.43 EC: 78.4 per 100,000 people | - | - |
| Anemia | RR: 0.79 EC: −18.7 per 100,000 people | - | - |
| Acute kidney injury | RR: 0.44 EC: −4.6 per 100,000 people | - | HR: 0.58 |
| Acute liver injury | - | - | |
| Pancreatitis | - | - | |
| Acute respiratory distress syndrome | - | - | HR: 0.21 |
| Neutropenia | Non-sig. | - | - |
| Lymphopenia | Non-sig. | - | - |
| Uveitis | Non-sig. | - | - |
| Arthritis | Non-sig. | - | Non-sig. |
| Kawasaki disease | - | Non-sig. | - |
| Thyroiditis | - | - | Non-sig. |
| Type 1 diabetes | - | - | Non-sig. |
| Erythema multiforme | - | - | Non-sig. |
| Chilblain | - | - | Non-sig. |
| Rhabdomyolysis | - | - | Non-sig. |
| Syncope | - | - | Non-sig. |
| Narcolepsy | - | - | Non-sig. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, S.-J.; Halim, A.; Halim, M.; Al Mutair, A.; Alhumaid, S.; Al-Sihati, J.; Albayat, H.; Alsaeed, M.; Garout, M.; Al Azmi, R.; et al. Rare Adverse Events Associated with BNT162b2 mRNA Vaccine (Pfizer-BioNTech): A Review of Large-Scale, Controlled Surveillance Studies. Vaccines 2022, 10, 1067. https://doi.org/10.3390/vaccines10071067
Yong S-J, Halim A, Halim M, Al Mutair A, Alhumaid S, Al-Sihati J, Albayat H, Alsaeed M, Garout M, Al Azmi R, et al. Rare Adverse Events Associated with BNT162b2 mRNA Vaccine (Pfizer-BioNTech): A Review of Large-Scale, Controlled Surveillance Studies. Vaccines. 2022; 10(7):1067. https://doi.org/10.3390/vaccines10071067
Chicago/Turabian StyleYong, Shin-Jie, Alice Halim, Michael Halim, Abbas Al Mutair, Saad Alhumaid, Jehad Al-Sihati, Hawra Albayat, Mohammed Alsaeed, Mohammed Garout, Reyouf Al Azmi, and et al. 2022. "Rare Adverse Events Associated with BNT162b2 mRNA Vaccine (Pfizer-BioNTech): A Review of Large-Scale, Controlled Surveillance Studies" Vaccines 10, no. 7: 1067. https://doi.org/10.3390/vaccines10071067
APA StyleYong, S.-J., Halim, A., Halim, M., Al Mutair, A., Alhumaid, S., Al-Sihati, J., Albayat, H., Alsaeed, M., Garout, M., Al Azmi, R., Aldakheel, N., Alshukairi, A. N., Al Ali, H. A., Almoumen, A. A., & Rabaan, A. A. (2022). Rare Adverse Events Associated with BNT162b2 mRNA Vaccine (Pfizer-BioNTech): A Review of Large-Scale, Controlled Surveillance Studies. Vaccines, 10(7), 1067. https://doi.org/10.3390/vaccines10071067







