The Big Potential of Small Particles: Lipid-Based Nanoparticles and Exosomes in Vaccination
Abstract
:1. Introduction
2. Ex Vivo-Prepared Lipid-Based Nanoparticles
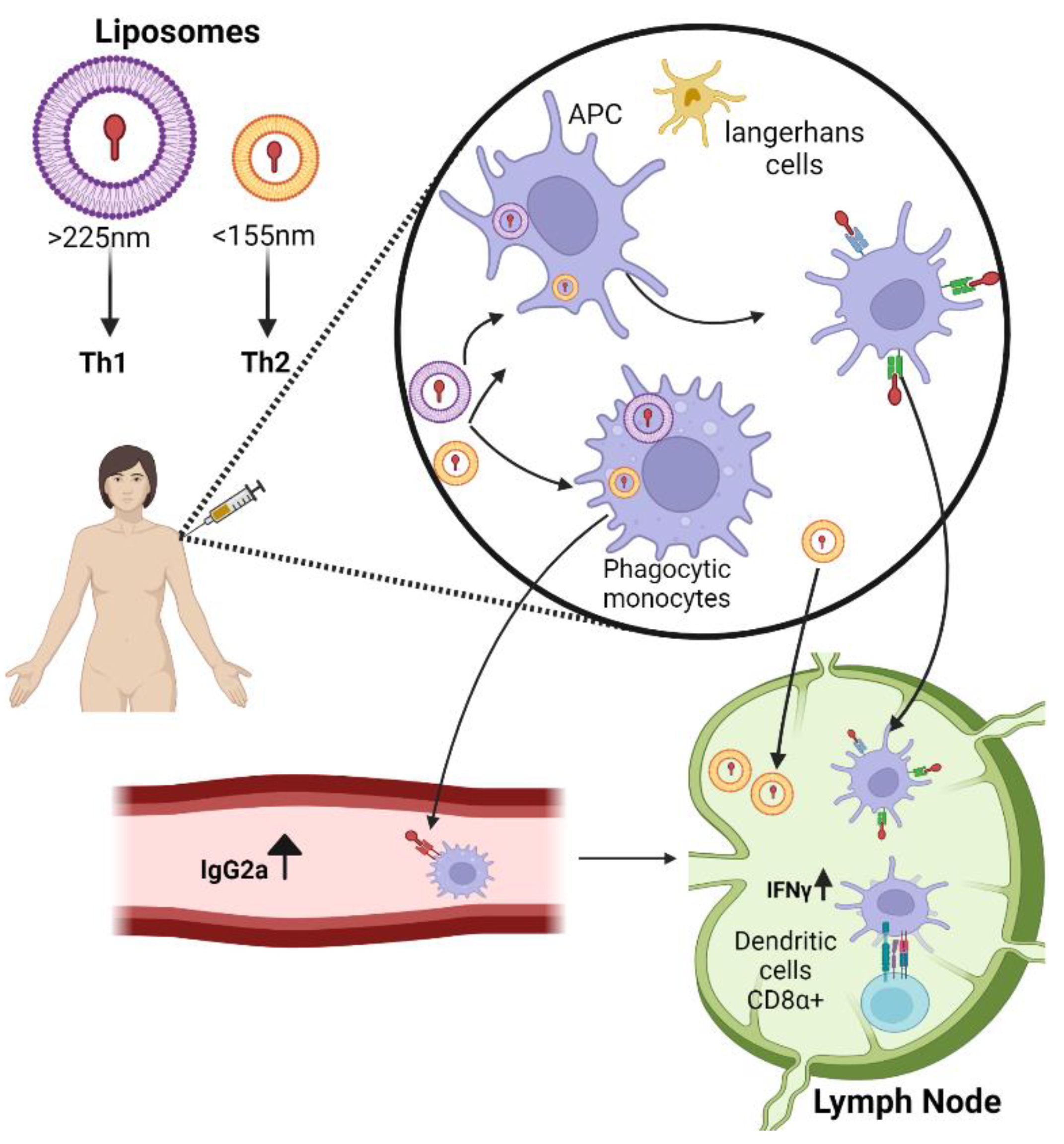
2.1. Liposomes
2.2. Lipid Nanoparticles
3. Cell-Derived Nanoparticles: Exosomes
3.1. Exosome-Based Therapies for COVID-19 in Clinical Trials
3.2. Exosome CD24 (EXO-CD24) Delivery System for COVID-19
3.3. Exosome-Based Therapies—Translational Challenges
4. Author Opinion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fortune Business Insights. Available online: https://www.fortunebusinessinsights.com/industry-reports/vaccines-market-101769 (accessed on 25 June 2022).
- Geall, A.J.; Mandl, C.W.; Ulmer, J.B. RNA: The New Revolution in Nucleic Acid Vaccines. Semin. Immunol. 2013, 25, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.P.; Leite, L.C.C. Recombinant Vaccines and the Development of New Vaccine Strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.J.; Wahren, B.; Liu, M.A. DNA Vaccines: Progress and Challenges. J. Immunol. 2005, 175, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef] [PubMed]
- Rahav, G.; Lustig, Y.; Lavee, J.; Benjamini, O.; Magen, H.; Hod, T.; Shem-Tov, N.; Shmueli, E.S.; Merkel, D.; Ben-Ari, Z.; et al. BNT162b2 MRNA COVID-19 Vaccination in Immunocompromised Patients: A Prospective Cohort Study. eClinicalMedicine 2021, 41, 101158. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- EMA EMA Recommends First COVID-19 Vaccine for Authorisation in the EU. Eur. Med. Agency 2020.
- Burton, D.R. Advancing an HIV Vaccine; Advancing Vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for Translation: Non-Viral Materials for Therapeutic MRNA Delivery. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Schwendener, R.A. Liposomes as Vaccine Delivery Systems: A Review of the Recent Advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, M.; Wang, T. Liposomes Used as a Vaccine Adjuvant-Delivery System: From Basics to Clinical Immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef] [PubMed]
- Janos Szebeni, Y.B. Complement Activation, Immunogenicity, and Immune Suppression as Potential Side Effects of Liposomes. In Advances in Clinical Immunology, Medical Microbiology, COVID-19, and Big Data; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2021. [Google Scholar]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.M.; Tetley, L.; Richmond, J.; Liew, F.Y.; Alexander, J. Lipid Vesicle Size Determines the Th1 or Th2 Response to Entrapped Antigen. J. Immunol. 1998, 161, 4000–4007. [Google Scholar]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- De Serrano, L.O.; Burkhart, D.J. Liposomal Vaccine Formulations as Prophylactic Agents: Design Considerations for Modern Vaccines. J. Nanobiotechnology 2017, 15, 83. [Google Scholar] [CrossRef] [Green Version]
- Askarizadeh, A.; Jaafari, M.R.; Khamesipour, A.; Badiee, A. Liposomal Adjuvant Development for Leishmaniasis Vaccines. Ther. Adv. Vaccines 2017, 5, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Barenholz, Y. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, F.; Persano, S. Advances in Lipid Nanoparticles for MRNA-Based Cancer Immunotherapy. Front. Chem. 2020, 8, 589959. [Google Scholar] [CrossRef]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of Nanotechnology behind the Success of MRNA Vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines-a New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Dolgin, E. The Tangled History of MRNA Vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm. Res. 2021, 38, 473–478. [Google Scholar] [CrossRef]
- Yuba, E.; Kojima, C.; Harada, A.; Tana; Watarai, S.; Kono, K. PH-Sensitive Fusogenic Polymer-Modified Liposomes as a Carrier of Antigenic Proteins for Activation of Cellular Immunity. Biomaterials 2010, 31, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.W.; Kim, J.-D.; Park, K. Polycation Gene Delivery Systems: Escape from Endosomes to Cytosol. J. Pharm. Pharmacol. 2010, 55, 721–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The MRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Huda, M.N.; Nurunnabi, M. Potential Application of Exosomes in Vaccine Development and Delivery. Pharm. Res. 2022, 7, 1–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and Other Extracellular Vesicles in Host–Pathogen Interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assil, S.; Webster, B.; Dreux, M. Regulation of the Host Antiviral State by Intercellular Communications. Viruses 2015, 7, 4707–4733. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Raab-Traub, N.; Dittmer, D.P. Viral Effects on the Content and Function of Extracellular Vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-Based Immunotherapy: A Promising Approach for Cancer Treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef]
- Yang, S.; Kittlesen, D.; Slingluff, C.L.; Vervaert, C.E.; Seigler, H.F.; Darrow, T.L. Dendritic Cells Infected with a Vaccinia Vector Carrying the Human Gp100 Gene Simultaneously Present Multiple Specificities and Elicit High-Affinity T Cells Reactive to Multiple Epitopes and Restricted by HLA-A2 and -A3. J. Immunol. 2000, 164, 4204–4211. [Google Scholar] [CrossRef] [Green Version]
- Lamparski, H.G.; Metha-Damani, A.; Yao, J.Y.; Patel, S.; Hsu, D.H.; Ruegg, C.; Le Pecq, J.B. Production and Characterization of Clinical Grade Exosomes Derived from Dendritic Cells. J. Immunol. Methods 2002, 270, 211–226. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, E.Y.; Chiou, T.W.; Harn, H.J. Exosomes in Clinical Trial and Their Production in Compliance with Good Manufacturing Practice. Tzu Chi Med. J. 2020, 32, 113–120. [Google Scholar]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell-Derived Exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Taïeb, J.; Schartz, N.E.C.; André, F.; Angevin, E.; Zitvogel, L. Exosome-Based Immunotherapy. Cancer Immunol. Immunother. 2004, 53, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.H.; Paz, P.; Villaflor, G.; Rivas, A.; Mehta-Damani, A.; Angevin, E.; Zitvogel, L.; Le Pecq, J.B. Exosomes as a Tumor Vaccine: Enhancing Potency through Direct Loading of Antigenic Peptides. J. Immunother. 2003, 26, 440–450. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of Metastatic Melanoma Patients with Autologous Dendritic Cell (DC) Derived-Exosomes: Results of the First Phase 1 Clinical Trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-Mediated MRNA Delivery in Vivo Is Safe and Can Be Used to Induce SARS-CoV-2 Immunity. J. Biol. Chem. 2021, 297, 101266. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Guo, C.; Atai, N.A.; Gould, S.J. Exosome-Mediated MRNA Delivery For SARS-CoV-2 Vaccination. bioRxiv 2020, 297, 101296. [Google Scholar]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal Therapy—a New Frontier in Regenerative Medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef]
- Huda, M.N.; Nafiujjaman, M.; Deaguero, I.G.; Okonkwo, J.; Hill, M.L.; Kim, T.; Nurunnabi, M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater. Sci. Eng. 2021, 7, 2106–2149. [Google Scholar] [CrossRef]
- Clinicaltrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/results?cond=&term=exosomes&cntry=&state=&city=&dist= (accessed on 16 April 2022).
- Clinicaltrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/results?term=exosome&age_v=&gndr=&type=Intr&rslt=&Search=Apply (accessed on 25 June 2022).
- Clinicaltrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04276987 (accessed on 25 June 2022).
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Álvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in Vitro Stimulated T Cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, F.A.; Saadeldin, I.M.; Ahmad, A.; Kumar, D.; Azhar, E.I.; Siddiqui, A.J.; Kurdi, B.; Sajini, A.; Alrefaei, A.F.; Jahan, S. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells Int. 2020, 2020, 8835986. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04389385 (accessed on 25 June 2022).
- Huber, S.R.; van Beek, J.; de Jonge, J.; Luytjes, W.; van Baarle, D. T Cell Responses to Viral Infections—Opportunities for Peptide Vaccination. Front. Immunol. 2014, 5, 171. [Google Scholar]
- Schmidt, M.E.; Varga, S.M. The CD8 T Cell Response to Respiratory Virus Infections. Front. Immunol. 2018, 9, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, L.A.; Herati, R.S.; Wherry, E.J. CD4+ T Cell Differentiation in Chronic Viral Infections: The Tfh Perspective. Trends Mol. Med. 2017, 23, 1072–1087. [Google Scholar] [CrossRef]
- Clinicaltrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04384445 (accessed on 22 June 2022).
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, X. Immunological Responses against SARS-Coronavirus Infection in Humans. Cell. Mol. Immunol. 2004, 1, 119–122. [Google Scholar]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, P.; Tang, J.; Liu, Y. CD24: From A to Z. Cell. Mol. Immunol. 2010, 7, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, G.Y.; Zheng, P. CD24-Siglec G/10 Discriminates Danger- from Pathogen-Associated Molecular Patterns. Trends Immunol. 2009, 30, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 Selectively Repress Tissue Damage—Induced Immune Responses. Science 2009, 323, 1722–1725. [Google Scholar] [CrossRef] [Green Version]
- Shapira, S.; Kazanov, D.; Weisblatt, S.; Starr, A.; Arber, N.; Kraus, S. The CD24 Protein Inducible Expression System Is an Ideal Tool to Explore the Potential of CD24 as an Oncogene and a Target for Immunotherapy in Vitro and in Vivo. J. Biol. Chem. 2011, 286, 40548–40555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evaluation of the Safety of CD24-Exosomes in Patients With COVID-19 Infection. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04747574 (accessed on 20 June 2022).
- Shapira, S.; Ben Shimon, M.; Hay-Levi, M.; Shenberg, G.; Choshen, G.; Bannon, L.; Tepper, M.; Kazanov, D.; Seni, J.; Lev-Ari, S.; et al. A Novel Platform for Attenuating Immune Hyperactivity Using EXO-CD24 in Covid-19 and Beyond. EMBO Mol. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- CD24Fc (MK-7110) as a Non-Antiviral Immunomodulator in COVID-19 Treatment (MK-7110-007) (SAC-COVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04317040 (accessed on 20 June 2022).
- Song, N.J.; Allen, C.; Vilgelm, A.E.; Riesenberg, B.P.; Weller, K.P.; Reynolds, K.; Chakravarthy, K.B.; Kumar, A.; Khatiwada, A.; Sun, Z.; et al. Treatment with Soluble CD24 Attenuates COVID-19-Associated Systemic Immunopathology. J. Hematol. Oncol. 2022, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Welker, J.; Pulido, J.D.; Catanzaro, A.T.; Malvestutto, C.D.; Li, Z.; Cohen, J.B.; Whitman, E.D.; Byrne, D.; Giddings, O.K.; Lake, J.E.; et al. Efficacy and Safety of CD24Fc in Hospitalised Patients with COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Infect. Dis. 2022, 22, 611–621. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Deng, W.; Klinke, D.J. Exosomes: Improved Methods to Characterize Their Morphology, RNA Content, and Surface Protein Biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef] [Green Version]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative Proteomics Identifies the Core Proteome of Exosomes with Syntenin-1 as the Highest Abundant Protein and a Putative Universal Biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef]
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440. [Google Scholar] [CrossRef]
- Miao, C.; Wang, X.; Zhou, W.; Huang, J. The Emerging Roles of Exosomes in Autoimmune Diseases, with Special Emphasis on MicroRNAs in Exosomes. Pharmacol. Res. 2021, 169, 105680. [Google Scholar] [CrossRef]
- Jadli, A.S.; Parasor, A.; Gomes, K.P.; Shandilya, R.; Patel, V.B. Exosomes in Cardiovascular Diseases: Pathological Potential of Nano-Messenger. Front. Cardiovasc. Med. 2021, 8, 767488. [Google Scholar] [CrossRef]
- Li, C.; Huang, N.; Luo, X.; Li, J.; Liao, S.; Lin, C.; Li, B.; Yang, F.; Liu, Y. Research Progress of RNA Carried by Exosomes in Malignant Bone Neoplasm. Chin. J. Orthop. 2020, 40, 1981–1996. [Google Scholar]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]

| Exosome Source | Disease | Loaded Component | Rout of Administration | Phase | End | Clinical Trial Identification Number |
| MSCs | Coronavirus pneumonia | None | Inhalation | I | 2020 | NCT04276987 |
| Human placenta MSCs | Complex perianal fistula | None | Fistula tact injection | I/II | Ongoing | NCT05402748 |
| Allogenic MSCs | Acute ischemic stroke | miR-124 | Stereotaxis/intraparenchymal | I/II | Ongoing | NCT03384433 |
| MSCs | COVID-19 | None | I.V | I/II | Ongoing | NCT04798716 |
| MSCs | COVID-19 | None | I.V | II/III | Ongoing | NCT05216562 |
| Mesenchymal progenitor cell | Microbial pulmonary infection | None | Inhalation | I/II | Ongoing | NCT04544215 |
| Mesenchymal stromal cells | Pancreas cancer | KrasG12D siRNA | I.V | I | Ongoing | NCT03608631 |
| MSCs | Epidermolysis bullosa | None | Dermal | I/II | Enrolled | NCT04173650 |
| Adipose MSCs | Alzheimer | None | Nasal drip | I/II | Ongoing | NCT04388982 |
| MSCs | COVID-19 | None | Inhalation | I/II | 2020 | NCT04491240 |
| Autologous adipose-derived stem cells | Periodontitis | None | Periodontal pockets injection | I | Ongoing | NCT04270006 |
| Umbilical cord blood-derived MSCs | Type I diabetes mellitus | None | I.V | I/III | Ongoing | NCT02138331 |
| MSCs | Knee osteoarthritis | None | Intra-articular injection | I | Ongoing | NCT05060107 |
| Autologous plasma | Cutaneous ulcers | None | Dermal | I | Ongoing | NCT02565264 |
| Platelet-rich plasma (PRP) enriched with exosomes | Chronic low back pain | None | Nucleus pulposus | I | Ongoing | NCT04849429 |
| Dendritic cells | Non-small cell lung cancer | Tumour antigen | n.d | II | 2018 | NCT01159288 |
| T cell | COVID-19 | None | Inhalation | I | Ongoing | NCT04389385 |
| Hek293 cell line | COVID-19 | CD24 * | Inhalation | II | Ongoing | NCT04969172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimon, M.B.; Shapira, S.; Seni, J.; Arber, N. The Big Potential of Small Particles: Lipid-Based Nanoparticles and Exosomes in Vaccination. Vaccines 2022, 10, 1119. https://doi.org/10.3390/vaccines10071119
Shimon MB, Shapira S, Seni J, Arber N. The Big Potential of Small Particles: Lipid-Based Nanoparticles and Exosomes in Vaccination. Vaccines. 2022; 10(7):1119. https://doi.org/10.3390/vaccines10071119
Chicago/Turabian StyleShimon, Marina Ben, Shiran Shapira, Jonathan Seni, and Nadir Arber. 2022. "The Big Potential of Small Particles: Lipid-Based Nanoparticles and Exosomes in Vaccination" Vaccines 10, no. 7: 1119. https://doi.org/10.3390/vaccines10071119





